The Need for Breathing Training Techniques: The Elephant in the Heart Failure Cardiac Rehabilitation Room: A Randomized Controlled Trial
Abstract
Highlights
- Standard cardiac rehabilitation (CR) programs do not typically consider respiratory symptoms.
- Breathing exercises (BE) have a positive physiological effect on chronic heart failure (CHF).
- Future CR programs for CHF have to manage respiratory symptoms.
- CR programs for CHF have to address patient-centered outcomes.
Abstract
1. Introduction
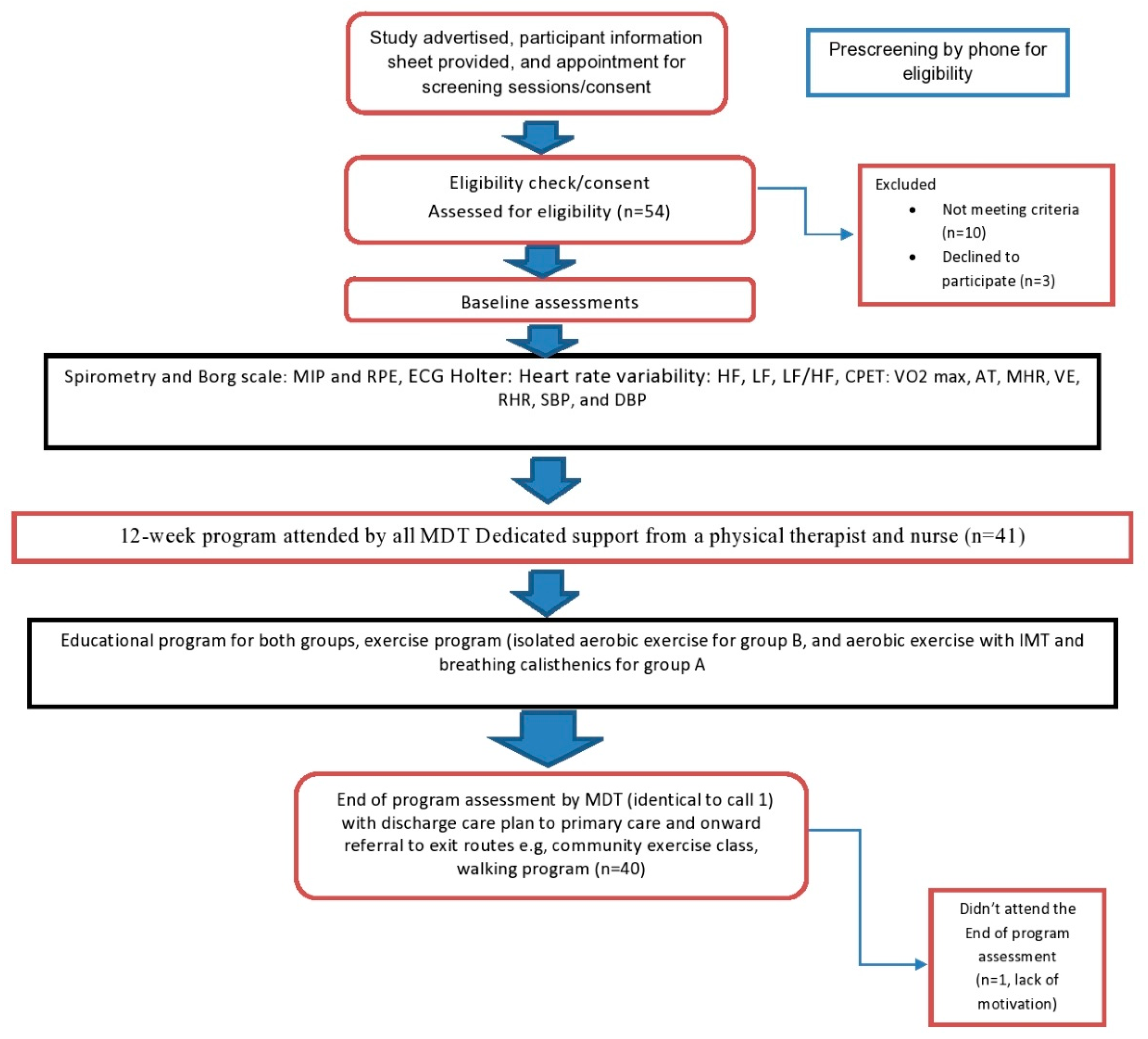
2. Methods
2.1. Study Design and Setting
2.2. Study Population
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Randomization and Grouping
2.4. Assessment
2.4.1. Pulmonary Function Testing (PFT): Spirometry System with Shutter (Zan Type 100-Germany)
2.4.2. Modified Borg Scale
2.4.3. Twenty-Four Hour Ambulatory Electrocardiography (ECG) Recording (Holter)
2.4.4. Cardiopulmonary Exercise Test (CPET)
2.5. Intervention
2.5.1. Standard Cardiac Rehabilitation Program (Groups A and B)
2.5.2. Cardiac Rehabilitation Integration with Breathing Training (Group A)
2.6. Outcome Measures
2.6.1. Primary Outcomes
2.6.2. Secondary Outcomes
2.7. Statistical Methods and Sample Size Calculations
3. Results
3.1. Respiratory Outcomes
3.2. Cardiovascular Outcomes
3.3. Cardiopulmonary Outcomes
4. Discussion
4.1. Respiratory Outcomes
4.2. Cardiovascular Outcomes
4.3. Cardiopulmonary Outcomes
4.4. Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| AT | Anaerobic threshold |
| BC | Breathing calisthenics |
| BE | Breathing exercises |
| CHF | Chronic heart failure |
| CPET | Cardiopulmonary exercise testing |
| CR | Cardiac rehabilitation |
| DBP | Diastolic blood pressure |
| ECG | Electrocardiography |
| FEV1 | Forced expiratory volume in the first second |
| FVC | Forced vital capacity |
| HF | High-frequency |
| HR | Heart rate |
| HRV | Heart rate variability |
| IMT | Inspiratory muscle training |
| LF | Low-frequency |
| MDT | Multidisciplinary team |
| MHR | Maximal heart rate |
| MIP | Maximum inspiratory pressure |
| NYHA | New York Heart Association |
| RCT | Randomized controlled study |
| RHR | Resting heart rate |
| RPE | Rate of perceived exertion |
| RSBP | Resting systolic blood pressure |
| RDBP | Resting diastolic blood pressure |
| RPE | Rating of perceived exertion |
| SA | Sinoatrial |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| VE | Maximal minute ventilation |
| VO2 max | Maximal oxygen capacity |
References
- Koshy, A.O.; Gallivan, E.R.; McGinlay, M.; Straw, S.; Drozd, M.; Toms, A.G.; Gierula, J.; Cubbon, R.M.; Kearney, M.T.; Witte, K.K. Prioritizing symptom management in the treatment of chronic heart failure. ESC Heart Fail. 2020, 7, 2193–2207. [Google Scholar] [CrossRef] [PubMed]
- Nayor, M.; Houstis, N.E.; Namasivayam, M.; Rouvina, J.; Hardin, C.; Shah, R.V.; Ho, J.E.; Malhotra, R.; Lewis, G.D. Impaired Exercise Tolerance in Heart Failure With Preserved Ejection Fraction: Quantification of Multiorgan System Reserve Capacity. JACC Heart Fail. 2020, 8, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Salyer, J.; Flattery, M.; Lyon, D.E. Heart failure symptom clusters and quality of life. Heart Lung 2019, 48, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Mordi, I.R.; Bridges, C.; Sagar, V.A.; Davies, E.J.; Coats, A.J.S.; Dalal, H.; Rees, K.; Singh, S.J.; Taylor, R.S. Exercise-based cardiac rehabilitation for adults with heart failure (Review). Cochrane Libr. Cochrane Database Syst. Rev. 2019, 1, CD003331. [Google Scholar] [CrossRef]
- Clark, A.L. Origin of symptoms in chronic heart failure. Heart 2006, 92, 12–16. [Google Scholar] [CrossRef]
- Aimo, A.; Saccaro, L.F.; Borrelli, C.; Fabiani, I.; Gentile, F.; Passino, C.; Emdin, M.; Piepoli, M.F.; Coats, A.J.; Giannoni, A. The ergoreflex: How the skeletal muscle modulates ventilation and cardiovascular function in health and disease. Eur. J. Heart Fail. 2021, 23, 1458–1467. [Google Scholar] [CrossRef]
- Neto, M.G.; Martinez, B.P.; Conceição, C.S.; Silva, P.E.; Carvalho, V. Combined Exercise and Inspiratory Muscle Training in Patients With Heart Failure. J. Cardiopulm. Rehabil. Prev. 2016, 36, 395–401. [Google Scholar] [CrossRef]
- Cornelis, J.; Taeymans, J.; Hens, W.; Beckers, P.; Vrints, C.; Vissers, D. Prognostic respiratory parameters in heart failure patients with and without exercise oscillatory ventilation—A systematic review and descriptive meta-analysis. Int. J. Cardiol. 2015, 182, 476–486. [Google Scholar] [CrossRef]
- Jennings, C.; Kotseva, K.; De Bacquer, D.; Hoes, A.; de Velasco, J.; Brusaferro, S.; Mead, A.; Jones, J.; Tonstad, S.; Wood, D.; et al. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: The EUROACTION PLUS varenicline trial. Eur. Heart J. 2014, 35, 1411–1420. [Google Scholar] [CrossRef]
- Connolly, S.B.; Kotseva, K.; Jennings, C.; Atrey, A.; Jones, J.; Brown, A.; Bassett, P.; Wood, D.A. Outcomes of an integrated community-based nurse-led cardiovascular disease prevention programme. Heart 2017, 103, 840–847. [Google Scholar] [CrossRef]
- Mcgaffin, S.; Taggart, M.; Smyth, D.; O’doherty, D.; Brown, J.; Teague, S.; Slevin, C.; Montgomery, L.; Coll, M.; Lindsay, C.; et al. Transitioning a cardiovascular health and rehabilitation programme to a virtual plat-form during COVID-19. Eur. J. Cardiovasc. Nurs. 2021, 20, zvab060.073. [Google Scholar] [CrossRef]
- Scherrenberg, M.; Wilhelm, M.; Hansen, D.; Völler, H.; Cornelissen, V.; Frederix, I.; Kemps, H.; Dendale, P. The future is now: A call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 28, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Ades, P.A.; Keteyian, S.J.; Wright, J.S.; Hamm, L.F.; Lui, K.; Newlin, K.; Shepard, D.S.; Thomas, R.J. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin. Proc. 2022, 92, 234–242. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Whellan, D.J.; Fiuzat, M.; Punjabi, N.M.; Tasissa, G.; Anstrom, K.J.; Benjafield, A.V.; Woehrle, H.; Blasé, A.B.; Lindenfeld, J. Cardiovascular Outcomes With Minute Ventilation–Targeted Adaptive Servo-Ventilation Therapy in Heart Failure: The CAT-HF Trial. J. Am. Coll. Cardiol. 2017, 69, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, N.; Masuda, T.; Kamiya, K.; Matsuzawa, R.; Nozaki, K.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; Ako, J. Respiratory muscle weakness increases dead-space ventilation ratio aggravating ventilation–perfusion mismatch during exercise in patients with chronic heart failure. Respirology 2019, 24, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Alkan, H.O.; Uysal, H.İ.; Enç, N.U.; Yigit, Z. Influence of Breathing Exercise Education Applied on Patients with Heart Failure on Dyspnoea and Quality of Sleep: A Randomized Controlled Study. Int. J. Med. Res. Health Sci. 2017, 6, 107–113. [Google Scholar]
- Wu, J.; Kuang, L.; Fu, L. Effects of inspiratory muscle training in chronic heart failure patients: A systematic review and meta-analysis. Congenit. Heart Dis. 2018, 13, 194–202. [Google Scholar] [CrossRef]
- Abeer, A.F.; Zeinab, M.H.; Haidy, M.; Aymn, S. Effect of Deep Breathing on Heart Rate Variability Following Coronary Artery Bypass Graft. Med. J. Cairo Univ. 2019, 87, 5179–5186. [Google Scholar] [CrossRef]
- McNamara, R.J.; Spencer, L.; Dale, M.; Leung, R.W.; McKeough, Z.J. Alternative Exercise and Breathing Interventions in Chronic Obstructive Pulmonary Disease: A Critical Review. EMJ Respir. 2018, 6, 117–127. [Google Scholar]
- CONSORT. CONSORT 2010 Flow Diagram Follow-Up. 2010; p. 2010. [Google Scholar]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Malaeva, V.V.; Korenbaum, V.I.; Pochekutova, I.A.; Kostiv, A.E.; Shin, S.N.; Katuntsev, V.P.; Baranov, V.M. A technique of forced expiratory noise time evaluation provides distinguishing human pulmonary ventilation dynamics during long-term head-down and head-up tilt bed rest tests simulating micro and lunar gravity. Front. Physiol. 2018, 9, 1255. [Google Scholar] [CrossRef] [PubMed]
- Hursh, D.G.; Baranauskas, M.N.; Wiggins, C.C.; Bielko, S.; Mickleborough, T.D.; Chapman, R. Chapman, Inspiratory Muscle Training: Improvement of Exercise Performance With Acute Hypoxic Exposure. Int. J. Sports Physiol. Perform. 2019, 14, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Atef, H.; Abdeen, H. Effect of exercise on sleep and cardiopulmonary parameters in patients with pulmonary artery hypertension. Sleep Breath. 2021, 25, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Pachon-M, J.C.; Pachon-M, E.I.; Pachon, C.T.C.; Santillana-P, T.G.; Lobo, T.J.; Pachon-M, J.C.; Zerpa-A, J.C.; Cunha-P, M.Z.; Higuti, C.; Ortencio, F.A.; et al. Long-Term Evaluation of the Vagal Denervation by Cardioneuroablation Using Holter and Heart Rate Variability. Circ. Arrhythmia Electrophysiol. 2020, 13, e008703. [Google Scholar] [CrossRef]
- Abolahrari-Shirazi, S.; Kojuri, J.; Bagheri, Z.; Rojhani-Shirazi, Z. Effect of exercise training on heart rate variability in patients with heart failure after percutaneous coronary intervention. J. Biomed. Phys. Eng. 2019, 9, 97–104. [Google Scholar] [CrossRef]
- Takken, T.; Mylius, C.; Paap, D.; Broeders, W.; Hulzebos, H.; Van Brussel, M.; Bongers, B. Reference values for cardiopulmonary exercise testing in healthy subjects—An updated systematic review. Expert Rev. Cardiovasc. Ther. 2019, 17, 413–426. [Google Scholar] [CrossRef]
- Giallauria, F.; Piccioli, L.; Vitale, G.; Sarullo, F.M. Exercise training in patients with chronic heart failure: A new challenge for cardiac rehabilitation community. Monaldi Arch. Chest Dis. 2018, 88, 38–44. [Google Scholar] [CrossRef]
- Sadek, Z.; Salami, A.; Joumaa, W.H.; Awada, C.; Ahmaidi, S.; Ramadan, W. Best mode of inspiratory muscle training in heart failure patients: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2018, 25, 1691–1701. [Google Scholar] [CrossRef]
- Sadek, Z.; Salami, A.; Youness, M.; Awada, C.; Hamade, M.; Joumaa, W.H.; Ramadan, W.; Ahmaidi, S. A randomized controlled trial of high-intensity interval training and inspiratory muscle training for chronic heart failure patients with inspiratory muscle weakness. Chronic Illn. 2020, 18, 140–154. [Google Scholar] [CrossRef]
- Barnett, L.; Prior, J.A.; Kadam, U.T.; Jordan, K. Chest pain and shortness of breath in cardiovascular disease: A prospective cohort study in UK primary care. BMJ Open 2017, 7, e015857. [Google Scholar] [CrossRef]
- Kleber, F.X.; Vietzke, G.; Wernecke, K.D.; Bauer, U.; Opitz, C.; Wensel, R.; Sperfeld, A.; Gläser, S. Impairment of ventilatory efficiency in heart failure: Prognostic impact. Circulation 2000, 101, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Catela, D.P.R. Effect of Slow Diaphragmatic Breathing Technique on Heart Rate, Blood Pressure and Peripheral Oxygen Saturation in Hypertensive Elderly. Open Access J. Biomed. Sci. 2021, 3, 780–784. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.P.; Danzmann, L.C.; Moraes, R.S.; Vieira, P.J.C.; Meurer, F.F.; Soares, D.S.; Chiappa, G.; Guimarâes, L.S.P.; Leitão, S.A.T.; Ribeiro, J.P.; et al. Yoga and breathing technique training in patients with heart failure and preserved ejection fraction: Study protocol for a randomized clinical trial. Trials 2018, 19, 405. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Lijauco, C.C.; Hearon, C.M.; Angadi, S.S.; Nelson, M.D.; Sarma, S.; Nanayakkara, S.; La Gerche, A.; Haykowsky, M.J. Mechanisms of the Improvement in Peak VO2 With Exercise Training in Heart Failure With Reduced or Preserved Ejection Fraction. Heart Lung Circ. 2018, 27, 9–21. [Google Scholar] [CrossRef]
- Borghi-Silva, A.; Goulart, C.D.L.; Carrascosa, C.R.; Oliveira, C.C.; Berton, D.C.; de Almeida, D.R.; Nery, L.E.; Arena, R.; Neder, J.A. Proportional Assist Ventilation Improves Leg Muscle Reoxygenation After Exercise in Heart Failure with Reduced Ejection Fraction. Front. Physiol. 2021, 12, 685274. [Google Scholar] [CrossRef]
- Abreu, A.; Mendes, M.; Dores, H.; Silveira, C.; Fontes, P.; Teixeira, M.; Clara, H.S.; Morais, J. Mandatory criteria for cardiac rehabilitation programs: 2018 guidelines from the Portuguese Society. Rev. Port. Cardiol. 2018, 37, 363–373. [Google Scholar] [CrossRef]
- Wenger, N.K. Current Status of Cardiac Rehabilitation. J. Am. Coll. Cardiol. 2008, 51, 1619–1631. [Google Scholar] [CrossRef]
| Points | Inspiratory Muscle Training (IMT) | Breathing Calisthenics |
|---|---|---|
| Definition | IMT is a device used for respiratory muscle training to improve the function of the respiratory muscles through specific exercises. The IMT device (Respironics, Chichester, UK) is very easy to use and practical and does not require large amounts of money. | A series of breathing exercises performed as eight repetitions of each exercise were completed. These exercises strengthened the abdominal muscles |
| Method of use | The patient who utilized this IMT was asked to inspire deeply through the mouthpiece of it against the selected load with all prescribed medications. | In the supine position, the legs were flexed to the abdomen as the patient exhaled (abdominal knee bend exercise). In a separate exercise, the head and shoulders were raised from the mat as the patient exhaled (abdominal sit-up exercise). In a third exercise, the patient was seated. He was asked to clench both hands behind the head and to move them back and forth during inspiration and expiration, respectively pulling his belly inward with each inhalation. |
| Intensity | The initial workload is measured as 30% and increased gradually to 60% of MIP. Subject trains in the initial workload for 2 weeks, then the target workload was increased by 5% every 2 weeks | The training intensity was adjusted over the weeks by using the sensation of dyspnea as a parameter, keeping it between 4 and 6 on the Borg CR 10 scale |
| Duration | Sessions were divided into six sets (12 weeks) five minutes in duration, and separated by 5 min of rest. | Sessions were divided into three sets, eight repetitions of each exercise, separated by 5 min of rest in between sets. |
| Frequency | six times/week [26] | six times/week [29] |
| Variable | IMT Group | Control Group | MD | t-Value | p-Value |
|---|---|---|---|---|---|
| X ± SD | X ± SD | ||||
| Age (year) | 55.55 ± 8.36 | 55.90 ± 6.54 | 0.40 | 1.49 | 0.883 |
| Height (cm) | 174 ± 12.49 | 173 ± 10.49 | 1.1 | 0.102 | 0.919 |
| Weight (kg) | 85.650 ± 17.623 | 79.45 ± 20.43 | 6.2 | 1.764 | 0.094 |
| EF (%) | 33.10 ± 4.52 | 33.30 ± 4.54 | 0.20 | 0.135 | 0.880 |
| NYHA | 2.65 ± 0.48 | 2.50 ± 0.51 | 0.15 | 1.00 | 0.330 |
| Respiratory and Cardiovascular Outcomes | Pre-Study Mean ± SD | Post-Study Mean ± SD | % of Change | p-Value |
|---|---|---|---|---|
| MIP | ||||
| Group A | 35.9 ± 2.7 | 43.3 ± 3.6 | 20.6% | 0.001 * |
| Group B | 35.6 ± 2.9 | 36.7 ± 3.3 | 3% | 0.277 |
| p-value | 0.727 | 0.001 * | ||
| RPE | ||||
| Group A | 4.3 ± 0.5 | 3.4 ± 0.6 | 20.9% | 0.001 * |
| Group B | 4.4 ± 0.6 | 3.8 ± 0.6 | 13.6% | 0.001 * |
| p-value | 0.584 | 0.039 * | ||
| LF | ||||
| Group A | 66.3 ± 2.5 | 65.1 ± 2 | 2% | 0.074 |
| Group B | 66.5 ± 1.9 | 65.6 ± 1.6 | 1.4% | 0.140 |
| p-value | 0.674 | 0.460 | ||
| HF | ||||
| Group A | 6.5 ± 0.6 | 9.1 ± 1.4 | 40% | 0.001 * |
| Group B | 6.5 ± 0.8 | 8.3 ± 1 | 27.7% | 0.001 * |
| p-value | 0.999 | 0.013 * | ||
| LF/HF | ||||
| Group A | 10.2 ± 1.1 | 7.2 ± 1.1 | 29.4% | 0.001 * |
| Group B | 10.4 ± 1.4 | 8 ± 1.1 | 23% | 0.001 * |
| p-value | 0.766 | 0.056 | ||
| RHR | ||||
| Group A | 86.1 ± 4 | 77.8 ± 4.7 | 9.6% | 0.001 * |
| Group B | 85.8 ± 4.9 | 81.4 ± 5.3 | 5.1% | 0.004 * |
| p-value | 0.868 | 0.019 * | ||
| RSBP | ||||
| Group A | 136.8 ± 4.3 | 126.4 ± 5.1 | 7.6% | 0.001 * |
| Group B | 136 ± 4 | 130.3 ± 5 | 4.2% | 0.001 * |
| p-value | 0.564 | 0.009 * | ||
| RDBP | ||||
| Group A | 81.8 ± 2.9 | 78.1 ± 2.4 | 4.5% | 0.001 * |
| Group B | 82 ± 2 | 81.1 ± 2 | 1% | 0.250 |
| p-value | 0.827 | 0.001 * |
| Cardiovascular Outcomes | Pre-Study Mean ± SD | Post-Study Mean ± SD | % of Change | p-Value |
|---|---|---|---|---|
| VO2 max | ||||
| Group A | 14.3 ± 1.4 | 15.8 ± 1.3 | 10.5% | 0.001 * |
| Group B | 14.5 ± 1.2 | 14.9 ± 1.2 | 3% | 0.307 |
| p-value | 0.735 | 0.045 * | ||
| AT | ||||
| Group A | 51.3 ± 1.8 | 60.7 ± 6.5 | 18.3% | 0.001 * |
| Group B | 51.1 ± 1.9 | 54.5 ± 3.2 | 6.6% | 0.007 * |
| p-value | 0.936 | 0.001 * | ||
| MHR (beat/min) | ||||
| Group A | 118.3 ± 6.6 | 123.1 ± 5.1 | 4% | 0.013 * |
| Group B | 120 ± 6 | 122.4 ± 5.8 | 2% | 0.205 |
| p-value | 0.354 | 0.730 | ||
| VE | ||||
| Group A | 46.4 ± 1.2 | 40.4 ± 1.4 | 12.9% | 0.001 * |
| Group B | 46.2 ± 1.3 | 46 ± 1.4 | 0.4% | 0.657 |
| p-value | 0.729 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farghaly, A.; Fitzsimons, D.; Bradley, J.; Sedhom, M.; Atef, H. The Need for Breathing Training Techniques: The Elephant in the Heart Failure Cardiac Rehabilitation Room: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 14694. https://doi.org/10.3390/ijerph192214694
Farghaly A, Fitzsimons D, Bradley J, Sedhom M, Atef H. The Need for Breathing Training Techniques: The Elephant in the Heart Failure Cardiac Rehabilitation Room: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(22):14694. https://doi.org/10.3390/ijerph192214694
Chicago/Turabian StyleFarghaly, Abeer, Donna Fitzsimons, Judy Bradley, Magda Sedhom, and Hady Atef. 2022. "The Need for Breathing Training Techniques: The Elephant in the Heart Failure Cardiac Rehabilitation Room: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 22: 14694. https://doi.org/10.3390/ijerph192214694
APA StyleFarghaly, A., Fitzsimons, D., Bradley, J., Sedhom, M., & Atef, H. (2022). The Need for Breathing Training Techniques: The Elephant in the Heart Failure Cardiac Rehabilitation Room: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 19(22), 14694. https://doi.org/10.3390/ijerph192214694






