Effectiveness of Autogenic Drainage in Improving Pulmonary Function in Patients with Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Applied Respiratory Therapies
2.3. Spirometric Examination and Saturation
2.4. Rating Scales for Dyspnea and Fatigue
2.5. Statistical Analysis
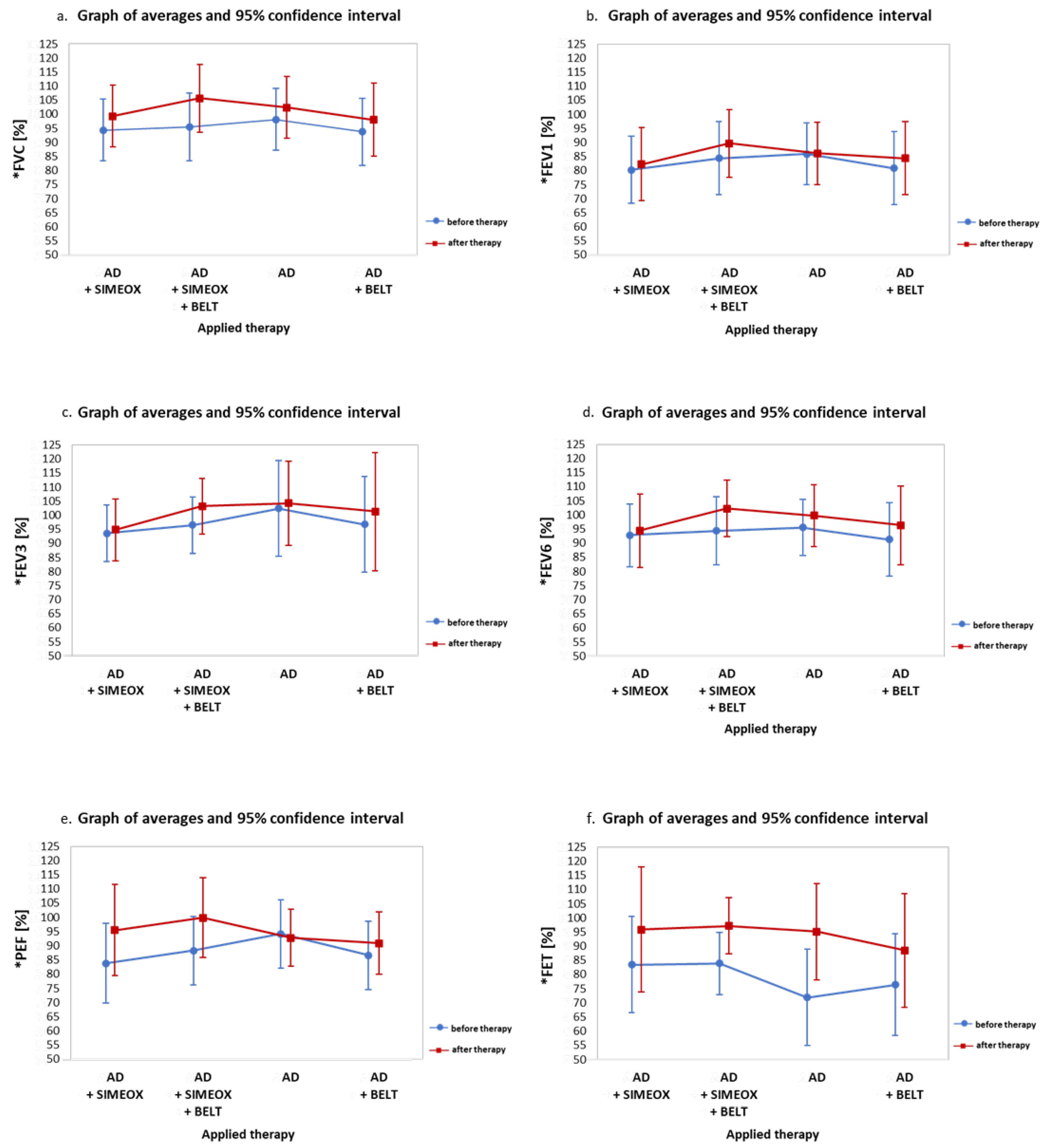
3. Results
3.1. Analysis of Respiratory Parameters
3.2. Analysis of Blood Oxygen Saturation Levels
3.3. Assessment of Dyspnea and Fatigue
3.4. Analysis of Therapy Efficiency Based on the Studied Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conway, S.; Balfour-Lynn, I.M.; De Rijcke, K.; Drevinek, P.; Foweraker, J.; Havermans, T.; Heijerman, H.; Lannefors, L.; Lindblad, A.; Macek, M.; et al. European Cystic Fibrosis Society Standards of Care: Framework for the Cystic Fibrosis Centre. J. Cyst. Fibros. 2014, 13, S3–S22. [Google Scholar] [CrossRef]
- Chevaillier, J. Autogenic Drainage: The Flow and Breathing Level Modulation Concept; Servei de Publicacions de la Universitat Autònoma de Barcelona: Barcelona, Spain, 2016; Volume 9. [Google Scholar]
- Barthe, J. Recommandations des journées internationales de kinésithérapie respiratoire instrumentale (JIKRI). Cah. De Kinésithérapie 2001, 209, 11–25. [Google Scholar]
- Cabillic, M.; Gouilly, P.; Reychler, G. Manual airway clearance techniques in adults and adolescents: What level of evidence? Rev. Mal. Respir. 2018, 35, 495–520. [Google Scholar] [CrossRef]
- Button, B.M.; Wilson, C.; Dentice, R.; Cox, N.S.; Middleton, A.; Tannenbaum, E.; Bishop, J.; Cobb, R.; Burton, K.; Wood, M.; et al. Physiotherapy for cystic fibrosis in Australia and New Zealand: A clinical practice guideline. Respirology 2016, 21, 656–667. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.; Burnham, P.; Southern, K.W. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database Syst. Rev. 2017, 10, CD009595. [Google Scholar] [CrossRef]
- Miossec, M. Drainage Autogène (DA) versus Expiration Lente et Totale Glotte Ouverte (ELTGOL) pour le Désencombrement Bronchique des Patients Atteints de Mucoviscidose. 2017. Available online: https://dumas.ccsd.cnrs.fr/dumas-01739642/document (accessed on 27 September 2021).
- Chevaillier, J. Autogenic Drainage (AD) “The Concept of Flow and Breathing Level Modulation”. Physiotherapy for People with Cystic Fibrosis: From Infant to Adult. Available online: https://www.ecfs.eu/sites/default/files/general-content-files/working-groups/IPG%20CF_Blue%20Booklet_7th%20edition%202019.pdf (accessed on 28 September 2021).
- McCormack, P.; Burnham, P.; Southern, K.W. A systematic Cochrane Review of autogenic drainage (AD) for airway clearance in cystic fibrosis. Paediatr. Respir. Rev. 2019, 29, 23–24. [Google Scholar] [CrossRef] [PubMed]
- McIlwaine, M.; Wong, L.T.; Chilvers, M.; Davidson, G.F. Long-Term Comparative Trial of Two Different Physiotherapy Techniques; Postural Drainage with Percussion and Autogenic Drainage, in the treatment of Cystic Fibrosis. Pediatr. Pulmonol. 2010, 45, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- App, E.M.; Kieselmann, R.; Reinhardt, D.; Lindemann, H.; Dasgupta, B.; King, M.; Brand, P. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy—Flutter vs autogenic drainage. Chest 1998, 114, 171–177. [Google Scholar] [CrossRef] [PubMed]
- McIlwaine, P.; Davidson, A.; Wong, L.; Pirie, G.; Nakielna, E. In Comparison of positive expiratory pressure and autogenic drainage with conventional percussion and drainage therapy in the treatment of cystic fibrosis. In Proceedings of the 17th European Cystic Fibrosis Conference, Copenhagen, Denmark, 18–21 June 1991; pp. 18–21. [Google Scholar]
- Osman, L.P.; Roughton, M.; Hodson, M.E.; Pryor, J.A. Short-term comparative study of high frequency chest wall oscillation and European airway clearance techniques in patients with cystic fibrosis. Thorax 2010, 65, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Hall, D.O.; Clayton, C.B.; Nelson, R. Chest physiotherapy in cystic fibrosis: A comparative study of autogenic drainage and the active cycle of breathing techniques with postural drainage. Thorax 1995, 50, 165–169. [Google Scholar] [CrossRef][Green Version]
- Pryor, J.A.; Tannenbaum, E.; Scott, S.F.; Burgess, J.; Cramer, D.; Gyi, K.; Hodson, M.E. Beyond postural drainage and percussion: Airway clearance in people with cystic fibrosis. J. Cyst. Fibros. 2010, 9, 187–192. [Google Scholar] [CrossRef]
- Sokol, G.; Vilozni, D.; Hakimi, R.; Lavie, M.; Sarouk, I.; Bar, B.-E.; Dagan, A.; Ofek, M.; Efrati, O. The Short-Term Effect of Breathing Tasks Via an Incentive Spirometer on Lung Function Compared with Autogenic Drainage in Subjects with Cystic Fibrosis. Respir. Care 2015, 60, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, A.; Theissl, B.; Oberwaldner, B.; Zach, M.S. Self-administered chest physiotherapy in cystic fibrosis: A comparative study of high-pressure PEP and autogenic drainage. Lung 1992, 170, 323–330. [Google Scholar] [CrossRef]
- Wallaert, E.; Perez, T.; Prevotat, A.; Reychler, G.; Wallaert, B.; Le Rouzic, O. The immediate effects of a single autogenic drainage session on ventilatory mechanics in adult subjects with cystic fibrosis. PLoS ONE 2018, 13, e0195154. [Google Scholar] [CrossRef]
- Walicka-Serzysko, K.M.; Sands, D.A. Application of the SIMEOX airway clearance technology in the treatment of cystic fibrosis pulmonary exacerbation-a case report. Simeox Clin. Noteb. 2018, 1, 1–8. [Google Scholar]
- Lafforgue, O.; Seyssiecq, I.; Poncet, S.; Favier, J. Rheological properties of synthetic mucus for airway clearance. J. Biomed. Mater. Res. A 2018, 106, 386–396. [Google Scholar] [CrossRef]
- Lafforgue, O.; Poncet, S.; Seyssiecq, I.; Favier, J. Rheological Characterization of Macromolecular Colloidal Gels as Simulant of Bronchial Mucus. Aip Conf. Proc. 2017, 1914, 110003. [Google Scholar]
- Lafforgue, O.; Bouguerra, N.; Poncet, S.; Seyssiecq, I.; Favier, J.; Elkoun, S. Thermo-physical properties of synthetic mucus for the study of airway clearance. J. Biomed. Mater. Res. A 2017, 105, 3025–3033. [Google Scholar] [CrossRef]
- MATIO Kwartalnik Fundacji Pomocy Rodzinom i Chorym na Mukowiscydozę. MATIO 2017, 4, 10–14.
- Przybylowski, T.; Tomalak, W.; Siergiejko, Z.; Jastrzebski, D.; Maskey-Warzechowska, M.; Piorunek, T.; Wojda, E.; Boros, P. Polish Respiratory Society guidelines for the methodology and interpretation of the 6 minute walk test (6MWT). Pneumonol. Alergol. Pol. 2015, 83, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, F.; Banakar, S.; Rostami, S. Effect of pre-cooling injection site on pain perception in pediatric dentistry: “A randomized clinical trial”. Dent. Res. J. 2013, 10, 790–794. [Google Scholar]
- Abbas, A.; Shahid, S.; Ahmed, S.W. An Insight to Chronic Obstructive Pulmonary Disorder COPD and its Pharmacotherapy. Br. Biomed. Bull. 2014, 1, 66–84. [Google Scholar]
- Hommerding, P.X.; Donadio, M.V.; Paim, T.F.; Marostica, P.J. The Borg scale is accurate in children and adolescents older than 9 years with cystic fibrosis. Respir. Care 2010, 55, 729–733. [Google Scholar] [PubMed]
- Moran, C.A.; Corso, S.D.; Bombig, M.T.; Serra, A.J.; Pereira, S.A.; Peccin, M.S. Heart rate agreement between the 20-meter shuttle run test and virtual system in healthy children: A cross-sectional study. BMC Pediatr. 2019, 19, 491. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.; Gravel, J.; Daoust, R. Reliability of the visual analog scale in children with acute pain in the emergency department. PAIN® 2012, 153, 839–842. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Langan, R.C.; Goodbred, A.J. Office Spirometry: Indications and Interpretation. Am. Fam. Physician 2020, 101, 362–368. [Google Scholar] [PubMed]
- Kerem, E.; Viviani, L.; Zolin, A.; MacNeill, S.; Hatziagorou, E.; Ellemunter, H.; Drevinek, P.; Gulmans, V.; Krivec, U.; Olesen, H.; et al. Factors associated with FEV1 decline in cystic fibrosis: Analysis of the ECFS Patient Registry. Eur. Respir. J. 2014, 43, 125–133. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation Patient Registry 2019 Annual Data Report Bethesda, Maryland. Available online: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Patient-Registry-Annual-Data-Report.pdf (accessed on 27 September 2021).
- Reynaud-Gaubert, M.; Averyanov, A.; Kolek, V.; Sliwinski, P.; Mihaltan, F.D.; Solovic, I. Simeox Clinical Notebook. Available online: https://www.physioassist.com/wp-content/uploads/2019/07/clinical_notebook_2_08_web.pdf (accessed on 27 September 2021).
- Sands, D.A. Mukowiscydoza: Choroba Wieloukładowa; Termedia sp. z oo: Poznań, Poland, 2018. [Google Scholar]
- Wang, X.B.; Dockery, D.W.; Wypij, D.; Fay, M.E.; Ferris, B.G. Pulmonary-Function between 6 and 18 Years of Age. Pediatr. Pulmonol. 1993, 15, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; O’Sullivan, B.P.; Robinson, K.A.; Goss, C.H.; Mogayzel, P.J.; Willey-Courand, D.B.; Bujan, J.; Finder, J.; Lesters, M.; Quittell, L.; et al. Cystic fibrosis pulmonary guidelines—Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care 2007, 176, 957–969. [Google Scholar] [CrossRef]
- Konstan, M.W.; Wagener, J.S.; VanDevanter, D.R.; Pasta, D.J.; Yegin, A.; Rasouliyan, L.; Morgan, W.J. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J. Cyst. Fibros. 2012, 11, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.A.; Crandall, R.; Seigler, N.; Rodriguez-Miguelez, P.; Mckie, K.T.; Forseen, C.; Thomas, J.; Harris, R.A. A single bout of maximal exercise improves lung function in patients with cystic fibrosis. J. Cyst. Fibros. 2017, 16, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Demir, T.; Borekci, S.; Uygun, M.; Demir, D.; Yildirim, N. FEV3, FEV3/FVC and 1-FEV3/FVC in Obstructive Lung Disease. Chest 2013, 144, 842A. [Google Scholar] [CrossRef]
- Rubinowicz, M.; Piotrowski, R.; Nowobilski, R. Evaluation of some anthropometric and clinical parameters in children with cystic fibrosis. Pneumonol. Alergol. Pol. 2005, 73, 172–177. [Google Scholar] [CrossRef]
- Corten, L.; Morrow, B.M. Autogenic Drainage in Children with Cystic Fibrosis. Pediatr. Phys. Ther. 2017, 29, 106–117. [Google Scholar] [CrossRef] [PubMed]


| Applied Therapy | Age (Year) | Avg | SD | Min. | Max. | Q25 | Me | Q75 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| FEV1 difference | |||||||||
| AD + SIMEOX | <10.5 | 6 | 4 | 0 | 9 | 6 | 8 | 9 | 0.02 |
| AD + SIMEOX | >10.5 | −2 | 5 | −8 | 7 | −5 | −2 | 0 | |
| AD + SIMEOX + BELT | <10.5 | 7 | 6 | 1 | 15 | 4 | 5 | 10 | NS |
| AD + SIMEOX + BELT | >10.5 | 4 | 9 | −7 | 19 | −2 | 4 | 7 | |
| AD | <10.5 | 1 | 3 | −1 | 5 | −1 | −1 | 4 | NS |
| AD | >10.5 | 0 | 4 | −7 | 5 | −2 | 0 | 2 | |
| AD + BELT | <10.5 | 9 | 9 | −2 | 20 | 3 | 8 | 15 | 0.02 |
| AD + BELT | >10.5 | −1 | 3 | −6 | 2 | −3 | −2 | 2 | |
| FEV3 difference | |||||||||
| AD + SIMEOX | <10.5 | 3 | 5 | −4 | 9 | −1 | 3 | 7 | NS |
| AD + SIMEOX | >10.5 | 0 | 8 | −11 | 10 | −5 | 0 | 5 | |
| AD + SIMEOX + BELT | <10.5 | 13 | 10 | 4 | 23 | 4 | 11 | 23 | 0.02 |
| AD + SIMEOX + BELT | >10.5 | 1 | 4 | −5 | 7 | 1 | 2 | 3 | |
| AD | <10.5 | 0 | 8 | −10 | 8 | −9 | 1 | 7 | NS |
| AD | >10.5 | 4 | 6 | −1 | 15 | 0 | 3 | 5 | |
| AD + BELT | <10.5 | 10 | 8 | 1 | 24 | 4 | 7 | 12 | 0.02 |
| AD + BELT | >10.5 | 0 | 5 | −7 | 4 | −5 | 1 | 4 | |
| FEV6 difference | |||||||||
| AD + SIMEOX | <10.5 | 2 | 7 | −8 | 10 | −1 | 3 | 6 | NS |
| AD + SIMEOX | >10.5 | 2 | 8 | −8 | 11 | −5 | 2 | 8 | |
| AD + SIMEOX + BELT | <10.5 | 14 | 8 | 5 | 22 | 6 | 15 | 20 | 0.02 |
| AD + SIMEOX + BELT | >10.5 | 4 | 5 | −5 | 8 | 1 | 4 | 7 | |
| AD | <10.5 | 2 | 8 | −8 | 14 | −5 | 2 | 8 | NS |
| AD | >10.5 | 6 | 6 | 0 | 15 | 3 | 5 | 10 | |
| AD + BELT | <10.5 | 7 | 5 | 1 | 14 | 4 | 7 | 9 | NS |
| AD + BELT | >10.5 | 3 | 6 | −6 | 11 | 0 | 4 | 6 | |
| PEF difference | |||||||||
| AD + SIMEOX | <10.5 | 25 | 27 | 4 | 71 | 8 | 19 | 21 | 0.008 |
| AD + SIMEOX | >10.5 | 1 | 3 | −4 | 4 | −1 | 1 | 3 | |
| AD + SIMEOX + BELT | <10.5 | 16 | 21 | −11 | 40 | 3 | 12 | 34 | NS |
| AD + SIMEOX + BELT | >10.5 | 8 | 13 | −5 | 26 | −2 | 5 | 18 | |
| AD | <10.5 | 2 | 17 | −27 | 17 | −8 | 6 | 15 | NS |
| AD | >10.5 | −5 | 8 | −15 | 9 | −8 | −7 | −1 | |
| AD + BELT | <10.5 | 9 | 6 | −2 | 15 | 6 | 10 | 13 | 0.04 |
| AD + BELT | >10.5 | 0 | 7 | −9 | 7 | −7 | 1 | 6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żak, M.; Gauchez, H.; Boberski, M.; Stangret, A.; Kempinska-Podhorodecka, A. Effectiveness of Autogenic Drainage in Improving Pulmonary Function in Patients with Cystic Fibrosis. Int. J. Environ. Res. Public Health 2023, 20, 3822. https://doi.org/10.3390/ijerph20053822
Żak M, Gauchez H, Boberski M, Stangret A, Kempinska-Podhorodecka A. Effectiveness of Autogenic Drainage in Improving Pulmonary Function in Patients with Cystic Fibrosis. International Journal of Environmental Research and Public Health. 2023; 20(5):3822. https://doi.org/10.3390/ijerph20053822
Chicago/Turabian StyleŻak, Magdalena, Hugues Gauchez, Marek Boberski, Anna Stangret, and Agnieszka Kempinska-Podhorodecka. 2023. "Effectiveness of Autogenic Drainage in Improving Pulmonary Function in Patients with Cystic Fibrosis" International Journal of Environmental Research and Public Health 20, no. 5: 3822. https://doi.org/10.3390/ijerph20053822
APA StyleŻak, M., Gauchez, H., Boberski, M., Stangret, A., & Kempinska-Podhorodecka, A. (2023). Effectiveness of Autogenic Drainage in Improving Pulmonary Function in Patients with Cystic Fibrosis. International Journal of Environmental Research and Public Health, 20(5), 3822. https://doi.org/10.3390/ijerph20053822







