The Autonomic Imbalance of Myocardial Ischemia during Exercise Stress Testing: Insight from Short-Term Heart Rate Variability Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exercise Stress Testing (EST)
2.3. RR Intervals Extraction
2.4. Analysis of Heart Rate Variability
2.5. Statistical Methods
3. Results
3.1. Comparison of HRV between RHC and sCAD Groups in Individual EST Stages
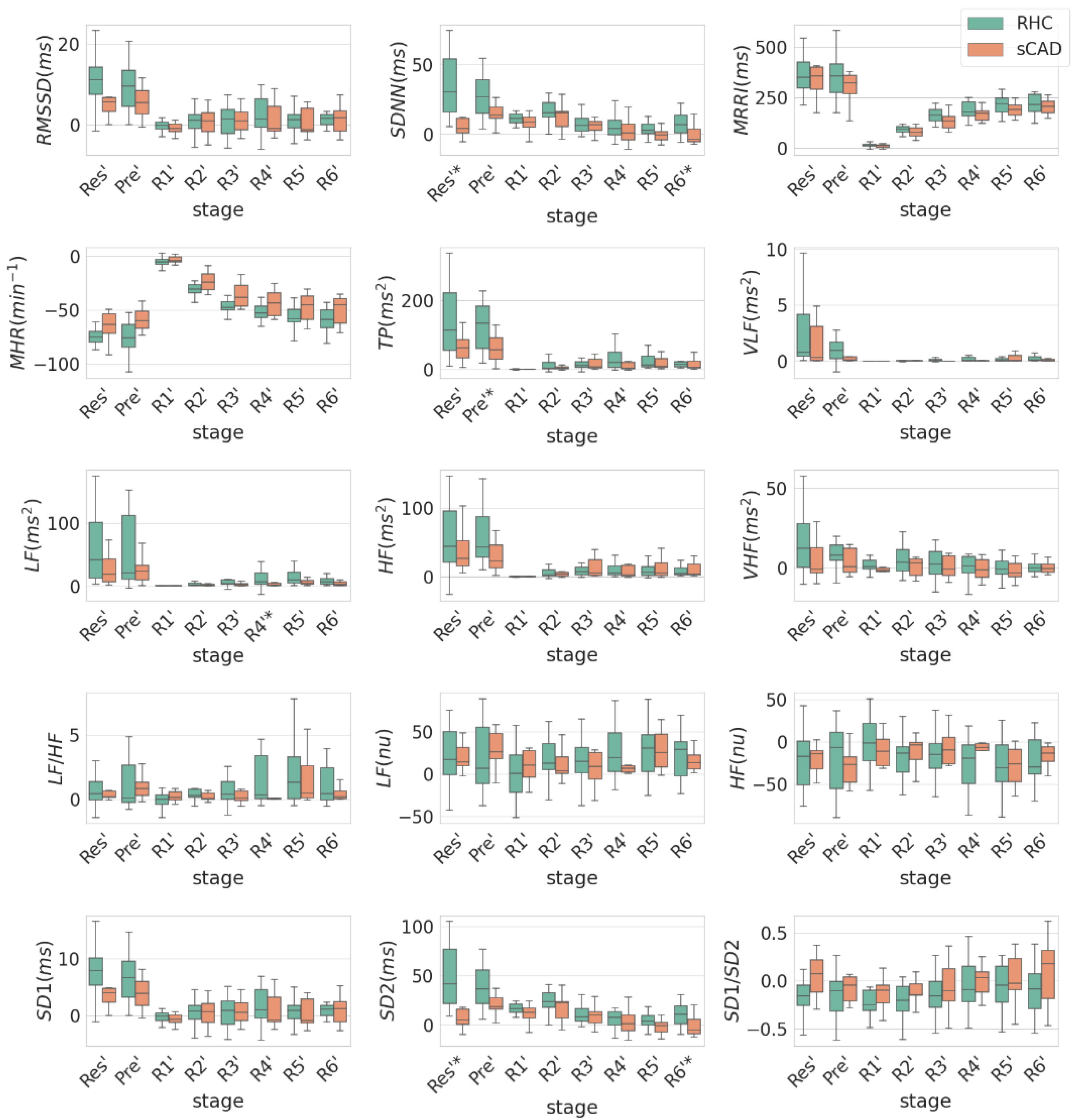
3.2. Comparison of HRV between RHC and sCAD Groups in Individual EST Stages with Respect to Max Stage
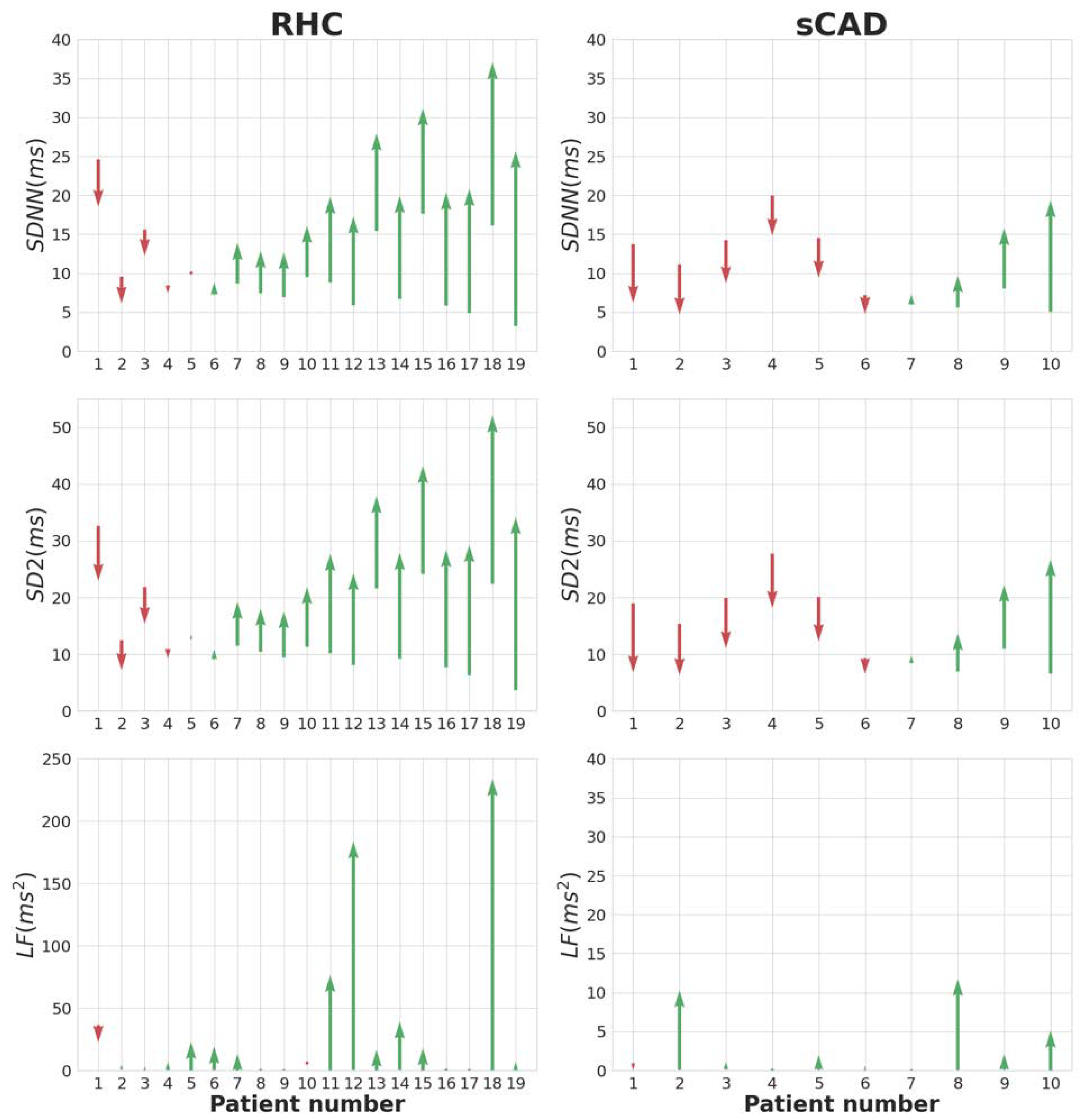
3.3. The Recovery Slope of HRV of the RHC, iCAD, and sCAD Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sun, Y.; Li, W.; Yin, L.; Wei, L.; Wang, Y. Diagnostic accuracy of treadmill exercise tests among Chinese women with coronary artery diseases: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 227, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kohli, P.; Gulati, M. An update on exercise stress testing. Curr. Probl. Cardiol. 2012, 37, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Newman, D.R.; Van Den Bruel, A.; Heneghan, C. Diagnostic accuracy of exercise stress testing for coronary artery disease: A systematic review and meta-analysis of prospective studies. Int. J. Clin. Pract. 2012, 66, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Revenco, V. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [Green Version]
- Detrano, R.; Gianrossi, R.; Froelicher, V. The diagnostic accuracy of the exercise electrocardiogram: A meta-analysis of 22 years of research. Prog. Cardiovasc. Dis. 1989, 32, 173–206. [Google Scholar] [CrossRef]
- Grad, C.; Zdrenghea, D. Heart rate recovery in patients with ischemic heart disease-risk factors. Clujul Med. 2014, 87, 220. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, I.; Goldkorn, R.; Shlomo, N.; Einhorn, M.; Levitan, J.; Kuperstein, R.; Klempfner, R.; Johnson, B. Heart rate variability for risk assessment of myocardial ischemia in patients without known coronary artery disease: The HRV-DETECT (heart rate variability for the detection of myocardial ischemia) study. J. Am. Heart Assoc. 2019, 8, e014540. [Google Scholar] [CrossRef]
- Zimatore, G.; Falcioni, L.; Gallotta, M.C.; Bonavolontà, V.; Campanella, M.; De Spirito, M.; Guidetti, L.; Baldari, C. Recurrence quantification analysis of heart rate variability to detect both ventilatory thresholds. PLoS ONE 2021, 16, e0249504. [Google Scholar] [CrossRef]
- Bailón, R.; Mateo, J.; Olmos, S.; Serrano, P.; García, J.; Del Río, A.; Ferreira, I.; Laguna, P. Coronary artery disease diagnosis based on exercise electrocardiogram indexes from repolarisation, depolarisation and heart rate variability. Med. Biol. Eng. Comput. 2003, 41, 561–571. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Zou, W.; Zhang, M.; Wang, Y.; Gu, Y. Association between cardiac autonomic nervous dysfunction and the severity of coronary lesions in patients with stable coronary artery disease. J. Int. Med. Res. 2018, 46, 3729–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtanen, M.; Kähönen, M.; Nieminen, T.; Karjalainen, P.; Tarvainen, M.; Lehtimäki, T.; Lehtinen, R.; Nikus, K.; Kööbi, T.; Niemi, M. Heart rate variability derived from exercise ECG in the detection of coronary artery disease. Physiol. Meas. 2007, 28, 1189. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Z.; Özdemir, S.; Altun, B.; Çelik, F. Assessment of Heart Rate Recovery with GATED-Myocardial Perfusion Scintigraphy Outcome in Patients with Coronary Artery Disease: A Retrospective Study and Institutional Experience. Mol. Imaging Radionucl. Ther. 2016, 25, 121. [Google Scholar] [CrossRef]
- Lipponen, J.A.; Tarvainen, M.P. A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J. Med. Eng. Technol. 2019, 43, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; John Camm, A.; Thomas Bigger, J.; Breithardt, G.n.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Zimatore, G.; Gallotta, M.C.; Campanella, M.; Skarzynski, P.H.; Maulucci, G.; Serantoni, C.; De Spirito, M.; Curzi, D.; Guidetti, L.; Baldari, C. Detecting Metabolic Thresholds from Nonlinear Analysis of Heart Rate Time Series: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12719. [Google Scholar] [CrossRef] [PubMed]
- Vallat, R. Pingouin: Statistics in Python. J. Open Source Softw. 2018, 3, 1026. [Google Scholar] [CrossRef]
- Lin, P.-L.; Chen, K.-Y.; Ma, H.; Wang, C.-L.; Lin, Y.J. Preliminary study a non-invasion method on early cardiac energy defect based on Hilbert Huang Transform. Med. Hypotheses 2020, 144, 110205. [Google Scholar] [CrossRef]
- Imai, K.; Sato, H.; Hori, M.; Kusuoka, H.; Ozaki, H.; Yokoyama, H.; Takeda, H.; Inoue, M.; Kamada, T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol. 1994, 24, 1529–1535. [Google Scholar] [CrossRef] [Green Version]
- Jouven, X.; Empana, J.-P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-Rate Profile during Exercise as a Predictor of Sudden Death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Vetrovec, G.W.; Froelicher, V.F. Importance of the first two minutes of heart rate recovery after exercise treadmill testing in predicting mortality and the presence of coronary artery disease in men. Am. J. Cardiol. 2004, 93, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yoshitama, T.; Makino, K.; Lee, T.; Saeki, F. Heart rate recovery after exercise is a predictor of silent myocardial ischemia in patients with type 2 diabetes. Diabetes Care 2011, 34, 724–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Vegte, Y.J.; van der Harst, P.; Verweij, N. Heart rate recovery 10 seconds after cessation of exercise predicts death. J. Am. Heart Assoc. 2018, 7, e008341. [Google Scholar] [CrossRef] [PubMed]
- Javorka, M.; Zila, I.; Balharek, T.; Javorka, K. Heart rate recovery after exercise: Relations to heart rate variability and complexity. Braz. J. Med. Biol. Res. 2002, 35, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Michael, S.; Graham, K.S.; Oam, G.M.D. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals-A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Elshazly, A.; Khorshid, H.; Hanna, H.; Ali, A. Effect of exercise training on heart rate recovery in patients post anterior myocardial infarction. Egypt. Heart J. 2018, 70, 283–285. [Google Scholar] [CrossRef]
- Ghaffari, S.; Kazemi, B.; Aliakbarzadeh, P. Abnormal heart rate recovery after exercise predicts coronary artery disease severity. Cardiol. J. 2011, 18, 47–54. [Google Scholar]
- Hottenrott, K.; Hoos, O.; Esperer, H.D. Heart rate variability and physical exercise. Current status. Herz 2006, 31, 544–552. [Google Scholar] [CrossRef]
- Hunt, K.J.; Saengsuwan, J. Changes in heart rate variability with respect to exercise intensity and time during treadmill running. Biomed. Eng. Online 2018, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dilaveris, P.E.; Zervopoulos, G.A.; Michaelides, A.P.; Sideris, S.K.; Psomadaki, Z.D.; Gialafos, E.J.; Gialafos, J.E.; Toutouzas, P.K. Ischemia-induced reflex sympathoexcitation during the recovery period after maximal treadmill exercise testing. Clin. Cardiol. 1998, 21, 585–590. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Mäkikallio, T.H. Heart rate variability in ischemic heart disease. Auton. Neurosci. Basic Clin. 2001, 90, 95–101. [Google Scholar] [CrossRef]
- Williams, D.P.; Joseph, N.; Gerardo, G.M.; Hill, L.K.; Koenig, J.; Thayer, J.F. Gender Differences in Cardiac Chronotropic Control: Implications for Heart Rate Variability Research. Appl. Psychophysiol. Biofeedback 2022, 47, 65–75. [Google Scholar] [CrossRef] [PubMed]
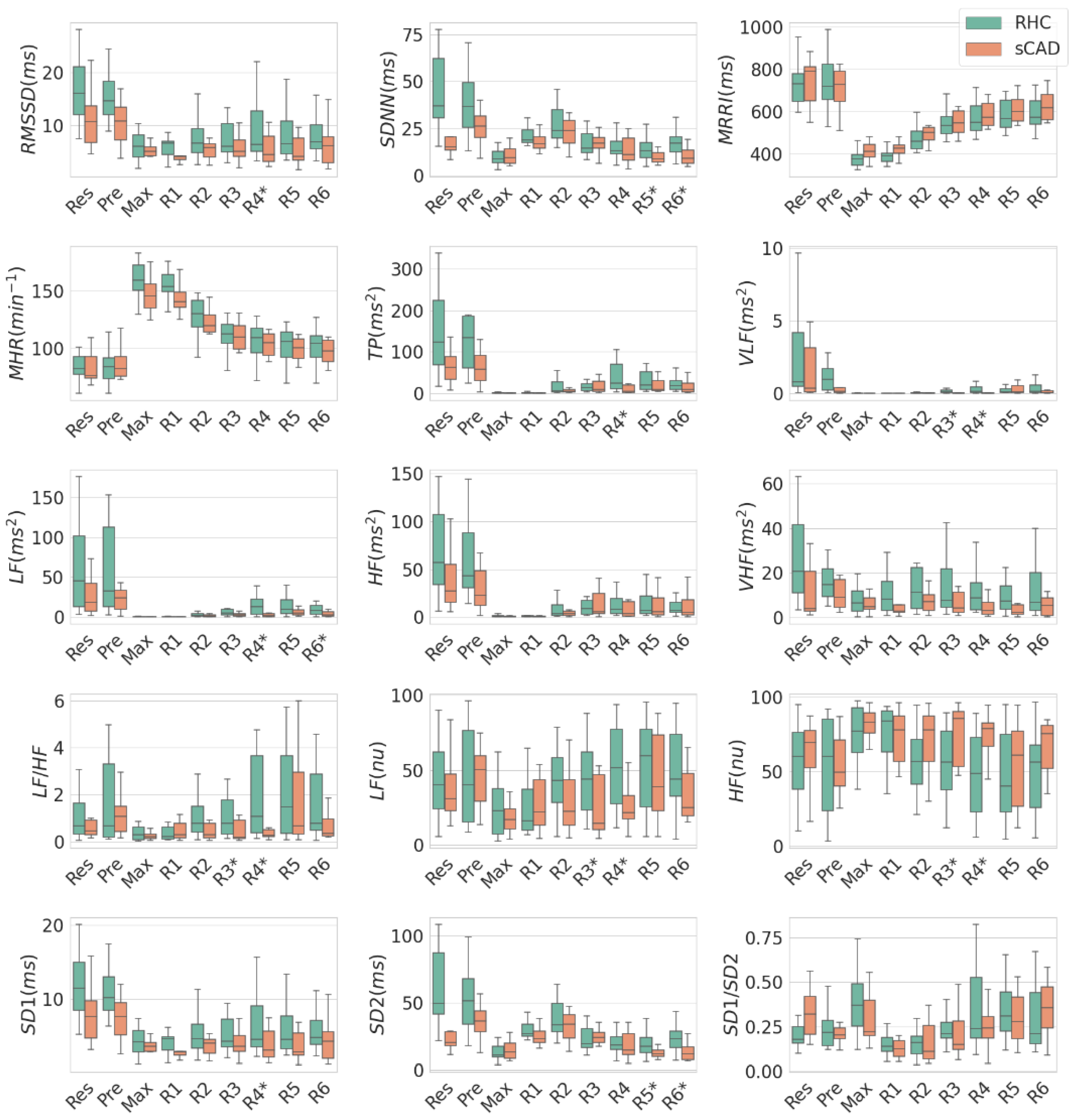

| Units | Stage | RHC (n = 19) | sCAD (n = 10) | p-Value | |
|---|---|---|---|---|---|
| VLF | ms2 | R3 | 0.23 ± 0.4 | 0.04 ± 0.08 | 0.03 |
| LF/HF | R3 | 1.57 ± 1.97 | 0.44 ± 0.46 | 0.04 | |
| LF (nu) | R3 | 45.02 ± 25.32 | 25.02 ± 20.3 | 0.04 | |
| HF (nu) | R3 | 54.98 ± 25.32 | 74.98 ± 20.3 | 0.04 | |
| VLF | ms2 | R4 | 0.85 ± 1.98 | 0.04 ± 0.06 | 0.01 |
| LF/HF | R4 | 3.41 ± 4.87 | 0.54 ± 0.67 | 0.02 | |
| LF | ms2 | R4 | 35.77 ± 64.06 | 3.39 ± 4.3 | <0.01 |
| TP | ms2 | R4 | 54.11 ± 69.41 | 16.31 ± 22.09 | 0.02 |
| LF (nu) | R4 | 52.18 ± 28.7 | 27.36 ± 20.32 | 0.02 | |
| HF (nu) | R4 | 47.82 ± 28.7 | 72.64 ± 20.32 | 0.02 | |
| RMSSD | ms | R4 | 8.67 ± 4.89 | 6.13 ± 4.54 | 0.05 |
| SD1 | ms | R4 | 6.16 ± 3.48 | 4.35 ± 3.23 | 0.05 |
| SDNN | ms | R5 | 14.12 ± 6.07 | 9.61 ± 3.28 | 0.05 |
| SD2 | ms | R5 | 18.92 ± 8.37 | 12.32 ± 3.67 | 0.03 |
| LF | ms2 | R6 | 13.6 ± 18.86 | 3.67 ± 3.38 | 0.02 |
| SDNN | ms | R6 | 17.8 ± 8.27 | 10.09 ± 4.98 | 0.01 |
| SD2 | ms | R6 | 24.23 ± 11.78 | 13.34 ± 6.99 | 0.01 |
| HRV | Units | Stage Level | RHC (n = 19) | sCAD (n = 10) | p-Value |
|---|---|---|---|---|---|
| SDNN | ms | Res—Max | 34.45 ± 21.28 | 11.08 ± 17.89 | <0.01 |
| SD2 | ms | Res—Max | 48.3 ± 30.04 | 14.89 ± 25.42 | <0.01 |
| Total Power | ms2 | Pre—Max | 182.83 ± 203.7 | 61.16 ± 42.45 | 0.03 |
| LF | ms2 | R4—Max | 33.35 ± 65.15 | 3.23 ± 4.38 | 0.04 |
| HF | ms2 | R4—Max | 9.98 ± 25.01 | 12.14 ± 17.93 | 0.54 |
| LF/HF | R4—Max | 2.98 ± 4.81 | 0.31 ± 0.62 | 0.15 | |
| SDNN | ms | R6—Max | 7.67 ± 8.34 | −0.46 ± 7.21 | 0.02 |
| SD2 | ms | R6—Max | 10.79 ± 11.96 | −1.07 ± 10.75 | 0.02 |
| RHC (n = 19) | iCAD (n = 25) | sCAD (n = 10) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| RHC vs. iCAD | RHC vs. sCAD | iCAD vs. sCAD | |||||
| Age | ys | 48.0 ± 10.4 | 49.7 ± 10.4 | 54.7 ± 10.9 | 0.96 | 0.17 | 0.21 |
| Gender (male/female) | 13/6 | 16/9 | 8/2 | ||||
| HRV indices | Units | ||||||
| RMSSD | ms/min | 0.39 ± 0.86 | 0.41 ± 0.41 | 0.28 ± 1.05 | 0.8 | 0.66 | 0.57 |
| SDNN | ms/min | −0.15 ± 1.13 | −0.57 ± −0.57 | −0.96 ± 0.88 | 0.17 | 0.04 * | 0.5 |
| MRRI | ms/min | 42.0 ± 13.58 | 43.01 ± 43.01 | 39.52 ± 10.41 | 0.71 | 0.7 | 0.32 |
| MHR | min−2 | −10.68 ± 1.94 | −10.0 ± −10.0 | −9.25 ± 2.33 | 0.24 | 0.12 | 0.3 |
| TP | ms2/min | 5.73 ± 6.96 | 6.85 ± 6.85 | 3.61 ± 4.56 | 0.76 | 0.11 | 0.12 |
| VLF | ms2/min | 0.08 ± 0.11 | 0.07 ± 0.07 | 0.04 ± 0.05 | 0.92 | 0.6 | 0.2 |
| LF | ms2/min | 3.93 ± 5.95 | 3.82 ± 3.82 | 0.85 ± 0.68 | 0.57 | 0.02 * | 0.06 |
| HF | ms2/min | 1.72 ± 2.66 | 2.96 ± 2.96 | 2.72 ± 3.93 | 0.39 | 0.8 | 0.39 |
| VHF | ms2/min | 0.46 ± 2.95 | 0.56 ± 0.56 | 0.5 ± 4.0 | 0.89 | 0.87 | 0.65 |
| LF/HF | min−1 | 0.52 ± 0.89 | 0.41 ± 0.41 | 0.16 ± 0.22 | 0.59 | 0.35 | 0.39 |
| LF (nu) | min−1 | 4.87 ± 5.03 | 4.24 ± 4.24 | 2.96 ± 3.31 | 0.76 | 0.32 | 0.35 |
| HF (nu) | min−1 | −4.87 ± 5.03 | −4.24 ± −4.24 | −2.96 ± 3.31 | 0.76 | 0.32 | 0.35 |
| SD1 | ms/min | 0.28 ± 0.62 | 0.29 ± 0.29 | 0.2 ± 0.75 | 0.79 | 0.66 | 0.57 |
| SD2 | ms/min | −0.29 ± 1.66 | −0.91 ± −0.91 | −1.49 ± 1.33 | 0.18 | 0.05 * | 0.37 |
| SD1/SD2 | min−1 | 0.01 ± 0.03 | 0.02 ± 0.02 | 0.03 ± 0.06 | 0.64 | 0.54 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-Y.; Tsai, C.-T.; Hsu, C.F.; Lee, Y.-H.; Huang, H.-P.; Huang, C.-C.; Liu, L.Y.-M.; Hsu, L.; Yang, T.-F.; Lin, P.-L. The Autonomic Imbalance of Myocardial Ischemia during Exercise Stress Testing: Insight from Short-Term Heart Rate Variability Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15096. https://doi.org/10.3390/ijerph192215096
Lin P-Y, Tsai C-T, Hsu CF, Lee Y-H, Huang H-P, Huang C-C, Liu LY-M, Hsu L, Yang T-F, Lin P-L. The Autonomic Imbalance of Myocardial Ischemia during Exercise Stress Testing: Insight from Short-Term Heart Rate Variability Analysis. International Journal of Environmental Research and Public Health. 2022; 19(22):15096. https://doi.org/10.3390/ijerph192215096
Chicago/Turabian StyleLin, Ping-Yen, Cheng-Ting Tsai, Chang Francis Hsu, Ying-Hsiang Lee, Han-Ping Huang, Chun-Che Huang, Lawrence Yu-Min Liu, Long Hsu, Ten-Fang Yang, and Po-Lin Lin. 2022. "The Autonomic Imbalance of Myocardial Ischemia during Exercise Stress Testing: Insight from Short-Term Heart Rate Variability Analysis" International Journal of Environmental Research and Public Health 19, no. 22: 15096. https://doi.org/10.3390/ijerph192215096






