Subacute Cardiac Tamponade Due to Tuberculous Pericarditis Diagnosed by Urine Lipoarabinomannan Assay in a Immunocompetent Patient in Oyam District, Uganda: A Case Report
Abstract
:1. Introduction
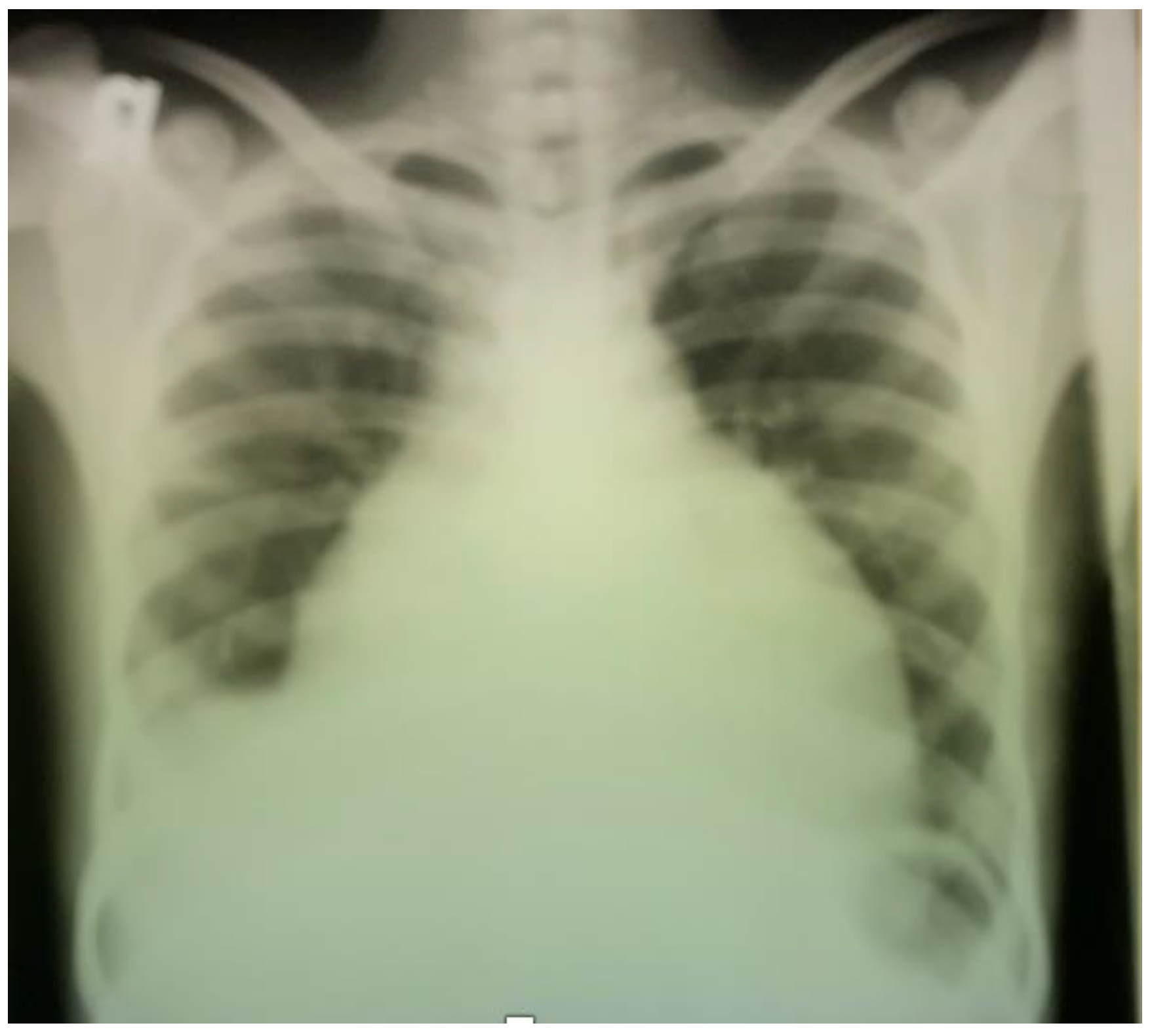
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report 2021. 2021. Available online: https://apps.who.int/iris/handle/10665/346387 (accessed on 14 June 2022).
- Gupta, R.K.; Lucas, S.B.; Fielding, K.L.; Lawn, S.D. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: A systematic review and meta-analysis. AIDS 2015, 29, 1987–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health Knowledge Management Portal. The Uganda National Tuberculosis Prevalence Survey, 2014–2015 Survey Report. Available online: http://library.health.go.ug/publications/tuberculosis/uganda-national-tuberculosis-prevalence-survey-2014-2015-survey-report (accessed on 14 June 2022).
- Cattamanchi, A.; Miller, C.R.; Tapley, A.; Haguma, P.; Ochom, E.; Ackerman, S.; Davis, J.L.; Katamba, A.; A Handley, M. Health worker perspectives on barriers to delivery of routine tuberculosis diagnostic evaluation services in Uganda: A qualitative study to guide clinic-based interventions. BMC Health Serv. Res. 2015, 15, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Gennaro, F.; Gualano, G.; Timelli, L.; Vittozzi, P.; Di Bari, V.; Libertone, R.; Cerva, C.; Pinnarelli, L.; Nisii, C.; Ianniello, S.; et al. Increase in Tuberculosis Diagnostic Delay during First Wave of the COVID-19 Pandemic: Data from an Italian Infectious Disease Referral Hospital. Antibiotics 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Mayosi, B.M.; Burgess, L.J.; Doubell, A.F. Tuberculous Pericarditis. Circulation 2005, 112, 3608–3616. [Google Scholar] [CrossRef] [Green Version]
- Pasipanodya, J.G.; Mubanga, M.; Ntsekhe, M.; Pandie, S.; Magazi, B.T.; Gumedze, F.N.; Myer, L.; Gumbo, T.; Mayosi, B.M. Tuberculous Pericarditis is Multibacillary and Bacterial Burden Drives High Mortality. EBioMedicine 2015, 2, 1634–1639. [Google Scholar] [CrossRef] [Green Version]
- Fowler, N.O. Tuberculous pericarditis. JAMA. 1991, 266, 99–103. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Agbor, V.N.; Ndoadoumgue, A.L.; Nkeck, J.R.; Kamguia, A.; Nyaga, U.F.; Ntsekhe, M. Epidemiology of pericardial diseases in Africa: A systematic scoping review. Heart 2019, 105, 180–188. [Google Scholar] [CrossRef]
- Bulterys, M.A.; Wagner, B.; Redard-Jacot, M.; Suresh, A.; Pollock, N.R.; Moreau, E.; Denkinger, C.M.; Drain, P.K.; Broger, T. Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update. J. Clin. Med. 2019, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Reuter, H.; Burgess, L.J.; Doubell, A.F. The role of chest radiography in diagnosing patients with tuberculous pericarditis: Cardiovascular topic. Cardiovasc. J. South Afr. 2005, 16, 108–111. [Google Scholar] [CrossRef]
- Suwanpimolkul, G.; Kawkitinarong, K.; Manosuthi, W.; Sophonphan, J.; Gatechompol, S.; Ohata, P.J.; Ubolyam, S.; Iampornsin, T.; Katerattanakul, P.; Avihingsanon, A.; et al. Utility of urine lipoarabinomannan (LAM) in diagnosing tuberculosis and predicting mortality with and without HIV: Prospective TB cohort from the Thailand Big City TB Research Network. Int. J. Infect. Dis. 2017, 59, 96–102. [Google Scholar] [CrossRef]
- Simieneh, A.; Tadesse, M.; Kebede, W.; Gashaw, M.; Abebe, G. Combination of Xpert® MTB/RIF and DetermineTM TB-LAM Ag improves the diagnosis of extrapulmonary tuberculosis at Jimma University Medical Center, Oromia, Ethiopia. PLoS ONE 2022, 17, e0263172. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.L.; Cheng, B.; Sheng, Y.; Jin, J. Diagnostic accuracy of adenosine deaminase for tuberculous pericarditis: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4411–4418. [Google Scholar] [PubMed]
- Light, R.W. Pleural Effusions. Med. Clin. N. Am. 2011, 95, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Adesina, A.; Chumba, D.; Nelson, A.M.; Orem, J.; Roberts, D.J.; Wabinga, H.; Wilson, M.; Rebbeck, T.R. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013, 14, e152–e157. [Google Scholar] [CrossRef]
- Briken, V.; Porcelli, S.A.; Besra, G.S.; Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol. Microbiol. 2004, 53, 391–403. [Google Scholar] [CrossRef]
- Pandie, S.; Peter, J.G.; Kerbelker, Z.S.; Meldau, R.; Theron, G.; Govender, U.; Ntsekhe, M.; Dheda, K.; Mayosi, B.M. The diagnostic accuracy of pericardial and urinary lipoarabinomannan (LAM) assays in patients with suspected tuberculous pericarditis. Sci. Rep. 2016, 6, 32924. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bozic, C.M.; McNeil, M.; Brennan, P.J. Structural features of the arabinan component of the lipoarabinomannan of Mycobacterium tuberculosis. J. Biol. Chem. 1991, 266, 9652–9660. [Google Scholar] [CrossRef]
- Mathabire Rucker, S.C.; Cossa, L.; Harrison, R.E.; Mpunga, J.; Lobo, S.; Kimupelenge, P.K.; Kol’Ampwe, F.M.; Quiles, I.A.; Molfino, L.; Szumilin, E.; et al. Feasibility of using Determine TB-LAM to diagnose tuberculosis in HIV-positive patients in programmatic conditions: A multisite study. Glob. Health Action 2019, 12, 1672366. [Google Scholar] [CrossRef] [Green Version]
- Rodger, A.; Jaffar, S.; Paynter, S.; Hayward, A.; Carless, J.; Maguire, H. Delay in the diagnosis of pulmonary tuberculosis, London, 1998–2000: Analysis of surveillance data. BMJ 2003, 326, 909–910. [Google Scholar] [CrossRef] [Green Version]
- Correia-Neves, M.; Fröberg, G.; Korshun, L.; Viegas, S.; Vaz, P.; Ramanlal, N.; Bruchfeld, J.; Hamasur, B.; Brennan, P.; Källenius, G. Biomarkers for tuberculosis: The case for lipoarabinomannan. ERJ Open Res. 2019, 5, 00115–02018. [Google Scholar] [CrossRef]
- Hamasur, B.; Bruchfeld, J.; Haile, M.; Pawlowski, A.; Bjorvatn, B.; Källenius, G.; Svenson, S.B. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J. Microbiol. Methods 2001, 45, 41–52. [Google Scholar] [CrossRef]
- Hamasur, B.; Bruchfeld, J.; van Helden, P.; Källenius, G.; Svenson, S. A sensitive urinary lipoarabinomannan test for tuberculosis. PLoS ONE 2015, 10, e0123457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscato, L.M.; Stuart, M.C. Heterophilic antibodies: A problem for all immunoassays. Clin. Chem. 1988, 34, 27–33. [Google Scholar] [CrossRef]
- Kebede, W.; Abebe, G.; Gudina, E.K.; Van Rie, A. The value of lateral flow urine lipoarabinomannan assay and empirical treatment in Xpert MTB/RIF ultra negative patients with presumptive TB: A prospective cohort study. Sci. Rep. 2021, 11, 24428. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Tsiaga, P.; Antoniadi, G.; Liakopoulos, V.; Kortsaris, A.; Giannatos, E.; Barbutis, K.; Stefanidis, I.; Vargemezis, V. The value of serum antilipoarabinomannan antibody detection in the diagnosis of latent tuberculosis in hemodialysis patients. Am. J. Kidney Dis. 2005, 46, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, F.; Pisani, L.; Veronese, N.; Pizzol, D.; Lippolis, V.; Saracino, A.; Monno, L.; Huson, M.A.; Copetti, R.; Putoto, G.; et al. Potential Diagnostic Properties of Chest Ultrasound in Thoracic Tuberculosis-A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2235. [Google Scholar] [CrossRef] [Green Version]
- Bobbio, F.; Di Gennaro, F.; Marotta, C.; Kok, J.; Akec, G.; Norbis, L.; Monno, L.; Saracino, A.; Mazzucco, W.; Lunardi, M. Focused ultrasound to diagnose HIV-associated tuberculosis (FASH) in the extremely resource-limited setting of South Sudan: A cross-sectional study. BMJ Open 2019, 9, e027179. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vita, E.; Segala, F.V.; Amone, J.; Samuel, K.; Marotta, C.; Putoto, G.; Nassali, R.; Lochoro, P.; Bavaro, D.F.; Ictho, J.; et al. Subacute Cardiac Tamponade Due to Tuberculous Pericarditis Diagnosed by Urine Lipoarabinomannan Assay in a Immunocompetent Patient in Oyam District, Uganda: A Case Report. Int. J. Environ. Res. Public Health 2022, 19, 15143. https://doi.org/10.3390/ijerph192215143
De Vita E, Segala FV, Amone J, Samuel K, Marotta C, Putoto G, Nassali R, Lochoro P, Bavaro DF, Ictho J, et al. Subacute Cardiac Tamponade Due to Tuberculous Pericarditis Diagnosed by Urine Lipoarabinomannan Assay in a Immunocompetent Patient in Oyam District, Uganda: A Case Report. International Journal of Environmental Research and Public Health. 2022; 19(22):15143. https://doi.org/10.3390/ijerph192215143
Chicago/Turabian StyleDe Vita, Elda, Francesco Vladimiro Segala, James Amone, Kabuga Samuel, Claudia Marotta, Giovanni Putoto, Ritah Nassali, Peter Lochoro, Davide Fiore Bavaro, Jerry Ictho, and et al. 2022. "Subacute Cardiac Tamponade Due to Tuberculous Pericarditis Diagnosed by Urine Lipoarabinomannan Assay in a Immunocompetent Patient in Oyam District, Uganda: A Case Report" International Journal of Environmental Research and Public Health 19, no. 22: 15143. https://doi.org/10.3390/ijerph192215143




