Hydrocarbon Degradation and Microbial Survival Improvement in Response to γ-Polyglutamic Acid Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Set-Up
- (1)
- PGA0: zeolite + nutrients + petroleum product (reference);
- (2)
- PGA0B: zeolite + nutrients + petroleum product + bacterial broth;
- (3)
- PGA1: zeolite + PGA at a 1:100 dilution (1%) + nutrients + petroleum product;
- (4)
- PGA1B: zeolite + PGA at a 1:100 dilution (1%) with bacterial broth + nutrients + petroleum product;
- (5)
- PGA10: zeolite + PGA at a 1:10 dilution (10%) + nutrients + petroleum product.
2.2. Media Characteristics
2.3. Analytical Methods
2.3.1. Samples Extraction and Preparation
2.3.2. Gas Chromatography-Mass Spectrometry (GC-MS)
- (1)
- Ratio of two acyclic isoprenoids differing in a chain length by a CH2 group, i.e., pristane (C19), abbreviated as Pr, and phytane (C20), abbreviated as Ph;
- (2)
- Ratios of acyclic isoprenoids to n-alkane: pristane (Pr) to n-heptadecane (n-C17) and phytane (Ph) to n-octadecane (n-C18);
- (3)
- Ratios of n-octadecane (n-C18) to 17α(H), 21β(H)-hopane (abbreviated as H30) and n-undecane (n-C11) to 17α(H), 21β(H)-hopane (H30);
- (4)
- Ratios of two n-alkanes differing in a chain length, i.e., n-heptadecane (n-C17) to n-octadecane (n-C18), n-undecane (n-C11) to n-octadecane (n-C18), n-undecane (n-C11) to n-dodecane (n-C12), n-undecane (n-C11) to n-henicosane (n-C21), and n-henicosane (n-C21) to n-hentriacontane (n-C31). The n-alkane ratios were selected so that the whole range of n-alkanes was covered, i.e., from n-C11 to n-C31, to better follow the biodegradation dynamics.
2.3.3. Electrical Conductivity (EC) and pH
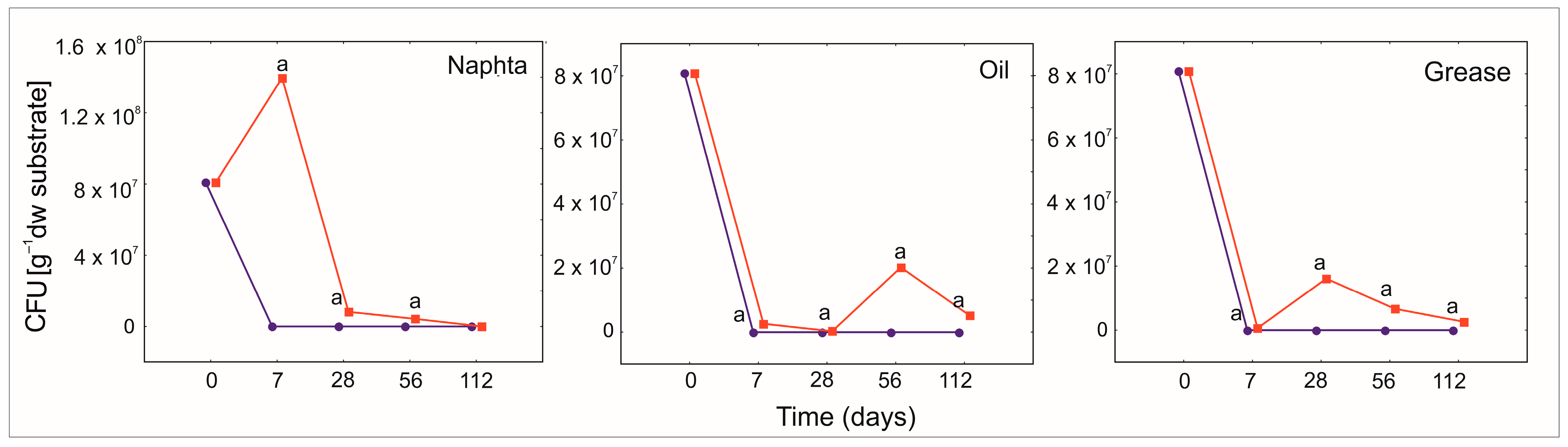
2.3.4. Microorganisms
2.4. Statistical Analysis
3. Results
3.1. Percentage Yield Extraction
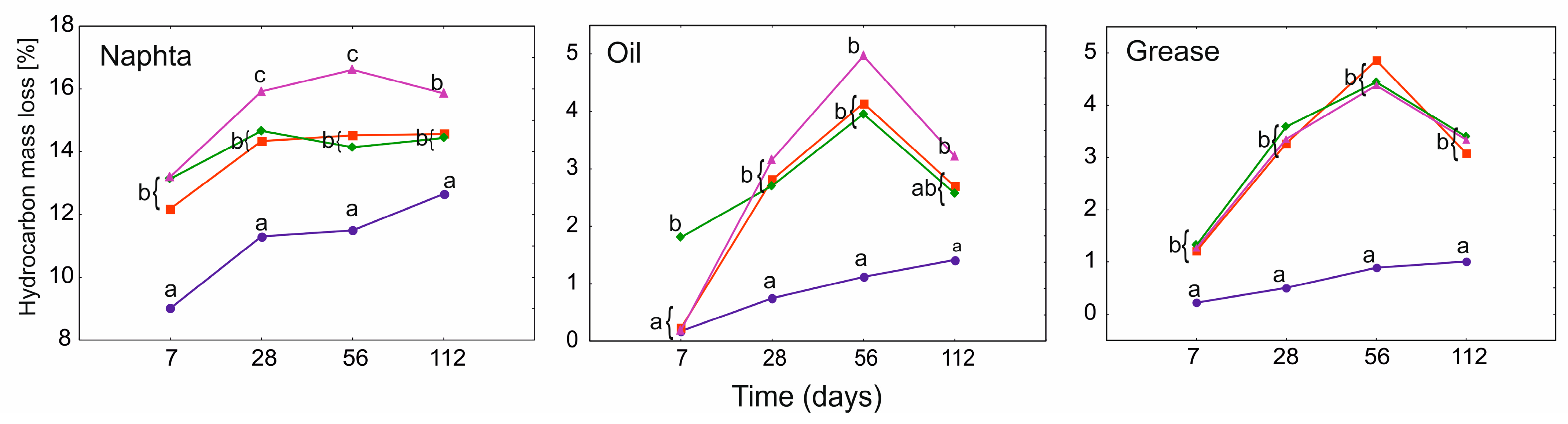
3.2. Hydrocarbon Mass Loss
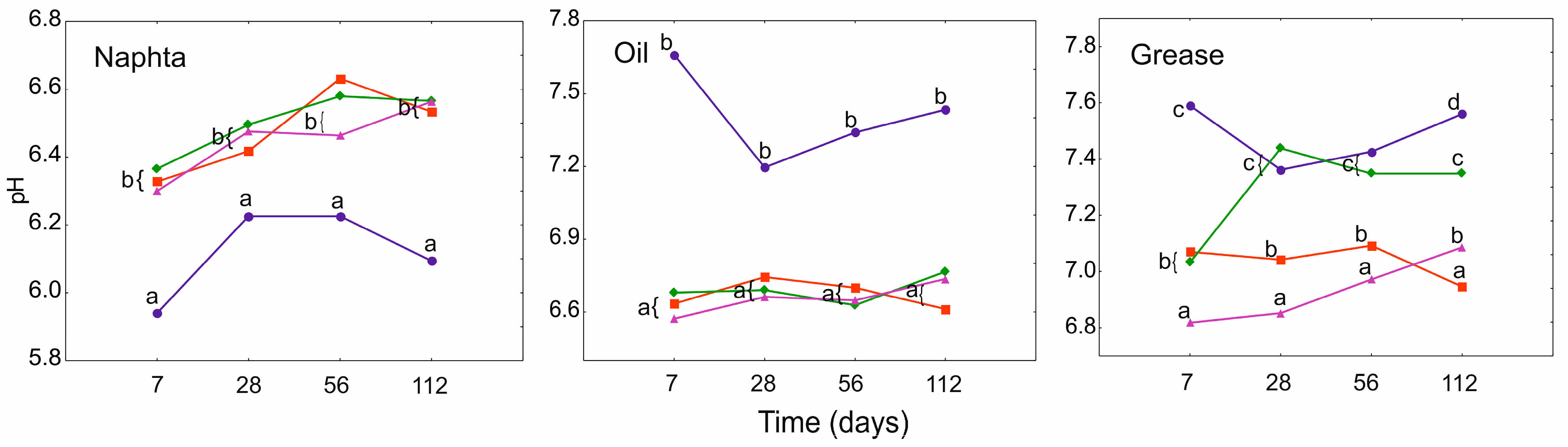
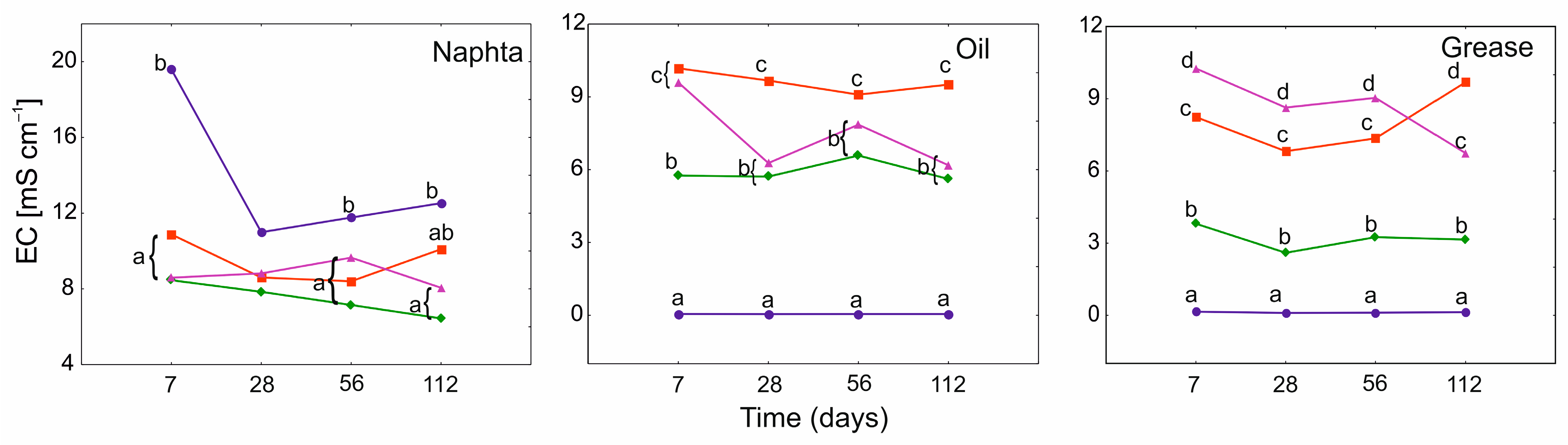
3.3. Electrical Conductivity (EC) and pH
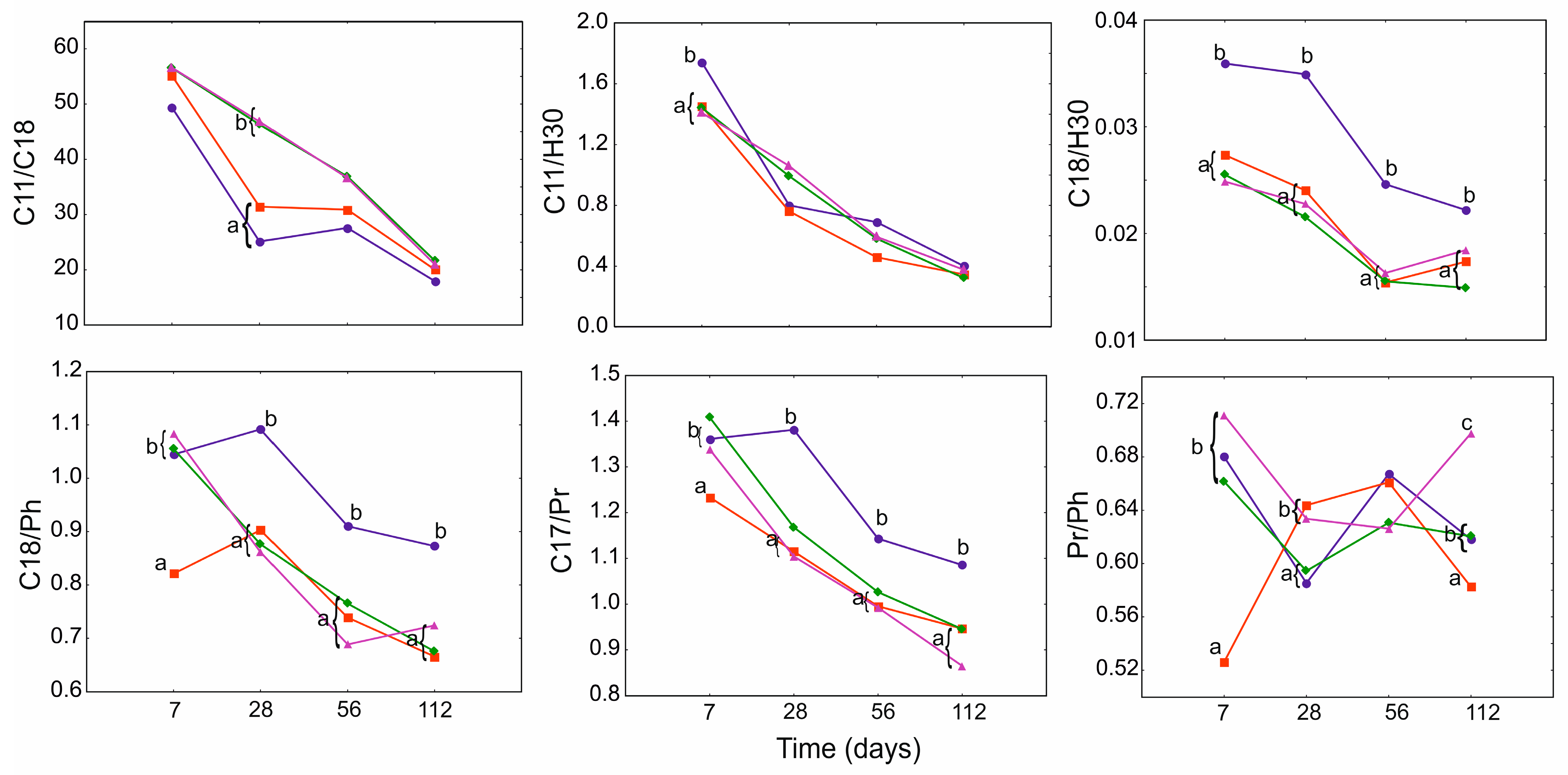
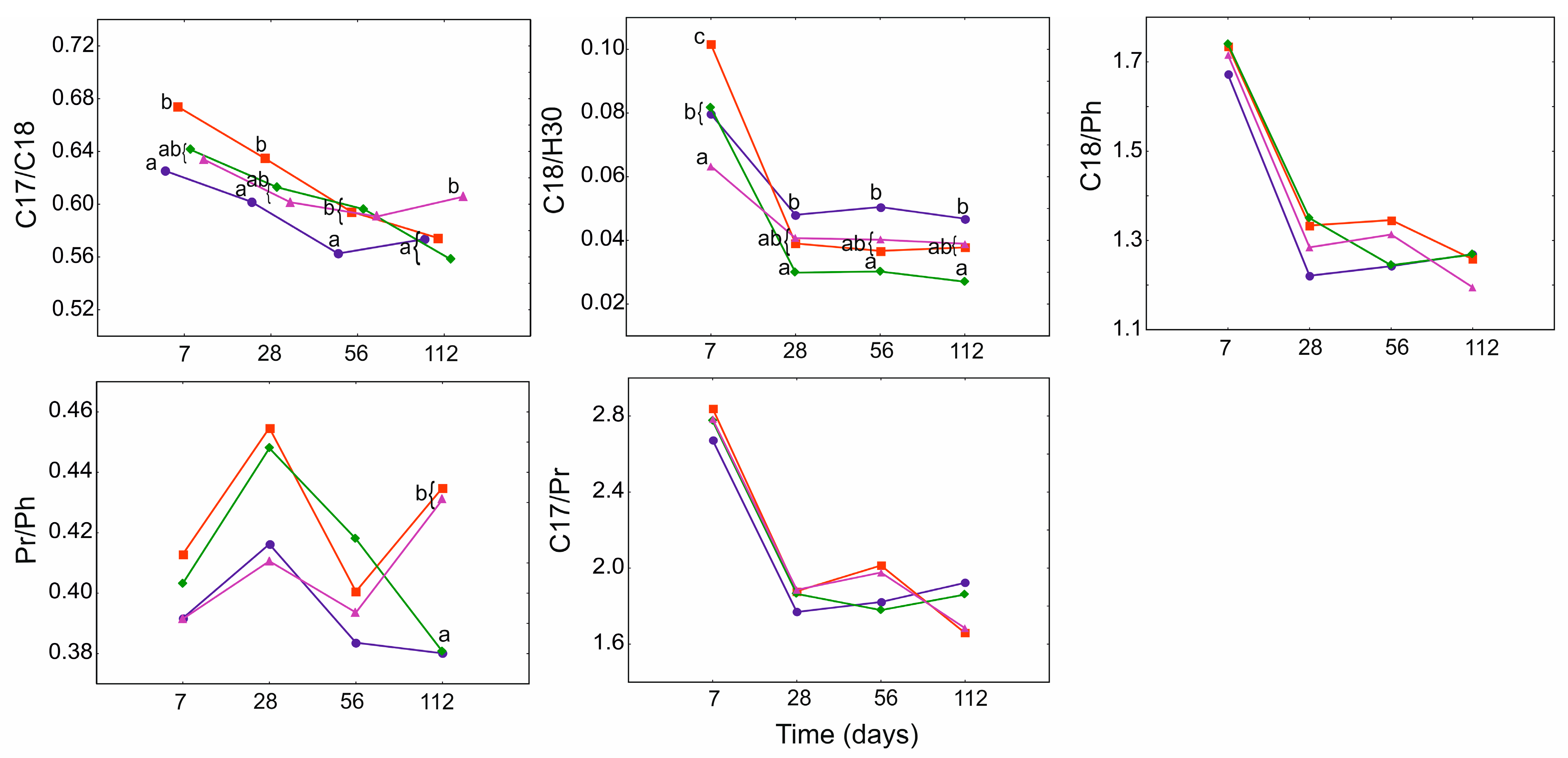
3.4. Geochemical Ratios
3.5. The Protective Role of PGA for Bacillus Strains
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Environment Agency (EEA). Overview of Contaminants Affecting Soil and Groundwater in Europe. 2011. Available online: http://www.eea.europa.eu/data-and-maps/figures/overview-of-contaminants-affecting-soil-and-groundwater-in-europe (accessed on 10 August 2022).
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Das, S. (Ed.) Microbial Biodegradation and Bioremediation, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; p. 612. [Google Scholar]
- De Almeida, F.F.; Freitas, D.; Motteran, F.; Fernandes, B.S.; Gavazza, S. Bioremediation of polycyclic aromatic hydrocarbons in contaminated mangroves: Understanding the historical and key parameter profiles. Mar. Pollut. Bull. 2021, 169, 112553. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Public Health Statement Total Petroleum Hydrocarbons; Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1999. [Google Scholar]
- Alegbeleye, O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Gossen, L.P.; Velichkina, L.M. Environmental problems of the oil-and-gas industry. Pet. Chem. 2006, 46, 67–72. [Google Scholar] [CrossRef]
- Galitchi, S. Major problems faced after the withdrawal of the soviet army from Moldova. In Environmental Security and Public Safety; Springer: Dordrecht, The Netherlands, 2007; pp. 197–205. [Google Scholar]
- Kuppusamy, S.; Maddela, N.R.; Mallavarapu, M.; Venkateswarlu, K. Fate of Total Petroleum Hydrocarbons in the Environment. In Total Petroleum Hydrocarbons. Environmental Fate, Toxicity and Remediation; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 57–78. [Google Scholar] [CrossRef]
- Jafarinejad, S. Oil-spill response. In Petroleum Waste Treatment and Pollution Control; Elsevier: Oxford, UK, 2017; pp. 117–148. [Google Scholar]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Approaches for Remediation of Sites Contaminated with Total Petroleum Hydrocarbons. In Total Petroleum Hydrocarbons. Environmental Fate, Toxicity, and Remediation; Springer Nature: Cham, Switzerland, 2020; pp. 167–205. [Google Scholar] [CrossRef]
- O’Brien, P.L.; De Sutter, T.M.; Casey, F.X.M.; Wick, A.F.; Khan, E. Evaluation of Soil Function Following Remediation of Petroleum Hydrocarbons—A Review of Current Remediation Techniques. Curr. Pollut. Rep. 2017, 3, 192–205. [Google Scholar] [CrossRef]
- Atlas, R.M. Microbial degradation of petroleum hydrocarbons: An environmental perspective. Microbiol. Rev. 1981, 45, 180–209. [Google Scholar] [CrossRef]
- Roy, A.S.; Baruah, R.; Borah, M.; Singh, A.K.; Boruah, H.P.D.; Saikia, N.; Deka, M.; Dutta, N.; Bora, T.C. Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int. Biodeterior. Biodegrad. 2014, 94, 79–89. [Google Scholar] [CrossRef]
- Koshlaf, E.; Ball, A.S. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2016, 3, 25–49. [Google Scholar] [CrossRef]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of Petroleum-Contaminated Soils with Microbial and Microbial Combined Methods: Advances, Mechanisms and Challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Wartell, B.; Boufadel, M.; Rodriguez-Freire, L. 2021. An effort to understand and improve the anaerobic biodegradation of petroleum hydrocarbons: A literature review. Int. Biodeterior. Biodegrad. 2021, 157, 105156. [Google Scholar] [CrossRef]
- Baidurah, S.; Takada, S.; Shimizu, K.; Yasue, K.; Arimoto, S.; Ishida, Y.; Yamane, T.; Ohtani, H. Evaluation of Biodegradability of Poly(Butylene succinate-co-butylene Adipate) on the Basis of Copolymer Composition Determined by Thermally Assisted Hydrolysis and Methylation-Gas Chromatography. Int. J. Polym. Anal. Charact. 2012, 17, 29–37. [Google Scholar] [CrossRef]
- Chrzanowski, Ł.; Owsianiak, M.; Szulc, A.; Marecik, R.; Piotrowska-Cyplik, A.; Olejnik-Schmidt, A.K.; Staniewski, J.; Lisiecki, P.; Ciesielczyk, F.; Jesionowski, T.; et al. Interactions between rhamnolipid biosurfactants and toxic chlorinated phenols enhance biodegradation of a model hydrocarbon-rich effluent. Int. Biodeterior. Biodegrad. 2011, 65, 605–611. [Google Scholar] [CrossRef]
- Kavitha, V.; Mandal, B.A.; Gnanamani, A. Microbial biosurfactant mediated removal and/or solubilization of crude oil contamination from soil and aqueous phase: An approach with Bacillus licheniformis MTCC 5514. Int. Biodeterior. Biodegrad. 2014, 94, 24–30. [Google Scholar] [CrossRef]
- Geetha, S.; Banat, I.; Joshi, S. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal. Agric Biotechnol. 2018, 14, 23–32. [Google Scholar] [CrossRef]
- Ali, N.; Wang, F.; Xu, B.; Safdar, B.; Ullah, A.; Naveed, M.; Wang, C.; Rashid, M.T. Production and Application of Biosurfactant Produced by Bacillus licheniformis Ali5 in Enhanced Oil Recovery and Motor Oil Removal from Contaminated Sand. Molecules 2019, 24, 4448. [Google Scholar] [CrossRef]
- Zdarta, A.; Smułek, W.; Pacholak, A.; Dudzińska-Bajorek, B.; Kaczorek, E. Surfactant addition in diesel oil degradation—How can it help the microbes? J. Environ. Health Sci. Eng. 2020, 18, 677–686. [Google Scholar] [CrossRef] [PubMed]
- April, T.M.; Foght, J.M.; Currah, R.S. Hydrocarbon-degrading filamentous fungi isolated from flare pit soils in northern and western Canada. Can. J. Microbiol. 2000, 46, 38–49. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Sutherland, J.B. Degradation of Polycyclic Aromatic Hydrocarbons by Fungi. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2080–2110. [Google Scholar] [CrossRef]
- Mao, J.; Guan, W. Fungal degradation of polycyclic aromatic hydrocarbons (PAHs) by Scopulariopsis brevicaulis and its application in bioremediation of PAH contaminated soil. Acta Agric. Scand. Sect. B 2016, 66, 399–405. [Google Scholar] [CrossRef]
- Govarthanan, M.; Fuzisawa, S.; Hosogai, T.; Chang, Y.C. Biodegradation of aliphatic and aromatic hydrocarbons using the filamentous fungus Penicillium sp. CHY-2 and characterization of its manganese peroxidase activity. RSC Adv. 2017, 7, 20716–20723. [Google Scholar] [CrossRef]
- Frick, C.M.; Farrell, R.E.; Germida, J.J. Assesment of Phytoremediation as an In-Situ Technique for Cleaning Oil Contaminated Sites; PTAC Petroleum Technology Alliance of Canada: Calgary, AB, Canada, 1999; p. 82. [Google Scholar]
- Gurska, J.; Wang, W.; Gerhardt, K.E.; Khalid, A.M.; Isherwood, D.M.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Three year field test of a plant growth promoting rhizobacteria enhanced phytoremediation system at a land farm for treatment of hydrocarbon waste. Environ. Sci. Technol. 2009, 43, 4472–4479. [Google Scholar] [CrossRef]
- Yenn, R.; Borah, M.; Boruah, D.H.P.; Roy, S.A.; Baruah, R.; Saikia, N.; Sahu, O.P.; Tamuli, A.K. Phytoremediation of abandoned crude oil contaminated drillsites of Assam with the aid of a hydrocarbon-degrading bacterial formulation. Int. J. Phytoremediat. 2014, 16, 909–925. [Google Scholar] [CrossRef]
- Turkovskaya, O.; Muratova, A. Plant-Bacterial Degradation of Polyaromatic Hydrocarbons in the Rhizosphere. Trends Biotechnol. 2019, 37, 926–930. [Google Scholar] [CrossRef]
- Abbaspour, A.; Zohrabi, F.; Dorostkar, V.; Faz, A.; Acosta, J.A. Remediation of an oil-contaminated soil by two native plants treated with biochar and mycorrhizae. J. Environ. Manag. 2020, 254, 109755. [Google Scholar] [CrossRef]
- Borde, X.; Guieysse, B.; Delgado, O.; Muñoz, R.; Hatti-Kaul, R.; Nugier-Chauvin, C.; Patin, H.; Mattiasson, B. Synergistic relationships in algal-bacterial microcosms for the treatment of aromatic pollutants. Bioresour. Technol. 2003, 86, 293–300. [Google Scholar] [CrossRef]
- Qin, G.; Gong, D.; Fan, M.Y. Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int. Biodeterior. Biodegrad. 2013, 201385, 150–155. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Zhang, X. Bioremediation of petroleum hydrocarbon-contaminated soil by petroleum-degrading bacteria immobilized on biochar. RSC Adv. 2019, 9, 35304–35311. [Google Scholar] [CrossRef]
- Mangwani, N.; Shukla, S.K.; Kumari, S.; Rao, T.S.; Das, S. Characterization of Stenotrophomonas acidaminiphila NCW-702 biofilm for implication in the degradation of polycyclic aromatic hydrocarbons. J. Appl. Microbiol. 2014, 117, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Shuklaa, S.K.; Mangwanib, N.; Raoa, T.S.; Das, S. Biofilm-Mediated Bioremediation of Polycyclic Aromatic Hydrocarbons. In Microbial Biodegradation and Bioremediation; Das, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 203–227. [Google Scholar]
- Najar, I.N.; Das, S. Poly-glutamic acid (PGA)—Structure, synthesis, genomic organization and its application: A review. Int. J. Pharm. Sci. Res. 2015, 6, 2258–2280. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T. The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Nair, P.; Navale, G.R.; Dharne, M.S. Poly-gamma-glutamic acid biopolymer: A sleeping giant with diverse applications and unique opportunities for commercialization. Biomass Conv. Bioref. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, I.B.; Singhal, R.S. Flocculation properties of poly (γ-glutamic acid) produced from Bacillus subtilis isolate. Food Bioprocess Technol. 2011, 4, 745–752. [Google Scholar] [CrossRef]
- Mark, S.S.; Crusberg, T.C.; DaCunha, C.M.; Iorio, A.A.D. A heavy metal biotrap for wastewater remediation using poly-γ-glutamic acid. Biotechnol. Progr. 2006, 22, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.R.; Irorere, V.U.; Bartlett, T.; Hill, D.; Kedia, G.; Charalampopoulos, D.; Nualkaekul, S.; Radecka, I. Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int. J. Food Microbiol. 2015, 196, 24–31. [Google Scholar] [CrossRef]
- Ye, H.; Jin, L.; Hu, R.; Yi, Z.; Li, J.; Wu, Y.; Xia, X.; Wu, Z. Poly (γ,l-glutamic acid)-cisplatin conjugate effectively inhibits human breast tumor xenografted in nude mice. Biomaterials 2006, 27, 5958–5965. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kato, K.; Shimauchi, A.; Ping, X.; Nakayama, H.; Fujita, K.-I.; Tanaka, T.; Tarui, Y.; Hirasawa, E. Proposals for Wastewater Treatment by Applying Flocculating Activity of Cross-linked Poly-Glutamic Acid. J. Biosci. Bioeng. 2005, 99, 245–251. [Google Scholar] [CrossRef]
- Campos, V.; Domingos, J.M.F.; Dos Anjos, D.N.; Lira, V. Study of fluvial water treatability using γ-polyglutamic acid based biopolymer coagulant Anais da Academia Brasileira de Ciências. Ann. Braz. Acad. Sci. 2019, 91, e20190051. [Google Scholar] [CrossRef]
- Hajdu, I.; Bodnár, M.; Csikós, Z.; Wei, S.; Daróczi, L.; Kovács, B.; Gyori, Z.; Tamás, J.; Borbél, J. Combined nanomembrane technology for removal of lead ions. J. Membr. Sci. 2012, 409, 44–53. [Google Scholar] [CrossRef]
- Thuy, L.T.X.; Yasuzawa, M.; Yabutani, T. Magnetic Removal of Cesium Ions Using γ-Poly(glutamic acid)-Coated Magnetite Particles with the Enhanced Effect of Zeolite Supplementation. Bull. Chem. Soc. Jpn. 2013, 86, 958–962. [Google Scholar] [CrossRef]
- Yang, Z.H.; Dong, C.D.; Chen, C.W.; Sheu, Y.T.; Kao, C.M. Using poly-glutamic acid as soil-washing agent to remediate heavy metal-contaminated soils. Environ. Sci. Pollut. Res. 2018, 25, 5231–5242. [Google Scholar] [CrossRef]
- Peng, Y.P.; Chang, Y.C.; Chen, K.F.; Wang, C.H. A field pilot-scale study on heavy metal-contaminated soil washing by using an environmentally friendly agent-poly-γ-glutamic acid (γ-PGA). Environ. Sci. Pollut. Res. 2020, 27, 34760–34769. [Google Scholar] [CrossRef]
- Sheu, Y.T.; Tsang, D.C.; Dong, C.D.; Chen, C.W.; Luo, S.G.; Kao, C.M. Enhanced bioremediation of TCE-contaminated groundwater using gamma poly-glutamic acid as the primary substrate. J. Clean. Prod. 2018, 178, 108–118. [Google Scholar] [CrossRef]
- Zhu, Y.; Ni, H.; Cao, J.; Li, K.; Yue, Q.; Zha, L. A New PGA-Seaweed Water-retaining Agent and Its Use in Saline-alkali Soils Repairing. AIP Conf. Proc. 2019, 2110, 020015. [Google Scholar] [CrossRef]
- Tan, Z.W.; Yang, D.; Zhang, K.; Zhao, L.; Xie, Z.; Xu, T.; Zhao, Y.; Wang, X.; Pan, X. Mitigation of soil salinization and alkalization by bacterium-induced inhibition of evaporation and salt crystallization. Sci. Total Environ. 2021, 755 Pt 1, 1142511. [Google Scholar] [CrossRef]
- Zhao, G.; Sheng, Y.; Wang, C.; Yang, J.; Wang, Q.; Chen, L. In situ microbial remediation of crude oil-soaked marine sediments using zeolite carrier with a polymer coating. Mar. Pollut. Bull. 2018, 129, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, K.; Steliga, T.; Kapusta, P.; Brzeszcz, J.; Skalski, T. Evaluation of the Effectiveness of the Biopreparation in Combination with the Polymer γ-PGA for the Biodegradation of Petroleum Contaminants in Soil. Materials 2022, 15, 400. [Google Scholar] [CrossRef]
- Ouriache, H.; Moumed, I.; Arrar, J.; Namane, A.; Lounici, H. Influence of C/N/P ratio evolution on biodegradation of petroleum hydrocarbons-contaminated soil. Alger. J. Environ. Sci. Technol. 2020, 6, 1604–1611. [Google Scholar]
- Upadhyay, S.K.; Ahmad, M.; Srivastava, A.K.; Abhilash, P.C.; Sharma, B. Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicer arietinum L.) plant. Chemosphere 2021, 267, 129216. [Google Scholar] [CrossRef]
- Khanpour-Alikelayeh, E.; Partovinia, A.; Talebi, A.; Kermanian, H. Investigation of Bacillus licheniformis in the biodegradation of Iranian heavy crude oil: A two-stage sequential approach containing factor-screening and optimization. Ecotox Environ. Safe 2020, 205, 111103. [Google Scholar] [CrossRef]
- Steliga, T.; Kapusta, P.; Jakubowicz, P. Effectiveness of Bioremediation Processes of Hydrocarbon Pollutants in Weathered Drill Wastes. Water Air Soil Pollut. 2009, 202, 211–228. [Google Scholar] [CrossRef]
- Capuccino, G.J.; Sherman, N. Microbiology (a Laboratory Manual); The Benyamin: San Francisco, CA, USA, 2001. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Release 4. Reference Manual and Users Guide to CANOCO for Windows: Software for Canonical Community Ordination; Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Reddy, B.A.; Shah, U.E.; Leckner, J.; Rutland, M.W.; Glavatskih, S. On electric conductivity of greases. Res. Sq. 2022; in preprint. [Google Scholar] [CrossRef]
- Sáenz-Marta, C.I.; de Lourdes Ballinas-Casarrubias, M.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Biosurfactants as Useful Tools in Bioremediation. In Advances in Bioremediation of Wastewater and Polluted Soil; Shiomi, N., Ed.; InTechOpen Publish: Wien, Austria, 2015; pp. 94–109. [Google Scholar] [CrossRef]
- Park, S.B.; Sung, M.H.; Uyama, H.; Han, D.K. Poly(glutamic acid): Production, composites, and medical applications of the next-generation biopolimer. Prog. Polym. Sci. 2021, 113, 101341. [Google Scholar] [CrossRef]
- Hamamura, N.; Olson, S.H.; Ward, D.M.; Inskeep, W.P. Diversity and functional analysis of bacteria communities associated with natural hydrocarbon seeps in acidic soils at Rainbow Springs, Yellowstone National Park. Appl. Environ. Microbiol. 2005, 7, 5943–5950. [Google Scholar] [CrossRef] [PubMed]
- Röling, W.F.M.; Oretga-Lucach, S.; Larter, S.R.; Head, I.M. Acidophilic microbial communities associated with a natural, biodegraded hydrocarbon seepage. J. Appl. Microbiol. 2006, 101, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Bhagobaty, R.K. Hydrocarbon-utilizing bacteria of natural crude oil seepages, Digboi oilfield, Northeastern region of India. J. Sediment Environ. 2020, 5, 177–185. [Google Scholar] [CrossRef]
- Shlimon, A.G.; Mansurbeg, H.; Othman, R.S.; Gittel, A.; Aitken, C.M.; Head, I.M.; Finster, K.W.; Kjeldsen, K.U. Microbial community composition in crude oils and asphalts from the Kurdistan Region of Iraq. Geomicrobiol. J. 2020, 37, 635–652. [Google Scholar] [CrossRef]
- Uche, E.C.; Dadrasnia, A. HC-0B-06: Biodegradation of Hydrocarbons. In Biodegradation and Bioconversion of Hydrocarbons. Environmental Footprints and Eco-Design of Products and Processes; Heimann, K., Karthikeyan, O., Muthu, S., Eds.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Gao, G.; Wang, L.; Li, J.; Wei, Z.; Shi, Y. Effects of poly-γ-glutamic acid (γ-PGA) on plant growth and its distribution in a controlled plant-soil system. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Chan, A.Y.; Kjellerup, B.V. Bio-and phytoremediation of persistent organic pollutants in stormwater containment systems and soil. In Microbial Biofilms in Bioremediation and Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2019; pp. 225–237. [Google Scholar]
- Morikawa, M.; Ito, M.; Imanaka, T. Isolation of a new surfactin producer Bacillus pumilus A-1 and cloning and nucleotide sequence of the regulator gene, psf-1. J. Ferment. Bioeng. 1992, 74, 255–261. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Iranzo, M.; Sainz-Pardo, I.; Boluda, R.; Sánchez, J.; Mormeneo, S. The use of microorganisms in environmental remediation. Ann. Microbiol. 2001, 51, 135–144. [Google Scholar]
- Mammitzsch, K.; Jost, G.; Jürgens, K. Impact of dissolved inorganic carbon concentrations and pH on growth of the chemolithoautotrophic epsilonproteobacterium Sulfurimonas gotlandica GD1T. Microbiologyopen 2014, 3, 80–88. [Google Scholar] [CrossRef]



| Parameters | Heavy Naphtha | Lubricating Oil | Grease | |||
|---|---|---|---|---|---|---|
| AX1 | AX2 | AX1 | AX2 | AX1 | AX2 | |
| PGA0 | 0.293 | 0.595 | 1.203 | −1.168 | 0.935 | 0.034 |
| PGA1 | −0.142 | 0.039 | 0.419 | 1.471 | −0.432 | 0.151 |
| PGA1B | −0.253 | −0.177 | −0.771 | −0.371 | −0.026 | −0.360 |
| PGA10 | 0.102 | −0.457 | −0.850 | 0.069 | −0.478 | 0.175 |
| T7 | 0.623 | −0.201 | −0.672 | −0.379 | 0.084 | 0.841 |
| T28 | −0.469 | 0.139 | 0.730 | 0.314 | 0.023 | −0.171 |
| T56 | −0.234 | 0.093 | 0.164 | 0.239 | −0.080 | −0.440 |
| T112 | 0.448 | −0.158 | −0.223 | −0.175 | −0.027 | −0.230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, E.; Fabiańska, M.J.; Jędrszczyk, E.; Skalski, T. Hydrocarbon Degradation and Microbial Survival Improvement in Response to γ-Polyglutamic Acid Application. Int. J. Environ. Res. Public Health 2022, 19, 15066. https://doi.org/10.3390/ijerph192215066
Zając E, Fabiańska MJ, Jędrszczyk E, Skalski T. Hydrocarbon Degradation and Microbial Survival Improvement in Response to γ-Polyglutamic Acid Application. International Journal of Environmental Research and Public Health. 2022; 19(22):15066. https://doi.org/10.3390/ijerph192215066
Chicago/Turabian StyleZając, Ewelina, Monika J. Fabiańska, Elżbieta Jędrszczyk, and Tomasz Skalski. 2022. "Hydrocarbon Degradation and Microbial Survival Improvement in Response to γ-Polyglutamic Acid Application" International Journal of Environmental Research and Public Health 19, no. 22: 15066. https://doi.org/10.3390/ijerph192215066
APA StyleZając, E., Fabiańska, M. J., Jędrszczyk, E., & Skalski, T. (2022). Hydrocarbon Degradation and Microbial Survival Improvement in Response to γ-Polyglutamic Acid Application. International Journal of Environmental Research and Public Health, 19(22), 15066. https://doi.org/10.3390/ijerph192215066







