Development and Validation of a Risk Prediction Tool to Identify People at Greater Risk of Having Hepatitis C among Drug Users
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Recruitment and Study Design
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethics
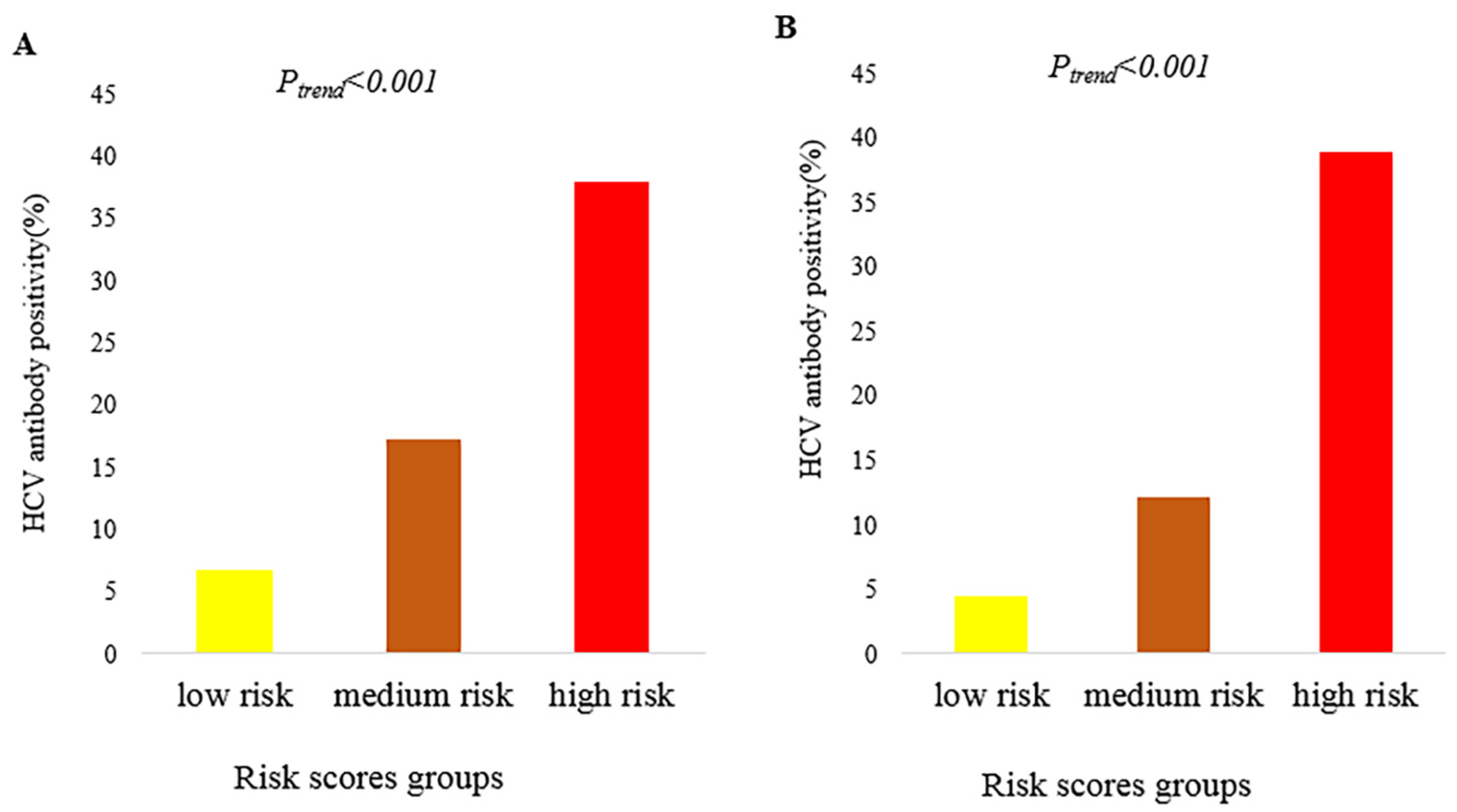
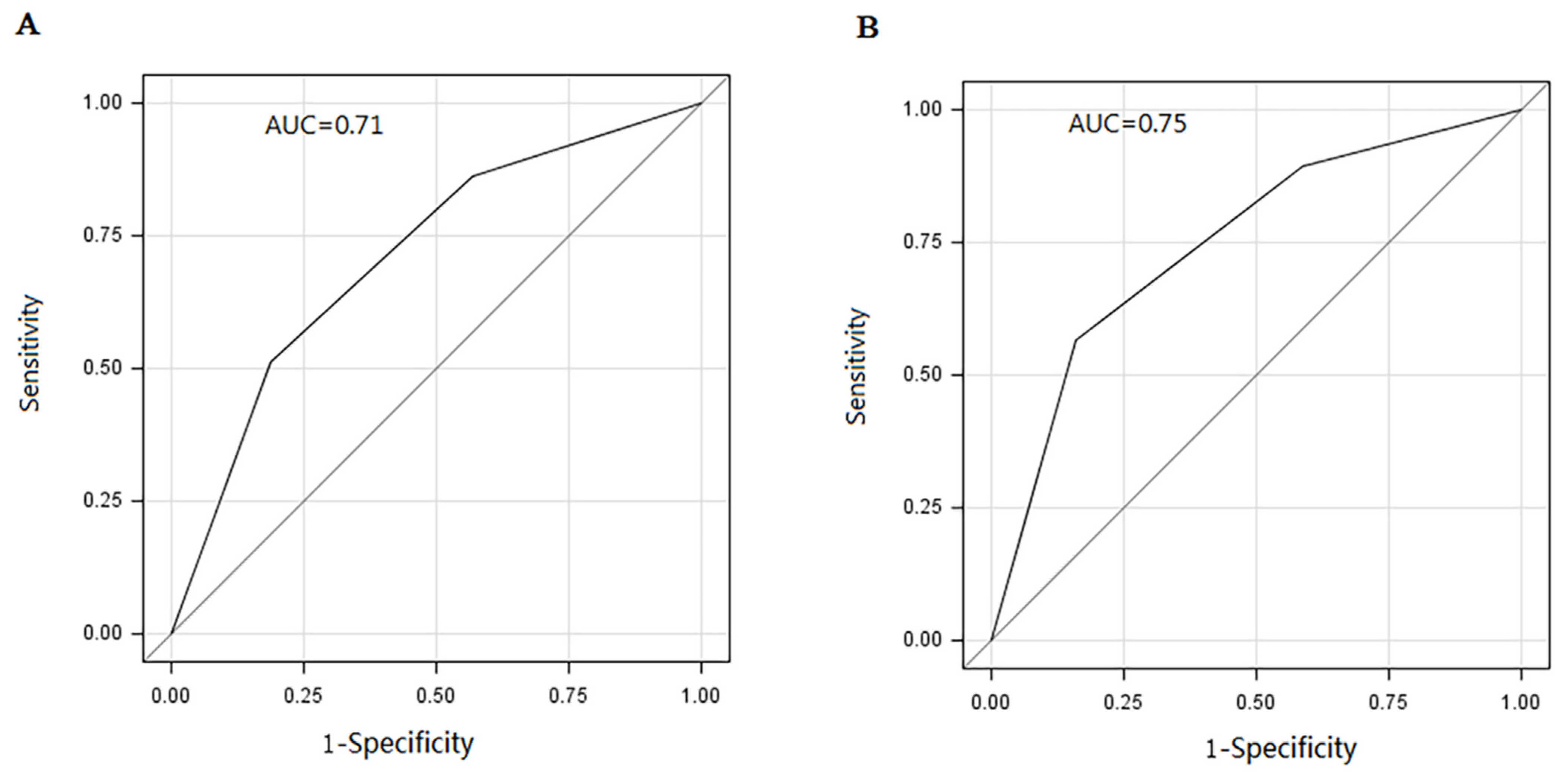
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 28 June 2022).
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Selvapatt, N.; Brown, A.; Thursz, M. Early diagnosis and treatment: The goal of hepatitis C screening. BMJ 2015, 350, h635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grebely, J.; Hajarizadeh, B.; Dore, G.J. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Capraru, C.; Feld, J.J. Remaining challenges in HCV elimination. J. Hepatol. 2021, 74, 964–965. [Google Scholar] [CrossRef]
- Tsui, J.I.; Miller, C.M.; Scott, J.D.; Corcorran, M.A.; Dombrowski, J.C.; Glick, S.N. Hepatitis C continuum of care and utilization of healthcare and harm reduction services among persons who inject drugs in Seattle. Drug Alcohol Depend. 2019, 195, 114–120. [Google Scholar] [CrossRef]
- Holtzman, D.; Barry, V.; Ouellet, L.J.; Des Jarlais, D.C.; Vlahov, D.; Golub, E.T.; Hudson, S.M.; Garfein, R.S. The influence of needle exchange programs on injection risk behaviors and infection with hepatitis C virus among young injection drug users in select cities in the United States, 1994–2004. Prev. Med. 2009, 49, 68–73. [Google Scholar] [CrossRef]
- Browne, D.C.; Clubb, P.A.; Wang, Y.; Wagner, F. Drug use and high-risk sexual behaviors among african american men who have sex with men and men who have sex with women. Am. J. Public Health 2009, 99, 1062–1066. [Google Scholar] [CrossRef]
- Office of China National Narcotics Control Commission. Drug Situation in China. 2020. Available online: http://www.nncc626.com/2021-07/16/c_1211244064.htm (accessed on 28 June 2022).
- Achakzai, M.; Kassi, M.; Kasi, P.M. Seroprevalences and co-infections of HIV, hepatitis C virus and hepatitis B virus in injecting drug users in Quetta, Pakistan. Trop. Dr. 2007, 37, 43–45. [Google Scholar] [CrossRef]
- Hser, Y.I.; Liang, D.; Lan, Y.C.; Vicknasingam, B.K.; Chakrabarti, A. Drug Abuse, HIV, and HCV in Asian Countries. J. Neuroimmune Pharmacol. 2016, 11, 383–393. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T.; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Donahue, K.; Doubeni, C.A.; Epling, J.W., Jr.; et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2020, 323, 970–975. [Google Scholar] [CrossRef]
- Chevaliez, S.; Pawlotsky, J.M. New virological tools for screening, diagnosis and monitoring of hepatitis B and C in resource-limited settings. J. Hepatol. 2018, 69, 916–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, A.; Smith, J.E. Modeling Hepatitis C Elimination Among People Who Inject Drugs in New Hampshire. JAMA Netw. Open 2021, 4, e2119092. [Google Scholar] [CrossRef] [PubMed]
- Busschots, D.; Bielen, R.; Koc, O.M.; Heyens, L.; Dercon, E.; Verrando, R.; Janssens, F.; Van den Bergh, L.; Van Lint, P.; Bruckers, L.; et al. On-site testing and case management to improve hepatitis C care in drug users: A prospective, longitudinal, multicenter study in the DAA era. BMC Public Health 2021, 21, 1574. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanz, J.; Vivancos-Gallego, M.J.; Fernandez-Felix, B.M.; Muriel, A.; Perez-Elias, P.; Uranga, A.; Romero, B.; Galan, J.C.; Moreno, S.; Perez-Elias, M.J. An Easy-to-Implement Risk Score for Targeted Hepatitis C Virus Testing in the General Population. Microbiol. Spectr. 2022, 10, e0228621. [Google Scholar] [CrossRef]
- Gebrezgi, M.T.; Fennie, K.P.; Sheehan, D.M.; Ibrahimou, B.; Jones, S.G.; Brock, P.; Ladner, R.A.; Trepka, M.J. Development and Validation of a Risk Prediction Tool to Identify People with HIV Infection Likely Not to Achieve Viral Suppression. AIDS Patient Care STDS 2020, 34, 157–165. [Google Scholar] [CrossRef]
- Newsum, A.M.; Stolte, I.G.; van der Meer, J.T.; Schinkel, J.; van der Valk, M.; Vanhommerig, J.W.; Buve, A.; Danta, M.; Hogewoning, A.; Prins, M.; et al. Development and validation of the HCV-MOSAIC risk score to assist testing for acute hepatitis C virus (HCV) infection in HIV-infected men who have sex with men (MSM). Eurosurveillance 2017, 22, 30540. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, M.J.; Cleland, C.M.; Lizcano, J.A.; Cherutich, P.; Kurth, A.E. Prevalence, estimated incidence, risk behaviours, and genotypic distribution of hepatitis C virus among people who inject drugs accessing harm-reduction services in Kenya: A retrospective cohort study. Lancet Infect. Dis. 2019, 19, 1255–1263. [Google Scholar] [CrossRef]
- Amon, J.J.; Garfein, R.S.; Ahdieh-Grant, L.; Armstrong, G.L.; Ouellet, L.J.; Latka, M.H.; Vlahov, D.; Strathdee, S.A.; Hudson, S.M.; Kerndt, P.; et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin. Infect. Dis. 2008, 46, 1852–1858. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, A.; Mirzazadeh, A.; Carter, G.M.; Esmaeili, A.; Hajarizadeh, B.; Sacks, H.S.; Page, K.A. Higher incidence of HCV in females compared to males who inject drugs: A systematic review and meta-analysis. J. Viral Hepat. 2017, 24, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Diaz, T.; Des Jarlais, D.C.; Vlahov, D.; Perlis, T.E.; Edwards, V.; Friedman, S.R.; Rockwell, R.; Hoover, D.; Williams, I.T.; Monterroso, E.R. Factors associated with prevalent hepatitis C: Differences among young adult injection drug users in lower and upper Manhattan, New York City. Am. J. Public Health 2001, 91, 23–30. [Google Scholar] [CrossRef]
- Alter, M.J.; Kruszon-Moran, D.; Nainan, O.V.; McQuillan, G.M.; Gao, F.; Moyer, L.A.; Kaslow, R.A.; Margolis, H.S. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N. Engl. J. Med. 1999, 341, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayomy Helal, H.E.; Yuonis, A.; Shaker, R.H.M.; Elawady, M.A. Prevalence of HCV Infection in Household Contacts of Chronic Liver Diseases Cases in Egypt. J. Environ. Public Health 2018, 2018, 2153537. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhu, L.; Tang, F.; Bao, C.; Zhu, Y.; Cao, M.; Du, G.; Xu, J.; Peng, H.; Zhai, X. Rate of infection and related risk factors on hepatitis C virus in three counties of Jiangsu province. Zhonghua Liu Xing Bing Xue Za Zhi 2014, 35, 1212–1217. [Google Scholar]
- Li, D.F.; Chen, H.C.; Jin, X.M.; Dai, J.; Zeng, Z.J.; Yang, M.; Sun, P.Y.; Dong, L.J.; Han, Y.; Ma, Y.L.; et al. HCV and Treponema pallidum infection status in HIV/AIDS cases in Yunnan province, January–June, 2020. Zhonghua Liu Xing Bing Xue Za Zhi 2021, 42, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Mo, P.K.H.; Fang, Y.; Ip, T.K.M.; Lau, J.T.F. Influence of Social Media on Sexualized Drug Use and Chemsex Among Chinese Men Who Have Sex With Men: Observational Prospective Cohort Study. J. Med. Internet Res. 2020, 22, e17894. [Google Scholar] [CrossRef]
- Tohme, R.A.; Holmberg, S.D. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology 2010, 52, 1497–1505. [Google Scholar] [CrossRef]
- Jin, F.; Matthews, G.V.; Grulich, A.E. Sexual transmission of hepatitis C virus among gay and bisexual men: A systematic review. Sex Health 2017, 14, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, M.G.; Chen, Y.; Shi, L.; Chen, Y.; Zhang, Z.; Xu, X.; Huan, X.; Fu, G.; McFarland, W. Changing trends in the types of drug used and infectious disease prevalence among drug users in jiangsu province, china. Int. J. Drug Policy 2021, 88, 103034. [Google Scholar] [CrossRef]
- Sun, H.Q.; Bao, Y.P.; Zhou, S.J.; Meng, S.Q.; Lu, L. The new pattern of drug abuse in China. Curr. Opin. Psychiatry 2014, 27, 251–255. [Google Scholar] [CrossRef]
- Zibbell, J.E.; Asher, A.K.; Patel, R.C.; Kupronis, B.; Iqbal, K.; Ward, J.W.; Holtzman, D. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am. J. Public Health 2018, 108, 175–181. [Google Scholar] [CrossRef]
- Degenhardt, L.; Peacock, A.; Colledge, S.; Leung, J.; Grebely, J.; Vickerman, P.; Stone, J.; Cunningham, E.B.; Trickey, A.; Dumchev, K.; et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health 2017, 5, e1192–e1207. [Google Scholar] [CrossRef] [PubMed]
- Moradi, G.; Hajarizadeh, B.; Rahmani, K.; Mohamadi-Bolbanabad, A.; Darvishi, S.; Zareie, B.; Zavareh, F.A.; Sharafi, H.; Alavian, S.M.; Ramazani, R.; et al. Drug use and risk behaviour profile, and the prevalence of HIV, hepatitis C and hepatitis B among people with methamphetamine use in Iran. Int. J. Drug Policy 2019, 73, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Crowley, D.; Lambert, J.S.; Betts-Symonds, G.; Cullen, W.; Keevans, M.; Kelly, E.; Laird, E.; McHugh, T.; McKiernan, S.; Miggin, S.J.; et al. The seroprevalence of untreated chronic hepatitis C virus (HCV) infection and associated risk factors in male Irish prisoners: A cross-sectional study, 2017. Eurosurveillance 2019, 24, 1800369. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 17,871) | Positive HCV Antibody Status, n (%) | Negative HCV Antibody Status, n (%) | Unadjusted OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Sex | <0.001 | ||||
| Female | 2432 | 511 (21.0) | 1921 (79.0) | 1.21 (1.09–1.34) | |
| Male | 15,439 | 2788 (18.1) | 12,651 (81.9) | ||
| Household registration | 0.610 | ||||
| Zhejiang Province | 11,638 | 2161 (18.6) | 9477 (81.4) | 0.98 (0.91–1.06) | |
| Other provinces | 6233 | 1138 (18.3) | 5095 (81.7) | ||
| Age (years) | <0.001 | ||||
| ≤30 | 4333 | 366 (8.4) | 3967 (91.6) | ||
| 31–40 | 6994 | 1180 (16.9) | 5814 (83.1) | 2.20 (1.94–2.49) | |
| ≥41 | 6544 | 1753 (26.8) | 4791 (73.2) | 3.97 (3.52–4.47) | |
| Marital status | 0.013 | ||||
| Married/cohabiting | 11,025 | 2098 (19.0) | 8927 (81.0) | 1.11 (1.02–1.20) | |
| Divorced/single | 6846 | 1201 (17.5) | 5645 (82.5) | ||
| Education | <0.001 | ||||
| Illiteracy | 1343 | 329 (24.5) | 1014 (75.5) | 2.79 (2.10–3.70) | |
| Primary school | 4446 | 928 (20.9) | 3518 (79.1) | 2.27 (1.74–2.96) | |
| Middle school | 8819 | 1553 (17.6) | 7266 (82.4) | 1.84 (1.41–2.38) | |
| High school/technical secondary school | 2630 | 423 (16.1) | 2207 (83.9) | 1.65 (1.25–2.17) | |
| College degree or above | 633 | 66 (10.4) | 567 (89.6) | ||
| Sexual activity after taking drugs | 0.914 | ||||
| Yes | 7122 | 1312 (18.4) | 5810 (81.6) | 1.00 (0.92–1.08) | |
| No | 10,749 | 1987 (18.5) | 8764 (81.5) | ||
| Heroin use | <0.001 | ||||
| Yes | 5359 | 1922 (35.9) | 3437 (64.1) | 4.52 (4.18–4.89) | |
| No | 12,512 | 1377 (11.0) | 11,135 (89.0) | ||
| Cocaine use | 0.215 | ||||
| Yes | 75 | 18 (24.0) | 57 (76.0) | 1.40 (0.82–2.38) | |
| No | 17,796 | 3281 (18.4) | 14,515 (81.6) | ||
| Opium use | 0.624 | ||||
| Yes | 33 | 5 (15.2) | 28 (84.8) | 0.79 (0.30–2.04) | |
| No | 17,838 | 3294 (18.5) | 14,544 (81.5) | ||
| Cannabis sativa use | 0.157 | ||||
| Yes | 150 | 21 (14.0) | 129 (86.0) | 0.72 (0.45–1.14) | |
| No | 17,721 | 3278 (18.5) | 14,443 (81.5) | ||
| Morphine use | 0.023 | ||||
| Yes | 139 | 36 (25.9) | 103 (74.1) | 1.55 (1.06–2.27) | |
| No | 17,732 | 3263 (18.4) | 14,469 (81.6) | ||
| Methamphetamine use | <0.001 | ||||
| Yes | 12,316 | 1514 (12.3) | 10,802 (87.7) | 0.30 (0.27–0.32) | |
| No | 5555 | 1785 (32.1) | 3770 (67.9) | ||
| Demerol | 0.256 | ||||
| Yes | 172 | 26 (15.1) | 146 (84.9) | 0.79 (0.52–1.19) | |
| No | 17,699 | 3273 (18.5) | 14,426 (81.5) | ||
| Ketamine use | 0.058 | ||||
| Yes | 240 | 33 (13.8) | 207 (86.2) | 0.70 (0.49–1.01) | |
| No | 17,631 | 3266 (18.5) | 14,365 (81.5) | ||
| Ecstasy use | 0.112 | ||||
| Yes | 98 | 12 (12.2) | 86 (87.8) | 0.62 (0.34–1.13) | |
| No | 17,773 | 3287 (18.5) | 14,486 (81.5) | ||
| Magu use | <0.001 | ||||
| Yes | 556 | 59 (10.6) | 497 (89.4) | 0.52 (0.39–0.68) | |
| No | 17,315 | 3240 (18.7) | 14,075 (81.3) |
| Variables in the Final Model | B | p-Value | Adjusted OR | 95% CI | Points |
|---|---|---|---|---|---|
| Sex (compared to males) | 0.39 | <0.001 | 1.48 | 1.32–1.65 | 2 |
| Marital status (compared to divorced/single) | |||||
| Married | 0.34 | <0.001 | 1.41 | 1.29–1.54 | 2 |
| Education (compared to college degree or above) | |||||
| Illiteracy | 0.41 | 0.007 | 1.51 | 1.12–2.04 | 3 |
| Primary school | 0.46 | 0.001 | 1.59 | 1.20–2.10 | 3 |
| Middle school | 0.45 | 0.001 | 1.56 | 1.19–2.05 | 3 |
| High school or technical secondary school | 0.34 | 0.022 | 1.4 | 1.05–1.87 | 2 |
| Heroin use | 1.5 | <0.001 | 4.5 | 3.88–5.22 | 9 |
| Morphine use | 0.54 | 0.011 | 1.71 | 1.14–2.59 | 3 |
| Methamphetamine use | 0.16 | 0.039 | 1.17 | 1.01–1.36 | 1 |
| Age group (compared to ≤30 years) | |||||
| 31–40 | 0.82 | <0.001 | 2.28 | 2.00–2.60 | 5 |
| >40 | 1.28 | <0.001 | 3.6 | 3.14–4.12 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Cheng, W.; Xu, Y.; Yang, J.; Jiang, J.; Pan, X.; Zhou, X.; Jiang, J.; Chai, C. Development and Validation of a Risk Prediction Tool to Identify People at Greater Risk of Having Hepatitis C among Drug Users. Int. J. Environ. Res. Public Health 2022, 19, 15677. https://doi.org/10.3390/ijerph192315677
Huang G, Cheng W, Xu Y, Yang J, Jiang J, Pan X, Zhou X, Jiang J, Chai C. Development and Validation of a Risk Prediction Tool to Identify People at Greater Risk of Having Hepatitis C among Drug Users. International Journal of Environmental Research and Public Health. 2022; 19(23):15677. https://doi.org/10.3390/ijerph192315677
Chicago/Turabian StyleHuang, Gang, Wei Cheng, Yun Xu, Jiezhe Yang, Jun Jiang, Xiaohong Pan, Xin Zhou, Jianmin Jiang, and Chengliang Chai. 2022. "Development and Validation of a Risk Prediction Tool to Identify People at Greater Risk of Having Hepatitis C among Drug Users" International Journal of Environmental Research and Public Health 19, no. 23: 15677. https://doi.org/10.3390/ijerph192315677





