Deleterious Effect of Air Pollution on Human Microbial Community and Bacterial Flora: A Short Review
Abstract
:1. Introduction
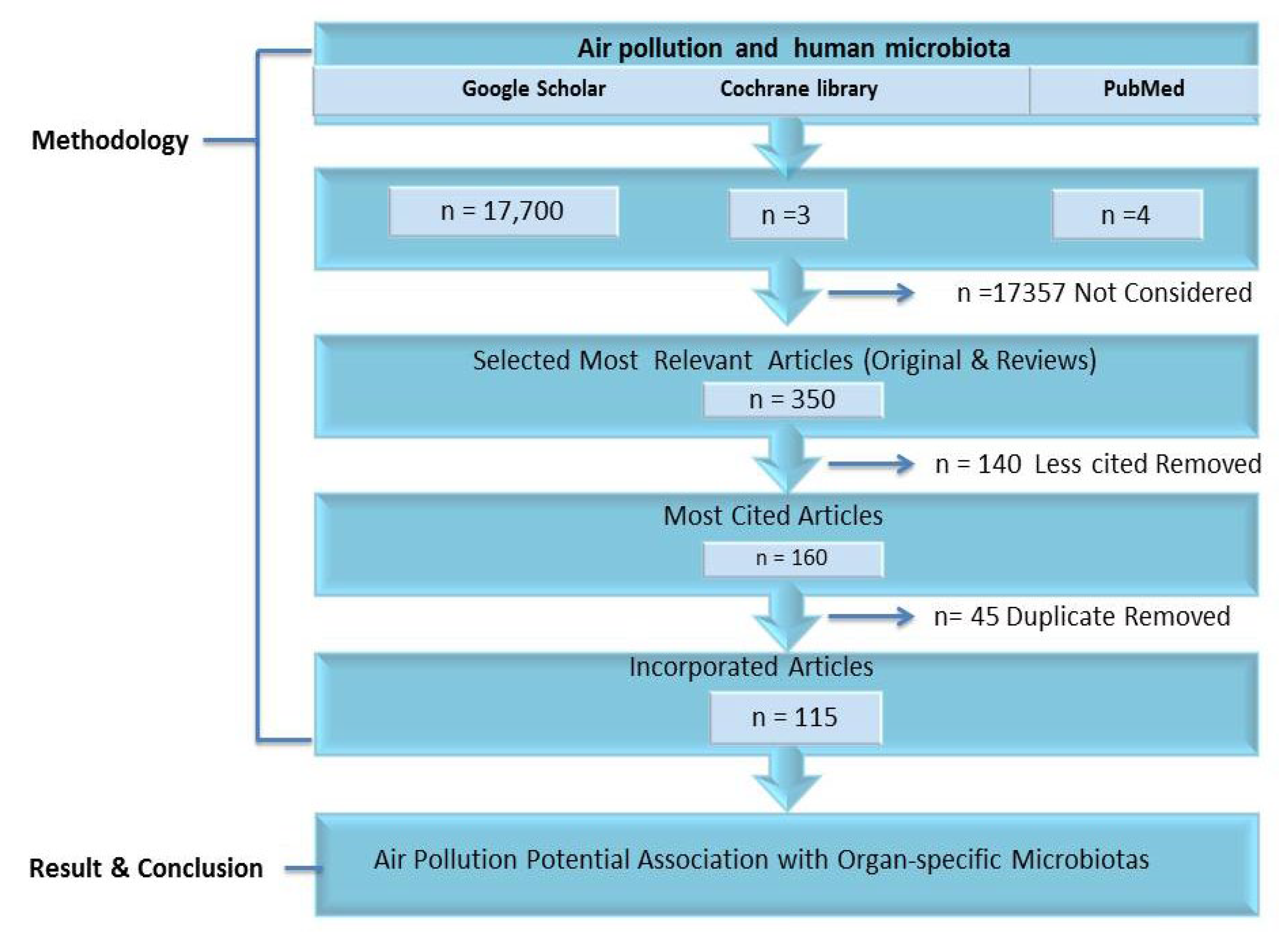
2. Methodology
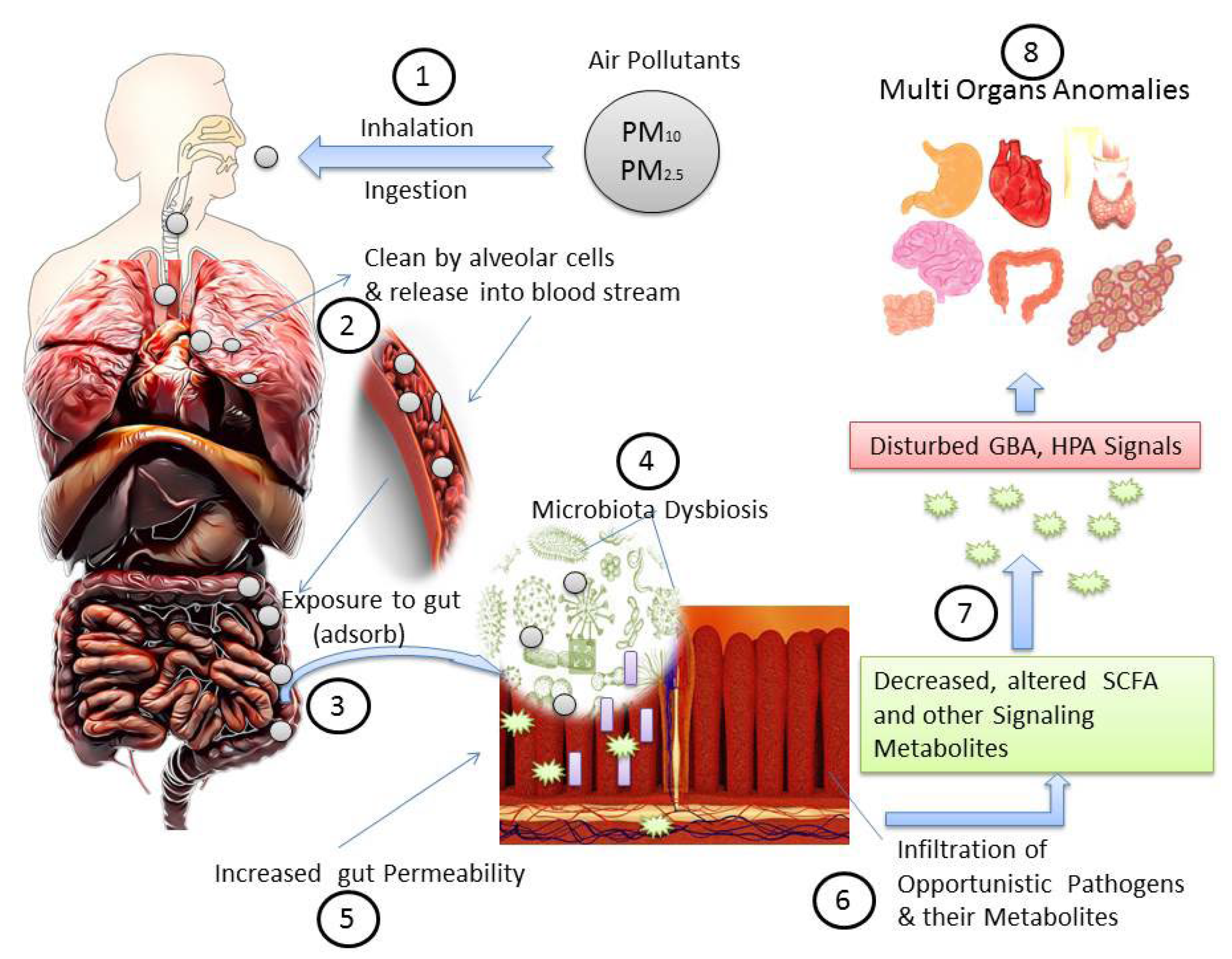
3. Air Pollutants and Dysbiosis of Human Microbiota
4. Air Pollution Impact on Nasal Microbiota
5. Air Pollution and Oral Microbiota Dysbiosis
6. Air Pollution Impact on Pharyngeal Microbiota
7. Air Pollution and Altered Respiratory Microbiota
8. Air Pollution and Skin Flora
9. Air Pollution, Gut Microbiota Dysbiosis, and Associated Diseases
10. Potential Joint Role of Air Pollution and Microbial Dysbiosis in Diseases’ Burdens
10.1. Irritable Bowel Syndrome and Inflammatory Bowel Diseases
10.2. Disturbed Thyroid Functions
10.3. Microbial Dysbiosis and Carcinogenesis
11. Rebalance and Prevention of Microbial Dysbiosis
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, Y.; Chu, J.; Li, Y.; Kong, X. The influence of air pollution on respiratory microbiome: A link to respiratory disease. Toxicol. Lett. 2020, 334, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.J.; Rifkin, R.F.; Cowan, D.A.; Potgieter, M. The healthy human blood microbiome: Fact or fiction? Front. Cell Infect. Microbiol. 2019, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.; Yadav, V.K.; Yadav, K.K.; Choudhary, N.; Kalasariya, H.; Alam, M.M.; Gacem, A.; Amanullah, M.; Ibrahium, H.A.; Park, J.-W.; et al. A Recent and Systemic Approach Towards Microbial Biodegradation of Dyes from Textile Industries. Water 2022, 14, 3163. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Link, C.D. Is There a Brain Microbiome? Neurosci. Insights 2021, 16, 26331055211018708. [Google Scholar] [CrossRef]
- Nardone, G.; Compare, D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United Eur. Gastroenterol. J. 2015, 3, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.Á.P.; Frizon, L.; Demeda, V.F. Ocular surface microbiome in health and disease. Asia-Pac. J. Ophthalmol. 2020, 9, 505–511. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Karimi, B.; Meyer, C.; Gilbert, D.; Bernard, N. Air pollution below WHO levels decreases by 40% the links of terrestrial microbial networks. Environ. Chem. Lett. 2016, 14, 467–475. [Google Scholar] [CrossRef]
- Ghio, A.J. Particle exposures and infections. Infection 2014, 42, 459–467. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef]
- Coker, E.S.; Cavalli, L.; Fabrizi, E.; Guastella, G.; Lippo, E.; Parisi, M.L.; Vergalli, S. The Effects of Air Pollution on COVID-19 Related Mortality in Northern Italy. Environ. Res. Econ. 2020, 76, 611–634. [Google Scholar] [CrossRef]
- Tsai, S.S.; Chiu, H.F.; Yang, C.Y. Ambient air pollution and hospital admissions for peptic ulcers in Taipei: A time-stratified case-crossover study. Int. J. Environ. Res. Public Health 2019, 16, 1916. [Google Scholar] [CrossRef] [Green Version]
- WHO. Air Quality and Health: Health Impact; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. Air Pollution; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- EPA. Criteria Air Pollutants; EPA: Washington, DC, USA, 2022. [Google Scholar]
- Mousavi, S.E.; Delgado-Saborit, J.M.; Adivi, A.; Pauwels, S.; Godderis, L. Air pollution and endocrine disruptors induce human microbiome imbalances: A systematic review of recent evidence and possible biological mechanisms. Sci. Total Environ. 2022, 816, 151654. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Vignal, C.; Guilloteau, E.; Gower-Rousseau, C.; Body-Malapel, M. Review article: Epidemiological and animal evidence for the role of air pollution in intestinal diseases. Sci. Total Environ. 2021, 757, 143718. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Bailey, M.J.; Naik, N.N.; Wild, L.E.; Patterson, W.B.; Alderete, T.L. Exposure to air pollutants and the gut microbiota: A potential link between exposure, obesity, and type 2 diabetes. Gut Microbes 2020, 11, 1188–1202. [Google Scholar] [CrossRef]
- Li, J.-J.; Lu, Z.-L.; Kou, W.-R.; Chen, Z.; Wu, Y.-F.; Yu, X.-H.; Zhao, Y.C. Long-Term Effects of Xuezhikang on Blood Pressure in Hypertensive Patients with Previous Myocardial Infarction: Data from the Chinese Coronary Secondary Prevention Study (CCSPS). Clin. Exp. Hypertens. 2010, 32, 491–498. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Zhang, M.; Feng, J. Gut microbiota dysbiosis exaggerates ammonia-induced tracheal injury Via TLR4 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 246, 114206. [Google Scholar] [CrossRef]
- Salim, S.Y.; Kaplan, G.G.; Madsen, K.L. Air pollution effects on the gut microbiota. Gut Microbes 2014, 5, 215–219. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal Pathways for Microbiome-Brain-Gut Axis Communication. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; pp. 115–133. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Al Mamun, A.; Rahaman, M.S.; Islam, M.M.; Cavalu, S. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, P.; Pal, N.; Kumawat, M.; Shubham, S.; Sarma, D.K.; Nagpal, R. Impact of Environmental Pollutants on Gut Microbiome and Mental Health via the Gut–Brain Axis. Microorganisms 2022, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Giambò, F.; Costa, C.; Teodoro, M.; Fenga, C. Role-Playing Between Environmental Pollutants and Human Gut Microbiota: A Complex Bidirectional Interaction. Front. Med. 2022, 9, 810397. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallès, Y.; Francino, M.P. Air Pollution, Early Life Microbiome, and Development. Curr. Environ. Health Rep. 2018, 5, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemon, K.P. Human nasal microbiota. Curr. Biol. 2020, 30, R1118–R1119. [Google Scholar] [CrossRef]
- Gisler, A.; Korten, I.; de Hoogh, K.; Vienneau, D.; Frey, U.; Decrue, F.; BILD Study Group. Associations of air pollution and greenness with the nasal microbiota of healthy infants: A longitudinal study. Environ. Res. 2021, 202, 111633. [Google Scholar] [CrossRef]
- Modi, S.; Inwati, G.K.; Gacem, A.; Saquib Abullais, S.; Prajapati, R.; Yadav, V.K.; Jeon, B.H. Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics. Antibiotics 2022, 11, 708. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sundaram Sivamaruthi, B.; Kesika, P.; Bharathi, M.; Chaiyasut, C. Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging—A Review. Microorganisms 2022, 10, 1405. [Google Scholar] [CrossRef]
- Pal, G.; Ramirez, V.; Engen, P.A.; Naqib, A.; Forsyth, C.B.; Green, S.J.; Keshavarzian, A. Deep nasal sinus cavity microbiota dysbiosis in Parkinson’s disease. NPJ Park. Dis. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Mariani, J.; Favero, C.; Spinazzè, A.; Cavallo, D.M.; Carugno, M.; Motta, V.; Bollati, V. Short-term particulate matter exposure influences nasal microbiota in a population of healthy subjects. Environ. Res. 2018, 162, 119–126. [Google Scholar] [CrossRef]
- Yadav, V.K.; Malik, P.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Islam, S.; Jeon, B.H. Health and Environmental Risks of Incense Smoke: Mechanistic Insights and Cumulative Evidence. J. Inflamm. Res. 2022, 15, 2665. [Google Scholar] [CrossRef]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, D.; Wu, H.; Li, R.; Xu, S.; Kang, Y.; Qiao, J. Epidemiology of infertility in China: A population-based study. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Foulon, A.; El Hage, W.; Dufour-Rainfray, D.; Denis, F. Is There a Link between Oropharyngeal Microbiome and Schizophrenia? A Narrative Review. Int. J. Mol. Sci. 2022, 23, 846. [Google Scholar] [CrossRef]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontol. 2000 2016, 70, 80–92. [Google Scholar] [CrossRef]
- Mohammed, H.; Varoni, E.M.; Cochis, A.; Cordaro, M.; Gallenzi, P.; Patini, R.; Rocchetti, V. Oral dysbiosis in pancreatic cancer and liver cirrhosis: A review of the literature. Biomedicines 2018, 6, 115. [Google Scholar] [CrossRef]
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, S.; Ray, J.G.; Saha, A. Dysbiosis of Oral Microbiota During Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448. [Google Scholar] [CrossRef]
- Sureda, A.; Daglia, M.; Argüelles Castilla, S.; Sanadgol, N.; Fazel Nabavi, S.; Khan, H.; Nabavi, S.M. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 2020, 151, 104582. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, F.; Zhou, F.; Li, H.; Ge, W.; Gan, R.; Huang, Z. Metagenomic analysis reveals oropharyngeal microbiota alterations in patients with COVID-19. Signal Transduct. Target. Ther. 2021, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Doşa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Bjørklund, G. Association between the gut and oral microbiome with obesity. Anaerobe 2021, 70, 102248. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Kang, Y.; Yu, J.; Ren, L. Human Pharyngeal Microbiome May Play a Protective Role in Respiratory Tract Infections. Genom. Proteom. Bioinform. 2014, 12, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, T.; Zhang, F.; Zhou, H.; Ren, H.; Du, Y.; Liang, S.; Xu, J. High-Level PM2.5/PM10 exposure is associated with alterations in the human pharyngeal microbiota composition. Front. Microbiol. 2019, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, Y.; An, Y.; Wang, R.; Lin, H.; Liu, M.; Xiao, C. Air pollution during the winter period and respiratory tract microbial imbalance in a healthy young population in Northeastern China. Environ. Pollut. 2019, 246, 972–979. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, C.; Luo, J.; Wang, J.; Zhang, D. 16SrDNA-Based Detection Technology in Patients with Chronic Pharyngitis to Analyze the Distribution Characteristics of Pharyngeal Bacteria. J. Healthc. Eng. 2022, 2022, 5186991. [Google Scholar] [CrossRef]
- Xin Pei Ho, E.; Ming Gemmy Cheung, C.; Sim, S.; Wenhan Chu, C.; Wilm, A.; Bitong Lin, C.; Hibberd, M. Human pharyngeal microbiota in age-related macular degeneration. PLoS ONE 2018, 13, e0201768. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Qiao, L.; Shi, J.; Xie, L.; Liu, Y.; Xiong, Y.; Liu, H. Clinical Study of Correlation for the Intestinal and Pharyngeal Microbiota in the Premature Neonates. Front. Pediatr. 2021, 9, 632573. [Google Scholar] [CrossRef]
- Adar, S.D.; Huffnagle, G.B.; Curtis, J.L. The respiratory microbiome: An underappreciated player in the human response to inhaled pollutants? Ann. Epidemiol. 2016, 26, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Hamidou Soumana, I.; Carlsten, C. Air pollution and the respiratory microbiome. J. Allergy Clin. Immunol. 2021, 148, 67–69. [Google Scholar] [CrossRef]
- Niemeier-Walsh, C.; Ryan, P.H.; Meller, J.; Ollberding, N.J.; Adhikari, A.; Reponen, T. Exposure to traffic-related air pollution and bacterial diversity in the lower respiratory tract of children. PLoS ONE 2021, 16, e0244341. [Google Scholar] [CrossRef]
- Rylance, J.; Kankwatira, A.; Nelson, D.E.; Toh, E.; Day, R.B.; Lin, H.; Gordon, S.B. Household air pollution and the lung microbiome of healthy adults in Malawi: A cross-sectional study. BMC Microbiol. 2016, 16, 182. [Google Scholar] [CrossRef] [Green Version]
- Thapa, A.; Kaushik, R.; Arora, S.; Jaglan, S.; Jaswal, V.; Yadav, V.K.; Singh, M.; Bains, A.; Chawla, P.; Khan, A.; et al. Biological Activity of Picrorhiza kurroa: A Source of Potential Antimicrobial Compounds against Yersinia enterocolitica. Int. J. Mol. Sci. 2022, 23, 14090. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, J.; Zhang, J.; Zhou, F.; He, X.; Shi, Y.; Tao, Y. The Role of Respiratory Microbiota in Lung Cancer. Int. J. Biol. Sci. 2021, 17, 3646–3658. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.-N.; Chu, C.-C.; Jing, Z.; Chen, Y.; Zhang, J.; Chen, L. Facial Skin Microbiota-Mediated Host Response to Pollution Stress Revealed by Microbiome Networks of Individual. MSystems 2021, 6, e00319-21. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wang, S.Q. Recognizing the impact of ambient air pollution on skin health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [Green Version]
- Alam, J.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Tavker, N.; Choudhary, N.; Hamid, A.A. Recent advances in methods for the recovery of carbon nanominerals and polyaromatic hydrocarbons from coal fly ash and their emerging applications. Crystals 2021, 11, 88. [Google Scholar] [CrossRef]
- Leung, M.H.Y.; Tong, X.; Bastien, P.; Guinot, F.; Tenenhaus, A.; Appenzeller, B.M.R.; Lee, P.K. Changes of the human skin microbiota upon chronic exposure to polycyclic aromatic hydrocarbon pollutants. Microbiome 2020, 8, 100. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Dreno, B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef]
- Olivares, M.; Sanz, Y. Chapter 3—Gut microbiota in the etiopathogenesis of celiac disease. In Biotechnological Strategies for the Treatment of Gluten Intolerance; Rossi, M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 45–64. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Li, C.; Zheng, D.; Chen, P. Gut Microbiota and Endocrine Disorder. In Biotechnological Strategies for the Treatment of Gluten Intolerance; Gut Microbiota and Pathogenesis of Organ Injury; Chen, P., Ed.; Springer: Singapore, 2020; pp. 143–164. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The influence of the gut microbiome on obesity in adults and the role of probiotics prebiotics and synbiotics for weight loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The human gut microbiome—A potential controller of wellness and disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2018, 216, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Maroni, L.; Fianchi, F.; Miele, L.; Svegliati Baroni, G. Chapter 5—The pathophysiology of gut–liver connection. In The Complex Interplay Between Gut-Brain, Gut-Liver, and Liver-Brain Axes; Stasi, C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 97–122. [Google Scholar] [CrossRef]
- Shin, C.; Kim, Y.-K. Chapter 3—The interactions between gut and brain in psychiatric and neurological disorders. In The Complex Interplay Between Gut-Brain, Gut-Liver, and Liver-Brain Axes; Stasi, C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 49–65. [Google Scholar] [CrossRef]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Fadlyana, E.; Soemarko, D.S.; Endaryanto, A.; Haryanto, B.; Darma, A.; Dewi, D.K.; Basrowi, R.W. The Impact of Air Pollution on Gut Microbiota and Children’s Health: An Expert Consensus. Children 2022, 9, 765. [Google Scholar] [CrossRef]
- Zheng, P.; Zhang, B.; Zhang, K.; Lv, X.; Wang, Q.; Bai, X. The Impact of Air Pollution on Intestinal Microbiome of Asthmatic Children: A Panel Study. Biomed. Res. Int. 2020, 2020, 5753427. [Google Scholar] [CrossRef]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef]
- Maria Tanase, D.; Maria Gosav, E.; Neculae, E.; Florida Costea, C.; Ciocoiu, M.; Liliana Hurjui, L.; Serban, I.L. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef]
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Molecular Sciences Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018, 19, 995. [Google Scholar] [CrossRef] [Green Version]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; el Aidy, S.; Deane, J.; Dinan, T.G. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, R.; Wang, W.; Qi, L.; Huang, T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflamm. 2020, 17, 288. [Google Scholar] [CrossRef]
- Canakis, A.; Haroon, M.; Weber, H.C. Irritable bowel syndrome and gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 28–35. [Google Scholar] [CrossRef]
- Tu, Y.; Yang, R.; Xu, X.; Zhou, X. The microbiota-gut-bone axis and bone health. J. Leukoc. Biol. 2021, 110, 525–537. [Google Scholar] [CrossRef]
- Shikata, F.; Shimada, K.; Sato, H.; Ikedo, T.; Kuwabara, A.; Furukawa, H.; Hashimoto, T. Potential Influences of Gut Microbiota on the Formation of Intracranial Aneurysm. Hypertension 2019, 73, 491–496. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.P.; Koh, A.Y. The gut microbiota in transplant patients. Blood Rev. 2020, 39, 100614. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Ni, J.J.; Han, B.X.; Yan, S.S.; Wei, X.T.; Feng, G.J.; Pei, Y.F. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2022, 12, 746998. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qi, Y.; Yang, X.; Zhao, L.; Wen, S.; Liu, Y.; Tang, L. Association between polycystic ovary syndrome and gut microbiota. PLoS ONE 2016, 11, e0153196. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.; Yang, Z.; Zhang, X.; Liu, Z.; Wang, M.; Wu, X.; Huang, R. Sustained Drug Treatment Alters the Gut Microbiota in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 704089. [Google Scholar] [CrossRef]
- Karl, J.P.; Berryman, C.E.; Young, A.J.; Radcliffe, P.N.; Branck, T.A.; Pantoja-Feliciano, I.G.; Pasiakos, S.M. Associations between the gut microbiota and host responses to high altitude. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G1003–G1015. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, J.; Wang, Y.; Bai, M.; Sun, S. Specific Alterations of Gut Microbiota in Chinese Patients with Hypertension: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2022, 47, 433–447. [Google Scholar] [CrossRef]
- Stock, J. Gut microbiota: An environmental risk factor for cardiovascular disease. Atherosclerosis 2013, 229, 440–442. [Google Scholar] [CrossRef]
- Dong, X.; Yao, S.; Deng, L.; Li, H.; Zhang, F.; Xu, J.; Wu, W. Alterations in the gut microbiota and its metabolic profile of PM2.5 exposure-induced thyroid dysfunction rats. Sci. Total Environ. 2022, 838, 156402. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Naghavi, M. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Marynowski, M.; Likońska, A.; Zatorski, H.; Fichna, J. Role of environmental pollution in irritable bowel syndrome. World J. Gastroenterol. 2015, 21, 11371–11378. [Google Scholar] [CrossRef]
- Chey, W.; Menees, S. The gut microbiome and irritable bowel syndrome. F1000Research 2018, 7, 1029. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opstelten, J.L.; Beelen, R.M.J.; Leenders, M.; Hoek, G.; Brunekreef, B.; van Schaik, F.D.M.; Oldenburg, B. Exposure to Ambient Air Pollution and the Risk of Inflammatory Bowel Disease: A European Nested Case–Control Study. Dig. Dis. Sci. 2016, 61, 2963–2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, G.G.; Szyszkowicz, M.; Fichna, J.; Rowe, B.H.; Porada, E.; Vincent, R.; Storr, M. Non-Specific Abdominal Pain and Air Pollution: A Novel Association. PLoS ONE 2012, 7, e47669. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.Z.; Song, H.; Wang, E.X.; Liu, Y.; Yuan, Y.J. Design and construction of synthetic microbial consortia in China. Synth. Syst. Biotechnol. 2016, 1, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [Green Version]
- The Lancet. Thyroid disease—More research needed. Lancet 2012, 379, 1076. [Google Scholar] [CrossRef]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Gong, B.; Wang, C.; Meng, F.; Wang, H.; Song, B.; Yang, Y.; Shan, Z. Association Between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 1. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sheflin, A.M.; Whitney, A.K.; Weir, T.L. Cancer-Promoting Effects of Microbial Dysbiosis. Curr. Oncol. Rep. 2014, 16, 406. [Google Scholar] [CrossRef] [Green Version]
- Borges-Canha, M.; Pedro Portela-Cidade, J.; Dinis-Ribeiro, M.; Leite-Moreira, A.F.; Pimentel-Nunes, P. Role of colonic microbiota in colorectal carcinogenesis: A systematic review. Rev. Española Enferm. Dig. 2015, 107, 659–671. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Ahasan, M.T.; Sarkar, N.; Khan, H.; Hasan, A.M.; Cavalu, S.; Rauf, A. Microbiome in cancer: Role in carcinogenesis and impact in therapeutic strategies. Biomed. Pharmacother. 2022, 149, 112898. [Google Scholar] [CrossRef]
- Parida, S.; Sharma, D. Microbial Alterations and Risk Factors of Breast Cancer: Connections and Mechanistic Insights. Cells 2020, 9, 1091. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Lu, X.; Zhang, Z.; Xue, Y.; Xu, J.; Xu, C. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med. 2019, 8, 6904–6914. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Schippa, S. Rebuilding the gut microbiota ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef] [Green Version]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Patel, N.B.; Gacem, A.; Alsufyani, T.; Reece, L.M.; Yadav, V.K.; Jeon, B.H. Marine Alga Ulva fasciata-Derived Molecules for the Potential Treatment of SARS-CoV-2: An in silico Approach. Mar. Drugs 2022, 20, 586. [Google Scholar] [CrossRef]


| Diseases Associated with Abnormal Gut Microbiota | Findings | References |
|---|---|---|
| Asthma in children | A little wave of air pollution may trigger asthma by impacting intestinal bacteria. | [76] |
| Weight gain and obesity | An imbalance in gut microbiota, especially Bacteroidetes and Firmicutes, is associated with obesity. | [77] |
| Diabetes, insulin resistance, inflammation | The imbalanced gut microbiome causes abnormal production of metabolites, inflammatory reactions, glucose metabolism alteration, and even insulin resistance. | [78] |
| Type 1 diabetes | Gut bacterial dysbiosis is highly associated with Type 1 diabetes. | [79] |
| Brain disorders | Impairment of gut microbiota may cause the development of autism and mood disorders. | [80] |
| Depression | Gut microbiota may be associated with depression. | [81] |
| Neuropsychiatric disorders | A link exists between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. | [82] |
| Irritable bowel syndrome (IBS) | Bacterial gut disturbances have been linked to the development and severity of IBS. | [83] |
| Bone disorders | Microbiota dysbiosis has been linked with multiple bone disorders. | [84] |
| Endocrine abnormalities | Gut microbiota has emerged as an important factor in endocrine system diseases. | [67] |
| Aneurysms | Gut microbiota contributes to the pathophysiology of aneurysms by modulating inflammation. | [85] |
| Sleep quality | A novel association exists between sleep health and gut microbiome diversity. | [86] |
| Adverse effects on organ transplantation | Disturbances in gut microbiota populations are associated with a number of adverse outcomes. | [87] |
| Inflammatory bowel disease (IBD) | The gut microbe’s dysbiosis may play a pivotal role in the pathogenesis of IBD. | [88] |
| Autoimmune diseases (AD) | Alterations in gut microbiota composition are associated with multiple autoimmune diseases. | [89] |
| Polycystic ovary syndrome (PCOS) | Dysbiosis of gut microbiota has been found to be associated with the pathogenesis of PCOS. | [90] |
| Rheumatoid arthritis (RA) | Change in gut microbiota has been associated with the pathogenesis of RA. | [91] |
| High-altitude (HA) sickness | Gut microbiota may contribute to variability in host responses to HA. | [92] |
| Hypertension | Variation in gut microbial parameters was likely associated with Chinese patients with hypertension. | [93] |
| Celiac disease | Dysbiosis of gut microbiota may lead to celiac disease. | [66] |
| Cardiovascular diseases | Disturbance in gut microbiota may lead to cardiovascular diseases. | [94] |
| Thyroid dysfunction | Animal study showed PM2.5 exposure disturbed the vital gut–thyroid axis and metabolic pathways related to thyroid thyrotoxicosis. | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Yadav, V.K.; Gacem, A.; Al-Dossari, M.; Yadav, K.K.; Abd El-Gawaad, N.S.; Ben Khedher, N.; Choudhary, N.; Kumar, P.; Cavalu, S. Deleterious Effect of Air Pollution on Human Microbial Community and Bacterial Flora: A Short Review. Int. J. Environ. Res. Public Health 2022, 19, 15494. https://doi.org/10.3390/ijerph192315494
Gupta N, Yadav VK, Gacem A, Al-Dossari M, Yadav KK, Abd El-Gawaad NS, Ben Khedher N, Choudhary N, Kumar P, Cavalu S. Deleterious Effect of Air Pollution on Human Microbial Community and Bacterial Flora: A Short Review. International Journal of Environmental Research and Public Health. 2022; 19(23):15494. https://doi.org/10.3390/ijerph192315494
Chicago/Turabian StyleGupta, Nishant, Virendra Kumar Yadav, Amel Gacem, M. Al-Dossari, Krishna Kumar Yadav, N. S. Abd El-Gawaad, Nidhal Ben Khedher, Nisha Choudhary, Pankaj Kumar, and Simona Cavalu. 2022. "Deleterious Effect of Air Pollution on Human Microbial Community and Bacterial Flora: A Short Review" International Journal of Environmental Research and Public Health 19, no. 23: 15494. https://doi.org/10.3390/ijerph192315494
APA StyleGupta, N., Yadav, V. K., Gacem, A., Al-Dossari, M., Yadav, K. K., Abd El-Gawaad, N. S., Ben Khedher, N., Choudhary, N., Kumar, P., & Cavalu, S. (2022). Deleterious Effect of Air Pollution on Human Microbial Community and Bacterial Flora: A Short Review. International Journal of Environmental Research and Public Health, 19(23), 15494. https://doi.org/10.3390/ijerph192315494











