Effects of BPZ, BPC, BPF, and BPS Exposure on Adult Zebrafish (Danio rerio): Accumulation, Oxidative Stress, and Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Zebrafish Maintenance and Solutions Preparation
2.3. Design of the Subacute Toxicity Test
2.4. Sampling
2.5. Samples Analysis
2.5.1. Total BPs Accumulation
2.5.2. Biochemical Analysis
2.5.3. mRNA Expression Analysis
2.6. Statistical Analysis
3. Results and Discussions
3.1. Method Validation
3.2. Residual Concentrations of BPs
3.3. Effects of BPs Exposure on Lipid Peroxidation
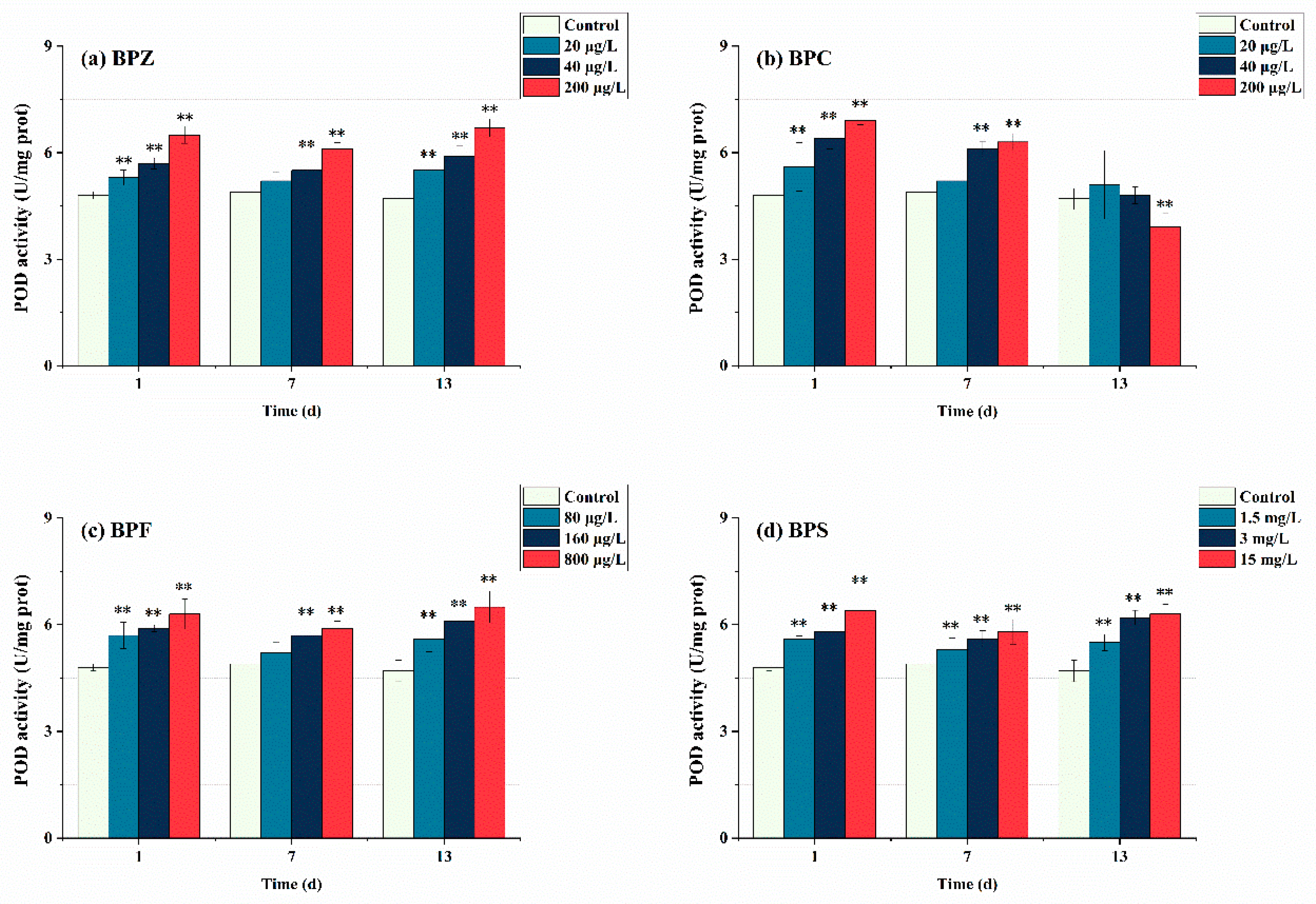
3.4. Effects of BPs Exposure on Antioxidant Enzyme Activities
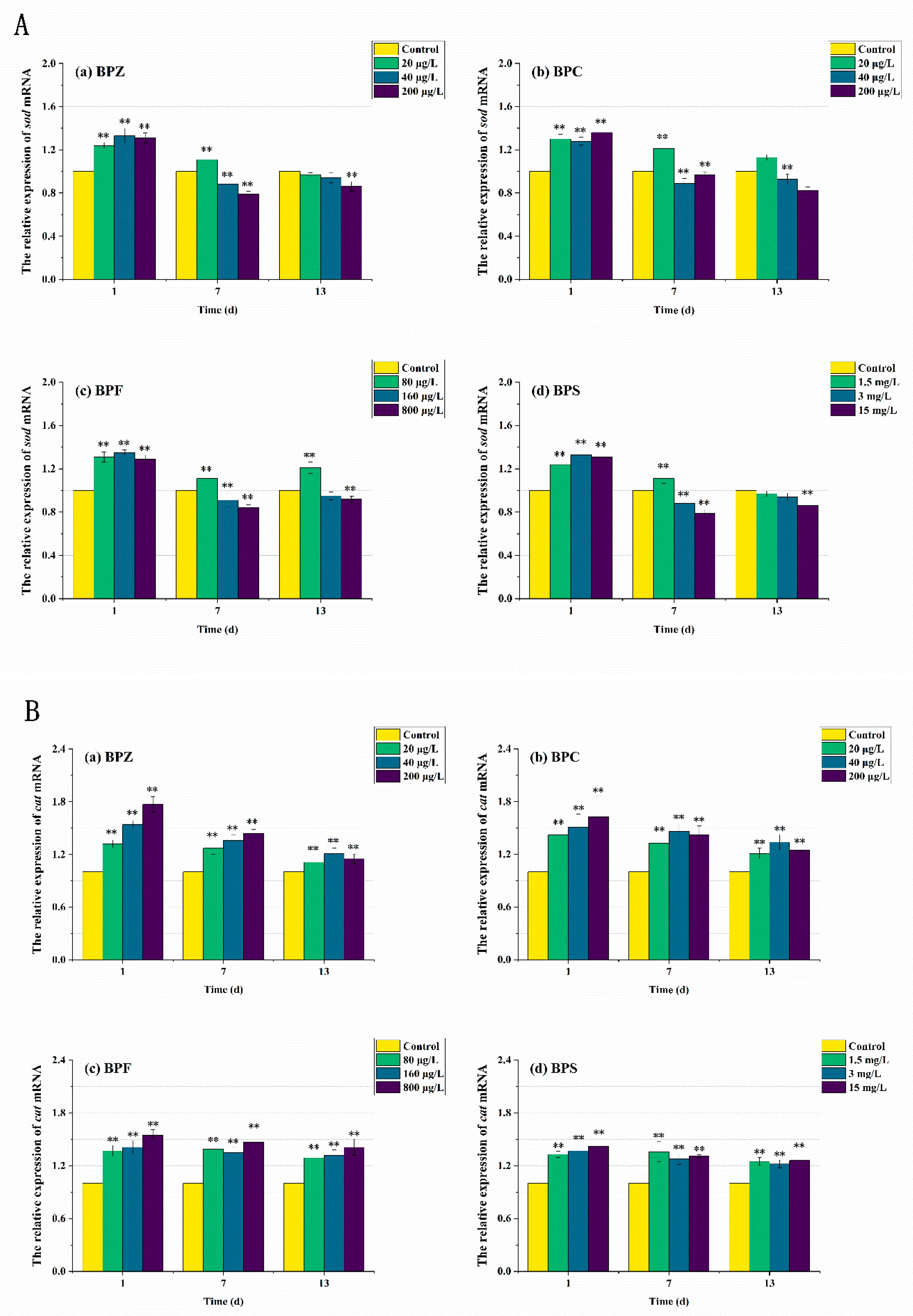
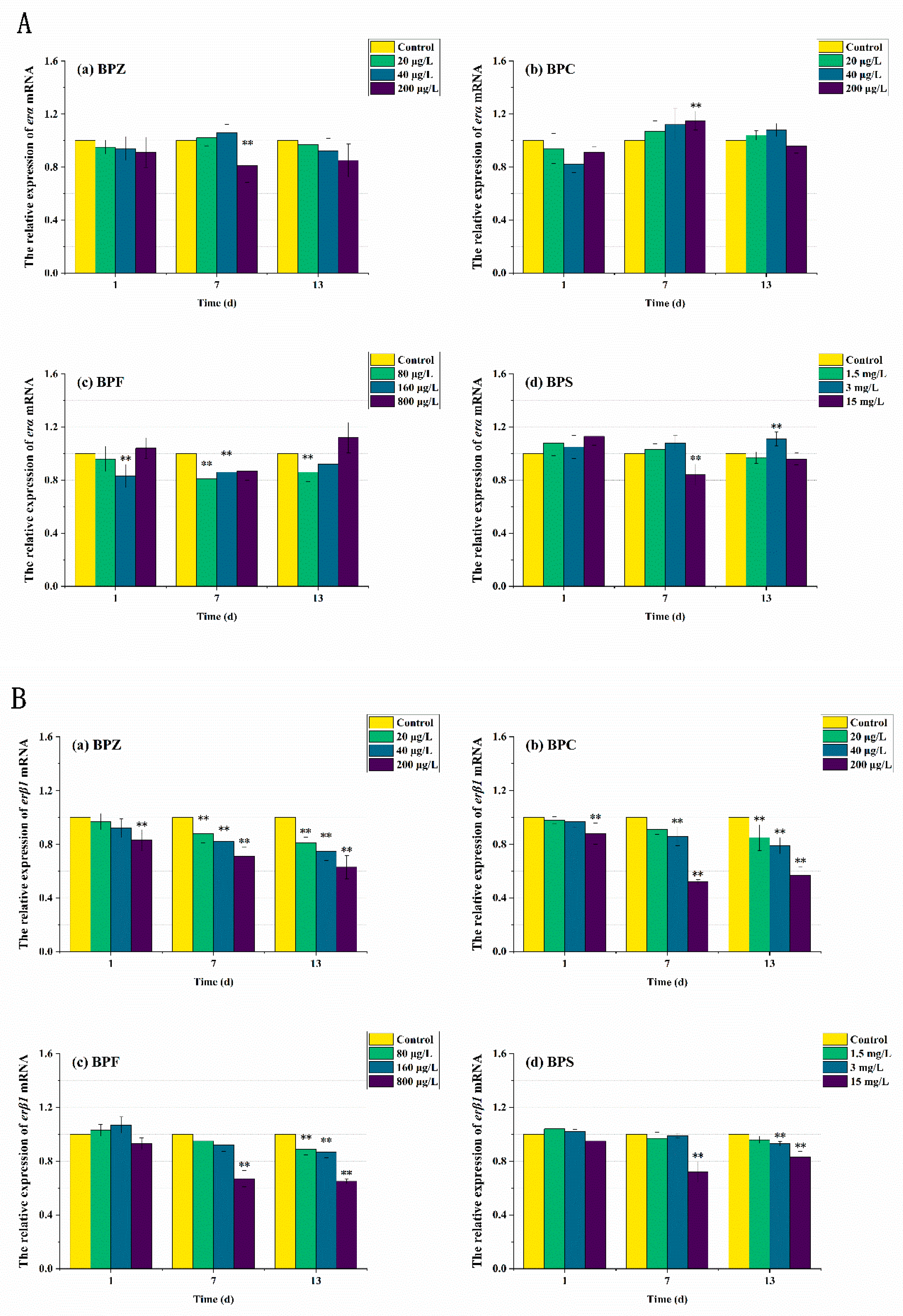
3.5. mRNA Expression Levels of Genes Related to Oxidative Stress Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghajanpour-Mir, S.M.; Zabihi, E.; Keyhani, E.; Akhavan-Niaki, H.; Bagherizadeh, I.; Biglari, S.; Behjati, F. The Genotoxic and Cytotoxic Effects of Bisphenol-A (BPA) in MCF-7 Cell Line and Amniocytes. Int. J. Mol. Cell. Med. 2016, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, W.-Q.; Zeng, X.; Tang, L. Dynamic Stocks and Flows Analysis of Bisphenol A (BPA) in China: 2000–2014. Environ. Sci. Technol. 2018, 52, 3706–3715. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Stir bar sorptive extraction coupled to gas chromatography–mass spectrometry for the determination of bisphenols in canned beverages and filling liquids of canned vegetables. J. Chromatogr. A 2012, 1247, 146–153. [Google Scholar] [CrossRef]
- Héliès-Toussaint, C.; Peyre, L.; Costanzo, C.; Chagnon, M.-C.; Rahmani, R. Is bisphenol S a safe substitute for bisphenol A in terms of metabolic function? An in vitro study. Toxicol. Appl. Pharmacol. 2014, 280, 224–235. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, L.S. Partitioning Behavior of Bisphenol Alternatives BPS and BPAF Compared to BPA. Environ. Sci. Technol. 2017, 51, 3725–3732. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Tyner, M.D.; Maloney, M.O.; Kelley, B.J.; Combelles, C.M. Comparing the effects of bisphenol A, C, and F on bovine theca cells in vitro. Reprod. Toxicol. 2022, 111, 27–33. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, J.-L.; Yang, Y.-Y.; Jia, Y.-W.; Zhang, Q.-Q.; Chen, C.-E.; Liu, Y.-S.; Yang, B.; Xie, L.; Ying, G.-G. Occurrence, mass loads and risks of bisphenol analogues in the Pearl River Delta region, South China: Urban rainfall runoff as a potential source for receiving rivers. Environ. Pollut. 2020, 263, 114361. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, D.; Yan, S.; Li, R.; Yan, J.; Teng, M.; Zhou, Z.; Zhu, W. Effects of perinatal exposure to BPA and its alternatives (BPS, BPF and BPAF) on hepatic lipid and glucose homeostasis in female mice adolescent offspring. Chemosphere 2018, 212, 297–306. [Google Scholar] [CrossRef]
- Park, C.; Song, H.; Choi, J.; Sim, S.; Kojima, H.; Park, J.; Iida, M.; Lee, Y. The mixture effects of bisphenol derivatives on estrogen receptor and androgen receptor. Environ. Pollut. 2020, 260, 114036. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, L.; Chen, Z.; Thalmann, B.; Zhou, S.; Hollert, H.; Seiler, T.-B. Evidence of increased estrogenicity upon metabolism of Bisphenol F—Elucidation of the key metabolites. Sci. Total Environ. 2021, 787, 147669. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Xia, P.; Zhang, J.; Wang, Y.; Zhang, R.; Giesy, J.P.; Shi, W.; Yu, H. A high-throughput, computational system to predict if environmental contaminants can bind to human nuclear receptors. Sci. Total Environ. 2017, 576, 609–616. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; de León, C.T.G.; García-Becerra, R.; Ambrosio, J.; Nava-Castro, K.E.; Morales-Montor, J. The chemical environmental pollutants BPA and BPS induce alterations of the proteomic profile of different phenotypes of human breast cancer cells: A proposed interactome. Environ. Res. 2020, 191, 109960. [Google Scholar] [CrossRef]
- Liu, S.; He, B.; Li, H. Bisphenol S promotes the progression of prostate cancer by regulating the expression of COL1A1 and COL1A2. Toxicology 2022, 472, 153178. [Google Scholar] [CrossRef]
- Harnett, K.G.; Moore, L.G.; Chin, A.; Cohen, I.C.; Lautrup, R.R.; Schuh, S.M. Teratogenicity and toxicity of the new BPA alternative TMBPF, and BPA, BPS, and BPAF in chick embryonic development. Curr. Res. Toxicol. 2021, 2, 399–410. [Google Scholar] [CrossRef]
- Hyun, M.; Rathor, L.; Kim, H.-J.; McElroy, T.; Hwang, K.H.; Wohlgemuth, S.; Curry, S.; Xiao, R.; Leeuwenburgh, C.; Heo, J.-D.; et al. Comparative toxicities of BPA, BPS, BPF, and TMBPF in the nematode Caenorhabditis elegans and mammalian fibroblast cells. Toxicology 2021, 461, 152924. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.-J. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J. Hazard. Mater. 2020, 406, 124303. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Shin, H.; Kho, Y.L.; Choi, K. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere 2019, 221, 115–123. [Google Scholar] [CrossRef]
- Huang, G.-M.; Tian, X.-F.; Fang, X.-D.; Ji, F.-J. Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere 2016, 147, 188–194. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sánchez, B.M.; Sendón, R.; de Quirós, A.R.-B.; Barbosa-Pereira, L. Study on the chemical behaviour of Bisphenol S during the in vitro gastrointestinal digestion and its bioaccessibility. Food Chem. 2021, 367, 130758. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Li, Z.; Gu, J.; Qin, J.; Li, J.; Zhang, X.; Ru, S. Bisphenol S exposure accelerates the progression of atherosclerosis in zebrafish embryo-larvae. J. Hazard. Mater. 2021, 426, 128042. [Google Scholar] [CrossRef]
- da Silva, B.S.; Pietrobon, C.B.; Bertasso, I.M.; Lopes, B.P.; Carvalho, J.C.; Peixoto-Silva, N.; Santos, T.R.; Claudio-Neto, S.; Manhães, A.C.; Oliveira, E.; et al. Short and long-term effects of bisphenol S (BPS) exposure during pregnancy and lactation on plasma lipids, hormones, and behavior in rats. Environ. Pollut. 2019, 250, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Salahinejad, A.; Attaran, A.; Naderi, M.; Meuthen, D.; Niyogi, S.; Chivers, D.P. Chronic exposure to bisphenol S induces oxidative stress, abnormal anxiety, and fear responses in adult zebrafish (Danio rerio). Sci. Total Environ. 2020, 750, 141633. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Khatib, I.; Kasianchuk, N.; Lushchak, O.; Horyn, O.; Sokolova, I.M. Toxic effects and mechanisms of common pesticides (Roundup and chlorpyrifos) and their mixtures in a zebrafish model (Danio rerio). Sci. Total Environ. 2022, 833, 155236. [Google Scholar] [CrossRef]
- Shi, Z.; Liang, X.; Zhao, Y.; Liu, W.; Martyniuk, C.J. Neurotoxic effects of synthetic phenolic antioxidants on dopaminergic, serotoninergic, and GABAergic signaling in larval zebrafish (Danio rerio). Sci. Total Environ. 2022, 830, 154688. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yun, Y.; Li, G.; Sang, N. Heavy metals in soil from gangue stacking areas increases children health risk and causes developmental neurotoxicity in zebrafish larvae. Sci. Total Environ. 2021, 794, 148629. [Google Scholar] [CrossRef]
- Han, Y.; Fei, Y.; Wang, M.; Xue, Y.; Chen, H.; Liu, Y. Study on the Joint Toxicity of BPZ, BPS, BPC and BPF to Zebrafish. Molecules 2021, 26, 4180. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- González-Rubio, S.; Vike-Jonas, K.; Gonzalez, S.V.; Ballesteros-Gómez, A.; Sonne, C.; Dietz, R.; Boertmann, D.; Rasmussen, L.M.; Jaspers, V.L.; Asimakopoulos, A.G. Bioaccumulation potential of bisphenols and benzophenone UV filters: A multiresidue approach in raptor tissues. Sci. Total Environ. 2020, 741, 140330. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, K.; Feng, T.; Han, Y.; Li, G.; Li, J.; Ma, H. Bioaccumulation, metabolism and endocrine-reproductive effects of metolachlor and its S-enantiomer in adult zebrafish (Danio rerio). Sci. Total Environ. 2021, 802, 149826. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Shu, L.; Chen, L.; Sun, L.; Qian, H.; Liu, W.; Fu, Z. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 2009, 78, 846–852. [Google Scholar] [CrossRef]
- Lin, T.; Yu, S.; Chen, Y.; Chen, W. Integrated biomarker responses in zebrafish exposed to sulfonamides. Environ. Toxicol. Pharmacol. 2014, 38, 444–452. [Google Scholar] [CrossRef]
- Macczak, A.; Cyrkler, M.; Bukowska, B.; Michalowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. In Vitro 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Jihen, E.H.; Imed, M.; Fatima, H.; Abdelhamid, K. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: Histology and Cd accumulation. Food Chem. Toxicol. 2008, 46, 3522–3527. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martínez, M.-A.; Dai, M.; Chen, D.; Ares, I.; Romero, A.; Castellano, V.; Martínez, M.; Rodríguez, J.L.; Martínez-Larrañaga, M.-R.; et al. Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ. Res. 2016, 149, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, M.; Suzuki, M.; Sato, I.; Tsukamoto, H.; Watanabe, K. Glutathione Peroxidase Activity in Endometrium: Effects of Sex Hormones and Cancer. Gynecol. Oncol. 1996, 60, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Koppal, T.; Howard, B.; Subramaniam, R.; Hall, N.; Hensley, K.; Yatin, S.; Allen, K.; Aksenov, M.; Aksenova, M.; et al. Structural and Functional Changes in Proteins Induced by Free Radical-mediated Oxidative Stress and Protective Action of the Antioxidants N-tert-Butyl--phenylnitrone and Vitamin E. Ann. N. Y. Acad. Sci. 1998, 854, 448–462. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, J.; Shuai, Z.; Zhu, L.; Wang, J. Acute and subchronic toxicity of pyraclostrobin in zebrafish (Danio rerio). Chemosphere 2017, 188, 510. [Google Scholar]
- Mao, L.; Jia, W.; Zhang, L.; Zhang, Y.; Zhu, L.; Sial, M.U.; Jiang, H. Embryonic development and oxidative stress effects in the larvae and adult fish livers of zebrafish (Danio rerio) exposed to the strobilurin fungicides, kresoxim-methyl and pyraclostrobin. Sci. Total Environ. 2020, 729, 139031. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, L.; Xue, T.; Long, M.; Su, Y.; Wu, N. Damage and recovery of the ovary in female zebrafish i.p.-injected with MC-LR. Aquat. Toxicol. 2014, 155, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zheng, J.L.; Wang, Y.H.; Xu, M.Y.; Zhu, A.Y.; Wu, C.W. The role of Nrf2/Keap1 signaling in inorganic mercury induced oxidative stress in the liver of large yellow croaker Pseudosciaena crocea. Ecotoxicol. Environ. Saf. 2016, 132, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Le Fol, V.; Aït-Aïssa, S.; Sonavane, M.; Porcher, J.-M.; Balaguer, P.; Cravedi, J.-P.; Zalko, D.; Brion, F. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol. Environ. Saf. 2017, 142, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-B.; Kim, G.-E.; On, J.; Pyo, H.; Park, J.-W.; Cho, S.-H. Sex-specific effects of bisphenol S with tissue-specific responsiveness in adult zebrafish: The antiandrogenic and antiestrogenic effects. Ecotoxicol. Environ. Saf. 2021, 229, 113102. [Google Scholar] [CrossRef]





| Name | 1/100 LC50 (μg/L) | 1/50 LC50 (μg/L) | 1/10 LC50 (μg/L) |
|---|---|---|---|
| BPZ | 20 | 40 | 200 |
| BPC | 20 | 40 | 200 |
| BPF | 80 | 160 | 800 |
| BPS | 1500 | 3000 | 15,000 |
| Gene | Sequence of the Primers (5′-3′) | Accession NO. | Size (bp) |
|---|---|---|---|
| rp17 | F: CAGAGGTATCAATGGTGTCAGCCC R: TTCGGAGCATGTTGATGGAGGC | NM213213644.2 | 119 |
| β-actin | F: CGAGCTGTCTTCCCATCCA R: TCACCAACGTAGCTGTCTTTCTG | AF025305.1 | 86 |
| erβ1 | F: GGG GAG AGT TCA ACC ACG GAG R: GCT TTC GGA CAC AGG AGG ACG | AJ414566 | 89 |
| erα | F: CCC ACA GGA CAA GAG GAA GA R: CCT GGT CAT GCA GAG ACA GA | AF268283 | 250 |
| cat | F: CTCCTGATGTGGCCCGATAC R: TCAGATGCCCGGCCATATTC | AF170069.1 | 126 |
| sod | F: GTCCGCACTTCAACCCTCA R: TCCTCATTGCCACCCTTCC | BX055516 | 217 |
| gpx | F: AGATGTCATTCCTGCACACG R: AAGGAGAAGCTTCCTCAGCC | AW232474 | 94 |
| Compound | Internal Standard Substance | Linear Range (μg/L) | Linear Equation | R2 | LOD (μg/kg) | LOQ (μg/kg) | Recovery Rate (%) | RSD (%) |
|---|---|---|---|---|---|---|---|---|
| BPZ | BPA-13C12 | 1–100 | y = 0.1044x + 0.0142 | 0.9998 | 0.60 | 1.89 | 87.92–96.13 | 2.84–16.36 |
| BPC | BPA-13C12 | 1–100 | y = 0.0478x – 0.1008 | 0.9989 | 0.12 | 0.38 | 85.98–100.10 | 4.63–11.83 |
| BPF | BPA-13C12 | 1–100 | y = 0.0568x – 0.4246 | 0.9994 | 0.16 | 0.54 | 83.30–98.23 | 1.47–8.63 |
| BPS | BPS-13C12 | 0.5–100 | y = 0.0616x + 0.3211 | 0.9990 | 0.019 | 0.06 | 92.79–102.91 | 4.27–14.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Liu, Y.; Wang, M.; Xue, Y. Effects of BPZ, BPC, BPF, and BPS Exposure on Adult Zebrafish (Danio rerio): Accumulation, Oxidative Stress, and Gene Expression. Int. J. Environ. Res. Public Health 2022, 19, 15784. https://doi.org/10.3390/ijerph192315784
Han Y, Liu Y, Wang M, Xue Y. Effects of BPZ, BPC, BPF, and BPS Exposure on Adult Zebrafish (Danio rerio): Accumulation, Oxidative Stress, and Gene Expression. International Journal of Environmental Research and Public Health. 2022; 19(23):15784. https://doi.org/10.3390/ijerph192315784
Chicago/Turabian StyleHan, Ying, Yuxuan Liu, Mingxin Wang, and Yingang Xue. 2022. "Effects of BPZ, BPC, BPF, and BPS Exposure on Adult Zebrafish (Danio rerio): Accumulation, Oxidative Stress, and Gene Expression" International Journal of Environmental Research and Public Health 19, no. 23: 15784. https://doi.org/10.3390/ijerph192315784




