Large Semi-Membrane Covered Composting System Improves the Spatial Homogeneity and Efficiency of Fermentation
Abstract
:1. Introduction
2. Methods and Materials
2.1. Raw Materials and Mixing Ratio
2.2. Experiment Design
2.3. Real-Time Collection Data of Temperature and Oxygen Content and Sample Collection
2.4. Physicochemical Methods of Samples
2.5. DNA Extraction and Illumina MiSeq Sequencing
2.6. Statistical Analysis
3. Results and Discussion
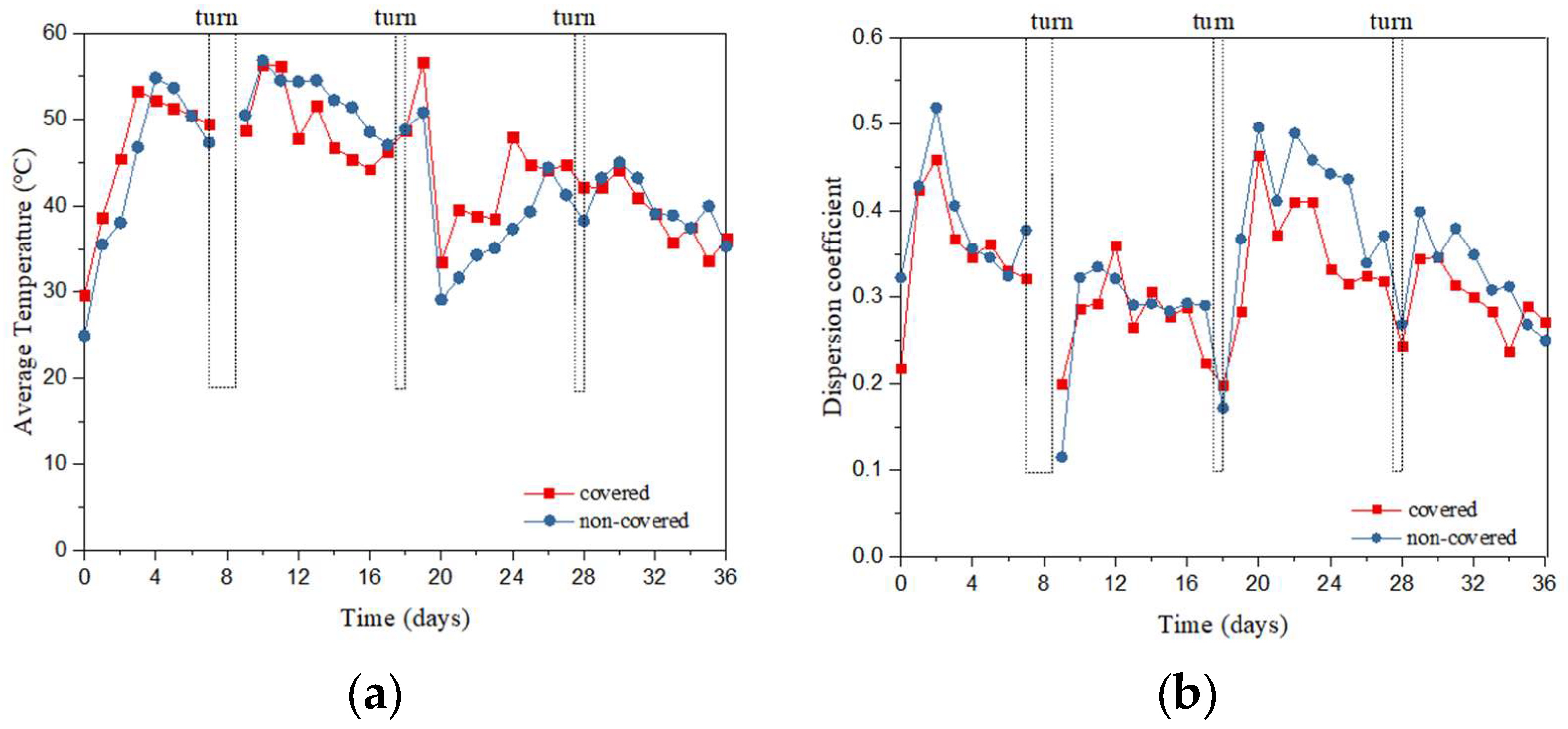
3.1. Spatial Distribution of Temperature in the Compost Pile
3.2. Spatial Distribution of Physicochemical Properties of Compost Samples
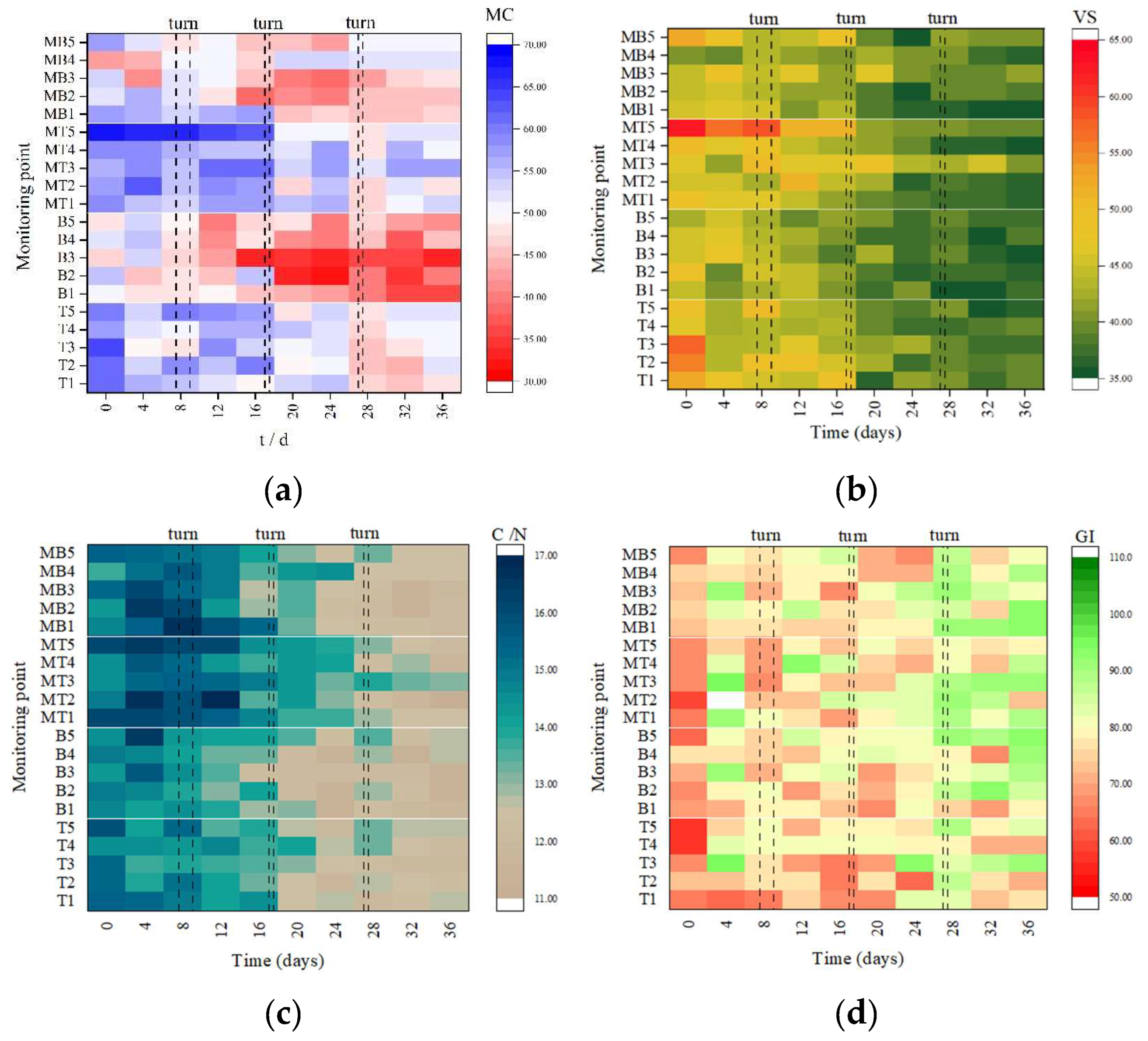
3.2.1. Moisture Content (MC)
3.2.2. Violate Solid (VS) and Ratio of Carbon to Nitrogen (C/N)
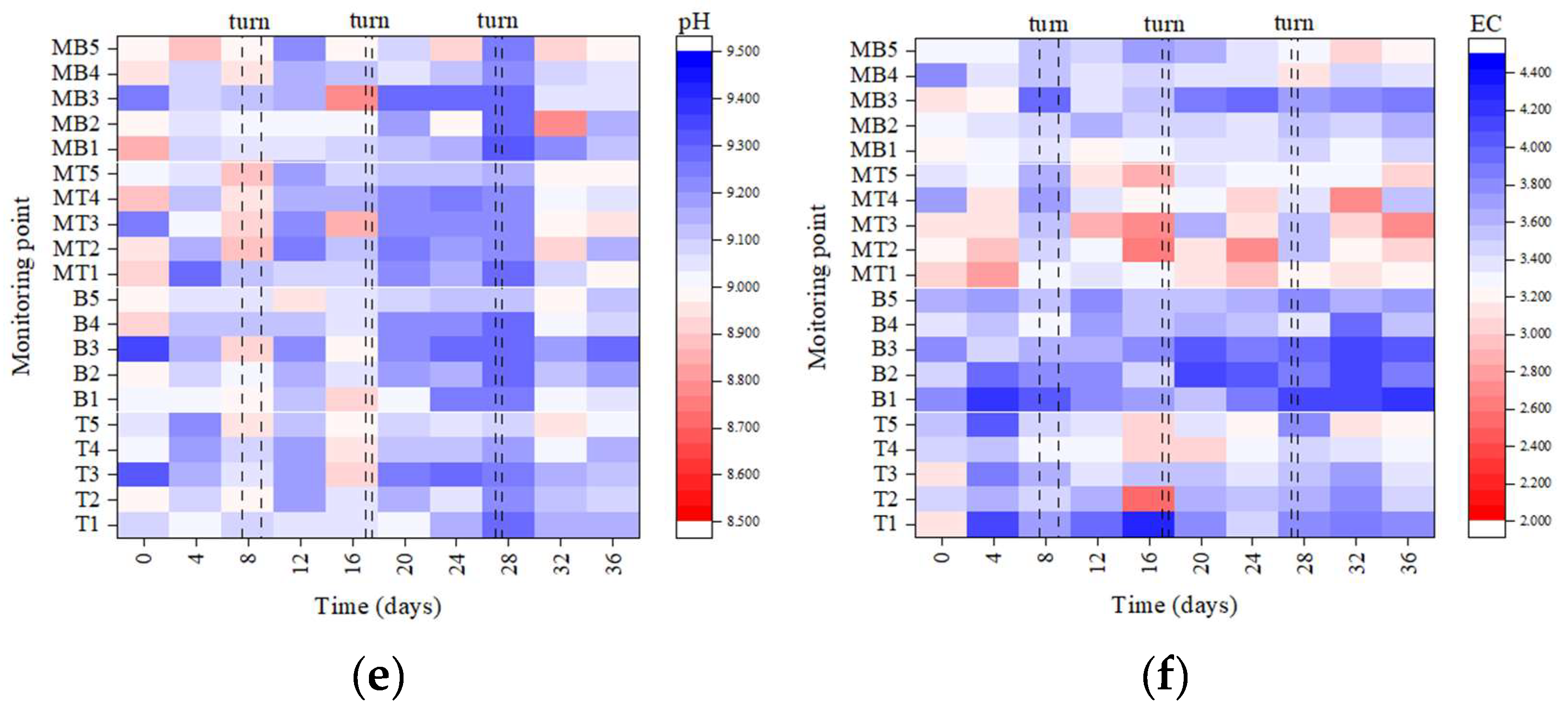
3.2.3. Germination Index (GI), pH, and Electrical Conductivity (EC)
3.3. Oxygen Concentration and Gases Emissions
3.3.1. Oxygen Concentration
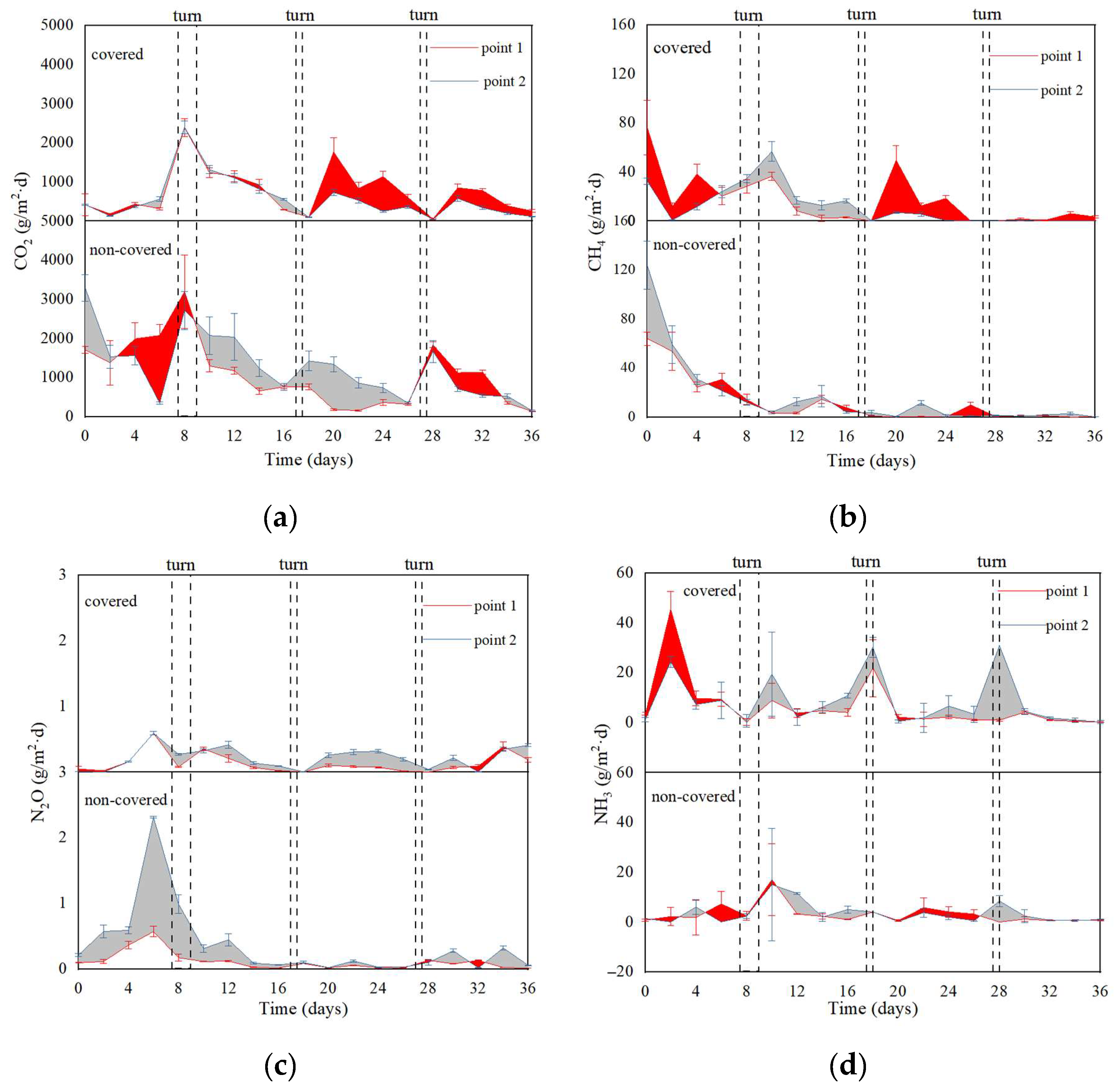
3.3.2. Greenhouse Gases (GHGs), Ammonia (NH3) Emissions, and Power Consumption Analysis
3.4. Spatial Distribution of Microbial Community Succession
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, M.; Zhou, H.; Shen, Y.; Meng, H.; Li, R.; Zhou, J.; Cheng, H.; Zhang, X.; Ding, J.; Wang, J.; et al. Effect of aeration rates on enzymatic activity and bacterial community succession during cattle manure composting. Bioresour. Technol. 2020, 304, 122928. [Google Scholar] [CrossRef]
- Zeng, J.; Shen, X.; Sun, X.; Liu, N.; Han, L.; Huang, G. Spatial and temporal distribution of pore gas concentrations during mainstream large-scale trough composting in China. Waste Manag. 2018, 75, 297–304. [Google Scholar] [CrossRef]
- Ma, S.; Xiong, J.; Cui, R.; Sun, X.; Han, L.; Xu, Y.; Kan, Z.; Gong, X.; Huang, G. Effects of intermittent aeration on greenhouse gas emissions and bacterial community succession during large-scale membrane-covered aerobic composting. J. Clean. Prod. 2020, 266, 121551. [Google Scholar] [CrossRef]
- He, X.; Chen, L.; Han, L.; Liu, N.; Cui, R.; Yin, H.; Huang, G. Evaluation of biochar powder on oxygen supply efficiency and global warming potential during mainstream large-scale aerobic composting. Bioresour. Technol. 2017, 245, 309–317. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Guo, J.; Zou, J.; Li, Y.; Zhang, L. Spatial distribution of dynamics characteristic in the intermittent aeration static composting of sewage sludge. Bioresour. Technol. 2011, 102, 5528–5532. [Google Scholar] [CrossRef]
- He, X.; Han, L.; Huang, G. Analysis of regulative variables on greenhouse gas emissions and spatial pore gas concentrations with modeling during large-scale trough composting. J. Clean. Prod. 2020, 277, 124066. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Li, X.; Ren, N. Spatial nitrifications of microbial processes during composting of swine, cow and chicken manure. Sci. Rep. 2015, 5, 14932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Ma, S.; Han, L.; Li, R.; Schlick, U.; Chen, P.; Huang, G. The effect of a semi-permeable membrane-covered composting system on greenhouse gas and ammonia emissions in the Tibetan Plateau. J. Clean. Prod. 2018, 204, 778–787. [Google Scholar] [CrossRef]
- Ma, S.; Xiong, J.; Wu, X.; Liu, H.; Han, L.; Huang, G. Effects of the functional membrane covering on the gas emissions and bacterial community during aerobic composting. Bioresour. Technol. 2021, 340, 125660. [Google Scholar] [CrossRef]
- Xiong, J.; Ma, S.; He, X.; Han, L.; Huang, G. Nitrogen transformation and dynamic changes in related functional genes during functional-membrane covered aerobic composting. Bioresour. Technol. 2021, 332, 125087. [Google Scholar] [CrossRef]
- Xiong, J.; Su, Y.; He, X.; Han, L.; Guo, J.; Qiao, W.; Huang, G. Effects of functional-membrane covering technique on nitrogen succession during aerobic composting: Metabolic pathways, functional enzymes, and functional genes. Bioresour. Technol. 2022, 354, 127205. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, L.; Liu, Y.; Xiong, J.; Su, Y.; Lan, Z.; Han, L.; Huang, G. Effect of micro-aerobic conditions based on semipermeable membrane-covered on greenhouse gas emissions and bacterial community during dairy manure storage at industrial scale. Environ. Pollut. 2022, 299, 118879. [Google Scholar] [CrossRef]
- Fang, C.; Su, Y.; Liang, Y.; Han, L.; He, X.; Huang, G. Exploring the microbial mechanism of reducing methanogenesis during dairy manure membrane-covered aerobic composting at industrial scale. Bioresour. Technol. 2022, 354, 127214. [Google Scholar] [CrossRef]
- Ge, J.; Huang, G.; Li, J.; Sun, X.; Han, L. Multivariate and Multiscale Approaches for Interpreting the Mechanisms of Nitrous Oxide Emission during Pig Manure–Wheat Straw Aerobic Composting. Environ. Sci. Technol. 2018, 52, 8408–8418. [Google Scholar] [CrossRef]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Chen, H.; Zhang, Z.; Wang, Q.; Ren, X.; Tu, Z.; Awasthi, M.K.; Taherzadeh, M.J. Dynamics of fungal diversity and interactions with environmental elements in response to wheat straw biochar amended poultry manure composting. Bioresour. Technol. 2018, 274, 410–417. [Google Scholar] [CrossRef]
- Hemati, A.; Aliasgharzad, N.; Khakvar, R.; Delangiz, N.; Asgari Lajayer, B.; van Hullebusch, E.D. Bioaugmentation of thermophilic lignocellulose degrading bacteria accelerate the composting process of lignocellulosic materials. Biomass Convers. Biorefin. 2022, 1–15. [Google Scholar] [CrossRef]
- Hemati, A.; Aliasgharzad, N.; Khakvar, R.; Khoshmanzar, E.; Asgari Lajayer, B.; van Hullebusch, E.D. Role of lignin and thermophilic lignocellulolytic bacteria in the evolution of humification indices and enzymatic activities during compost production. Waste Manag. 2021, 119, 122–134. [Google Scholar] [CrossRef]
- He, X.Q.; Han, L.J.; Fu, B.; Du, S.R.; Liu, Y.; Huang, G.Q. Effect and microbial reaction mechanism of rice straw biochar on pore methane production during mainstream large-scale aerobic composting in China. J. Clean. Prod. 2019, 215, 1223–1232. [Google Scholar] [CrossRef]
- He, X.; Han, L.; Ge, J.; Huang, G. Modelling for reactor-style aerobic composting based on coupling theory of mass-heat-momentum transport and Contois equation. Bioresour. Technol. 2018, 253, 165–174. [Google Scholar] [CrossRef]
- Denes, J. Numerical simulation of organic waste aerobic biodegradation: A new way to correlate respiration kinetics and organic matter fractionation. Waste Manag. 2015, 36, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.B.; Chen, T.B.; Gao, D.; Zheng, G.D.; Chen, J.; Pan, T.H.; Liu, H.T.; Gu, R.Y. Simulation of water removal process and optimization of aeration strategy in sewage sludge composting. Bioresour. Technol. 2014, 171, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Seng, B.; Kaneko, H.; Hirayama, K.; Katayama-Hirayama, K. Development of water movement model as a module of moisture content simulation in static pile composting. Environ. Technol. 2012, 33, 1685–1694. [Google Scholar] [CrossRef]
- Bongochgetsakul, N.; Ishida, T. A new analytical approach to optimizing the design of large-scale composting systems. Bioresour. Technol. 2008, 99, 1630–1641. [Google Scholar] [CrossRef]
- El Kader, N.A.; Robin, P.; Paillat, J.-M.; Leterme, P. Turning, compacting and the addition of water as factors affecting gaseous emissions in farm manure composting. Bioresour. Technol. 2007, 98, 2619–2628. [Google Scholar] [CrossRef]
- Sun, X.; Cui, R.; Shuangshuang, M.A.; Han, L.; Huang, G.; University, C.A. Design and Test on Large-scale Semi-membrane-covered Compost System. Trans. Chin. Soc. Agric. Mach. 2018. [Google Scholar]
- Selim, S.M.; Zayed, M.S.; Atta, H.M. Evaluation of phytotoxicity of compost during composting process. Nat. Sci. 2012, 10, 69–77. [Google Scholar]
- Ma, S.; Fang, C.; Sun, X.; Han, L.; He, X.; Huang, G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour. Technol. 2018, 259, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P. Studying beta diversity: Ecological variation partitioning by multiple regression and canonical analysis. J. Plant Ecol. 2008, 1, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illmer, P.; Schinner, F. Compost turning—A central factor for a rapid and high-quality degradation in household composting. Bioresour. Technol. 1997, 59, 157–162. [Google Scholar] [CrossRef]
- Fang, C.; Yin, H.; Han, L.; Ma, S.; He, X.; Huang, G. Effects of semi-permeable membrane covering coupled with intermittent aeration on gas emissions during aerobic composting from the solid fraction of dairy manure at industrial scale. Waste Manag. 2021, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Yong, X.; Wu, X.; Jia, H.; Wong, J.W.C.; Wu, H.; Zhou, J. Odor emission and microbial community succession during biogas residue composting covered with a molecular membrane. Bioresour. Technol. 2020, 297, 122518. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Tang, J.; Wu, J.; Shen, C.; Shangguan, H.; Zeng, R.J.; Zhou, S. Alternating electric field enables hyperthermophilic composting of organic solid wastes. Sci. Total Environ. 2022, 828, 154439. [Google Scholar] [CrossRef]
- Ahn, H.K.; Richard, T.L.; Choi, H.L. Mass and thermal balance during composting of a poultry manure—Wood shavings mixture at different aeration rates. Process Biochem. 2007, 42, 215–223. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, J.; Han, L.; Ma, S.; Sun, X.; Huang, G. Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour. Technol. 2017, 241, 190–199. [Google Scholar] [CrossRef]
- Ge, J.Y.; Huang, G.Q.; Yang, Z.; Huang, J.; Han, L.J. Characteriaztion of the Dymatic Thickness of the Aerobic Layer during Pig manure Aerobic Composting by Fourier Transform Infrared Microspectroscopy. Environ. Sci. Technol. 2014, 48, 5043–5050. [Google Scholar] [CrossRef]
- Jiang, T.; Schuchardt, F.; Li, G.; Guo, R.; Zhao, Y. Effect of C/N ratio, aeration rate and moisture content on ammonia and greenhouse gas emission during the composting. J. Environ. Sci. 2011, 23, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Pagans, E.; Barrena, R.; Font, X.; Sánchez, A. Ammonia emissions from the composting of different organic wastes. Dependency on process temperature. Chemosphere 2006, 62, 1534–1542. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Kong, Y.; Liu, Y.; Li, D.; Zhang, X.; Yuan, J.; Li, G. Evolution of phytotoxicity during the active phase of co-composting of chicken manure, tobacco powder and mushroom substrate. Waste Manag. 2020, 114, 25–32. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
- Walker, D.J.; Bernal, M.P. The effects of olive mill waste compost and poultry manure on the availability and plant uptake of nutrients in a highly saline soil. Bioresour. Technol. 2008, 99, 396–403. [Google Scholar] [CrossRef]
- Andreae, M.O.; Browell, E.V.; Garstang, M.; Gregory, G.L.; Harriss, R.C.; Hill, G.F.; Jacob, D.J.; Pereira, M.C.; Sachse, G.W.; Setzer, A.W.; et al. Biomass-burning emissions and associated haze layers over Amazonia. J. Geophys. Res. Atmos. 1988, 93, 1509–1527. [Google Scholar] [CrossRef]
- Ge, J.; Huang, G.; Huang, J.; Zeng, J.; Han, L. Particle-Scale Modeling of Methane Emission during Pig Manure/Wheat Straw Aerobic Composting. Environ. Sci. Technol. 2016, 50, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yin, H.; Fang, C.; Xiong, J.; Han, L.; Yang, Z.; Huang, G. Metagenomic and q-PCR analysis reveals the effect of powder bamboo biochar on nitrous oxide and ammonia emissions during aerobic composting. Bioresour. Technol. 2021, 323, 124567. [Google Scholar] [CrossRef]
- Richard, T.L.; Hamelers, H.V.M.; Veeken, A.; Silva, T. Moisture Relationships in Composting Processes. Compost. Sci. Util. 2002, 10, 286–302. [Google Scholar] [CrossRef]
- Wang, K.; Chu, C.; Li, X.; Wang, W.; Ren, N. Succession of bacterial community function in cow manure composing. Bioresour. Technol. 2018, 267, 63–70. [Google Scholar] [CrossRef]
- Su, C.; Deng, Q.; Lu, Y.; Pan, J.; Chen, W.; Chen, S.; Deng, X.; Lin, X.; Huang, Z. Effect of circulation and micro-aeration on sludge characteristics and microbial community in an ABR for treating traditional Chinese medicine wastewater. Environ. Technol. 2020, 41, 3284–3296. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, X.; Qiu, G.; Feng, J.; Zhang, L.; Brookes, P.C.; Xu, J.; He, Y. Synchronous response in methanogenesis and anaerobic degradation of pentachlorophenol in flooded soil. J. Hazard. Mater. 2019, 374, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Tran, L.T.H.; Idemoto, Y.; Abe, M.; Rollon, A.P. Comparison of organic matter degradation and microbial community during thermophilic composting of two different types of anaerobic sludge. Bioresour. Technol. 2009, 100, 676–682. [Google Scholar] [CrossRef] [PubMed]
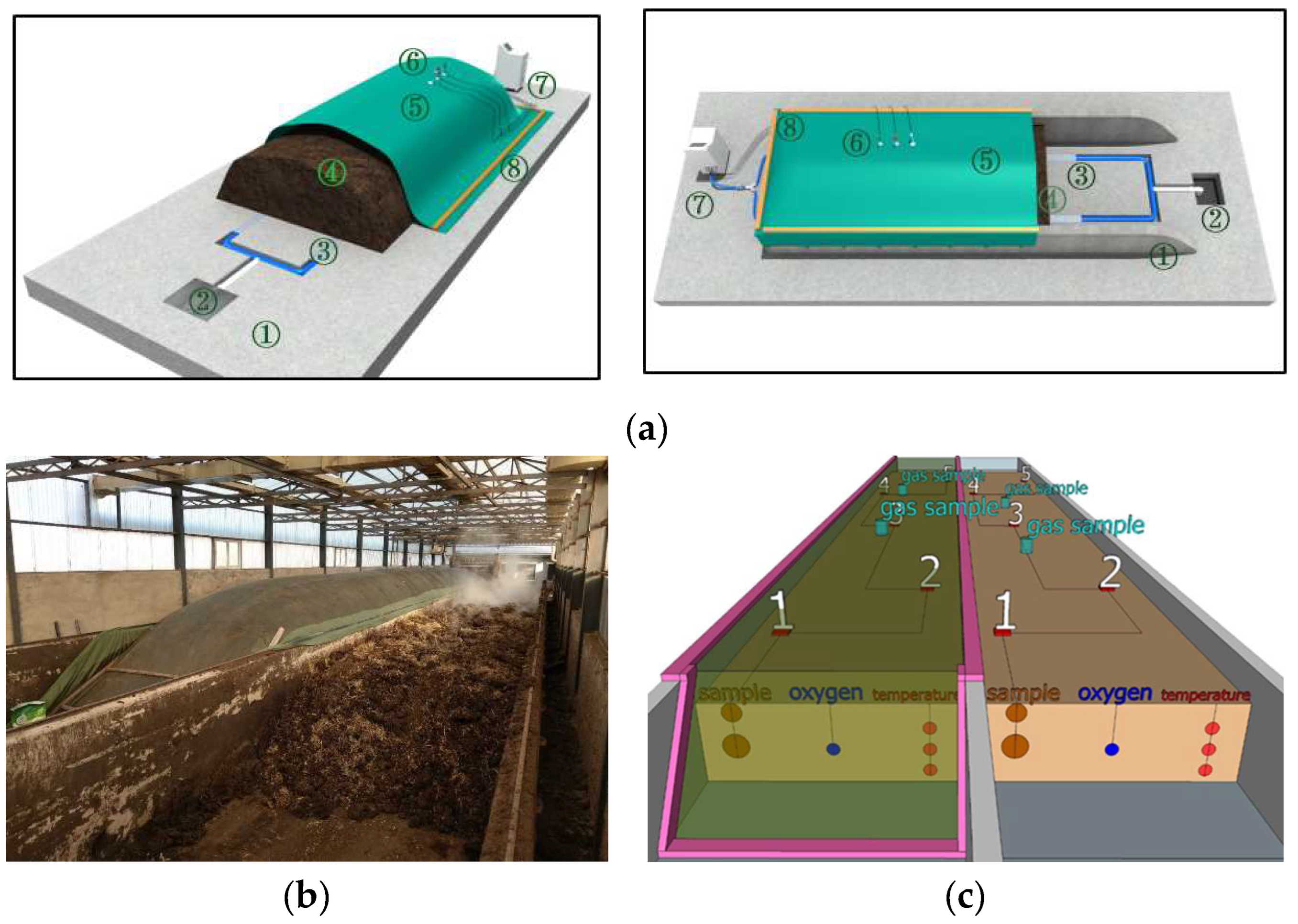




| Parameter | Units | Type/Value |
|---|---|---|
| Functional layer material | - | Polytetrafluoroethylene |
| Protective layer material | - | Polyester |
| Unit mass | g·m−2 | 260~450 |
| Breathability at 125 Pa | m3·min−1·m−1 | 0~0.028 |
| Moisture permeability at 200 Pa | g/m−2/h−1 | 0~104 |
| Waterproof | KPa | 100 |
| Width tensile strength at 50 cm | kg·mm−2 | 9.3 (radial), 9.7 (latitudinal) |
| Elongation | % | 2.1 (radial), 3.6 (latitudinal) |
| Water absorption | % | ≤8 |
| UV resistance | - | 5000 |
| Group Name | Monitoring Points | CO2/(g/m2·d) | CH4/(g/m2·d) | N2O/(g/m2·d) | NH3/(g/m2·d) | Power Consumption (KWh /m3·Day) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Maximum | Average | Maximum | Average | Maximum | Average | Maximum | Average | |||
| Covered | Out-membrane 1 | 2624.16 | 762.91 | 98.41 | 15.38 | 0.62 | 0.14 | 38.32 | 6.83 | 0.26 |
| Out-membrane 2 | 2553.21 | 589.28 | 64.80 | 11.29 | 0.62 | 0.22 | 52.69 | 8.93 | ||
| Non-covered | 1 | 4137.68 | 1093.46 | 69.26 | 10.99 | 0.65 | 0.12 | 31.34 | 3.23 | 0.30 |
| 2 | 3203.52 | 1229.82 | 143.39 | 13.78 | 2.33 | 0.36 | 37.52 | 3.64 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Huang, G.; Huang, Y.; Fang, C.; He, X.; Zheng, Y. Large Semi-Membrane Covered Composting System Improves the Spatial Homogeneity and Efficiency of Fermentation. Int. J. Environ. Res. Public Health 2022, 19, 15503. https://doi.org/10.3390/ijerph192315503
Sun X, Huang G, Huang Y, Fang C, He X, Zheng Y. Large Semi-Membrane Covered Composting System Improves the Spatial Homogeneity and Efficiency of Fermentation. International Journal of Environmental Research and Public Health. 2022; 19(23):15503. https://doi.org/10.3390/ijerph192315503
Chicago/Turabian StyleSun, Xiaoxi, Guangqun Huang, Yuanping Huang, Chen Fang, Xueqin He, and Yongjun Zheng. 2022. "Large Semi-Membrane Covered Composting System Improves the Spatial Homogeneity and Efficiency of Fermentation" International Journal of Environmental Research and Public Health 19, no. 23: 15503. https://doi.org/10.3390/ijerph192315503
APA StyleSun, X., Huang, G., Huang, Y., Fang, C., He, X., & Zheng, Y. (2022). Large Semi-Membrane Covered Composting System Improves the Spatial Homogeneity and Efficiency of Fermentation. International Journal of Environmental Research and Public Health, 19(23), 15503. https://doi.org/10.3390/ijerph192315503







