The Role of Platelet-Rich Plasma on the Chondrogenic and Osteogenic Differentiation of Human Amniotic-Fluid-Derived Stem Cells
Abstract
1. Introduction
1.1. Platelet-Rich Plasma
1.2. Mesenchymal Stem Cells + Platelet-Rich Plasma
1.3. Amniotic-Fluid-Derived Stem Cells
2. Materials and Methods
2.1. Preparation of Platelet-Rich Plasma
2.2. Amniotic-Fluid-Derived Stem Cells
2.2.1. Isolation and Culture
2.2.2. Study Protocols
2.2.3. Staining
2.2.4. Real-Time PCR
3. Results
3.1. Molecular Characterization of the AFSCs
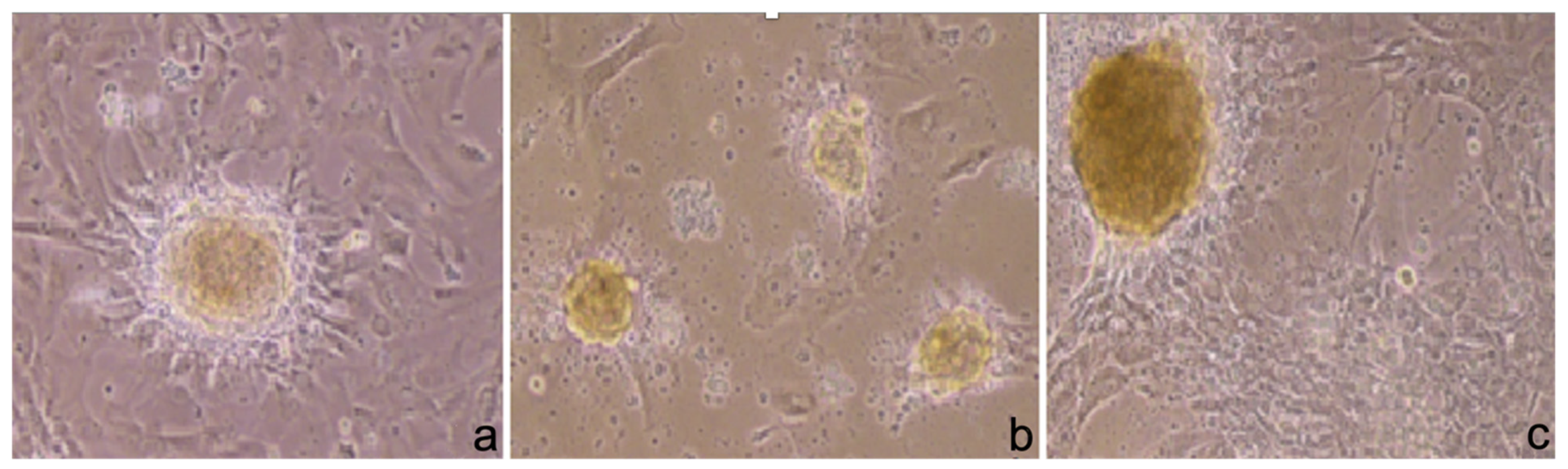
3.2. Phase-Contrast Microscopy
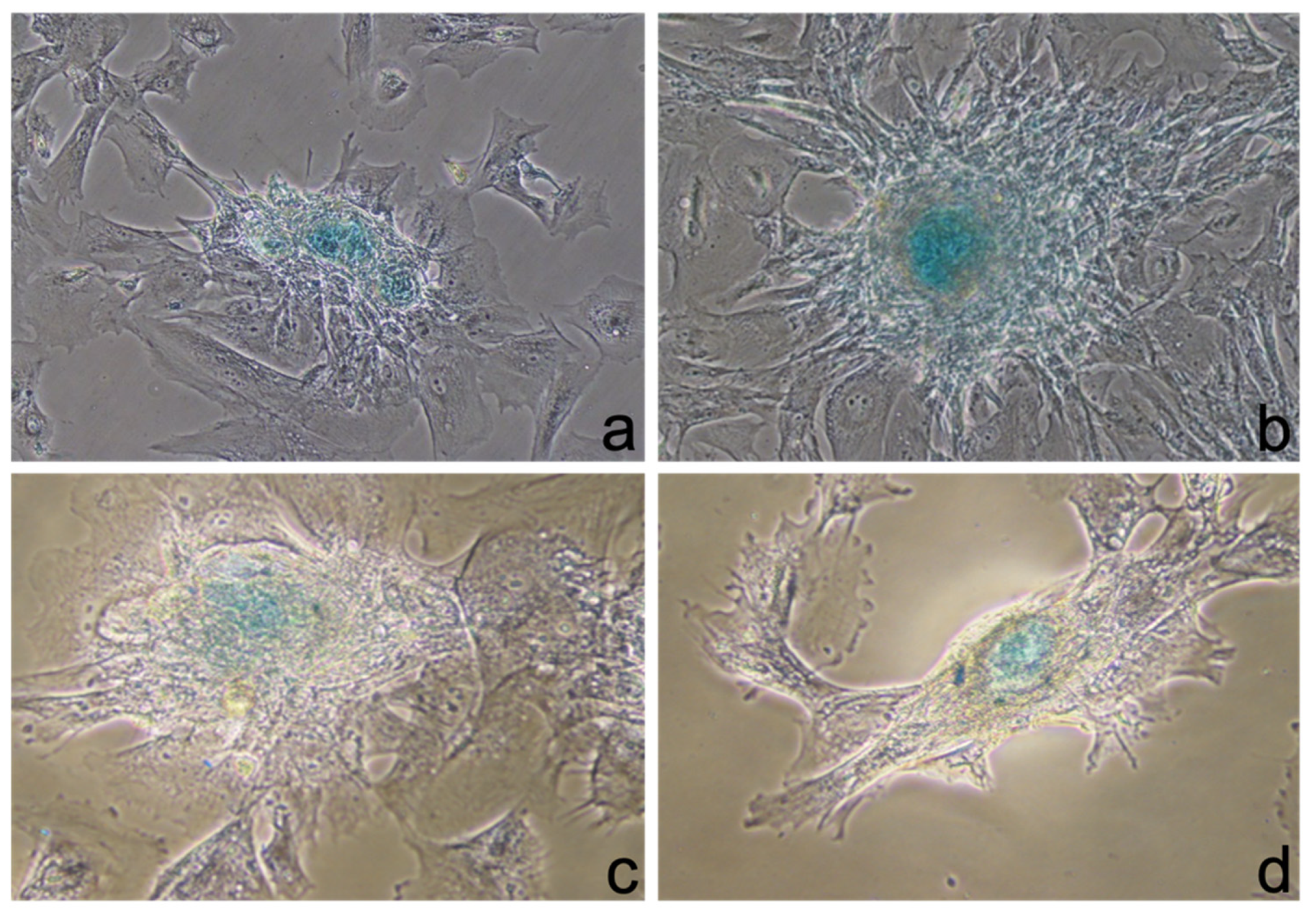
3.3. Staining
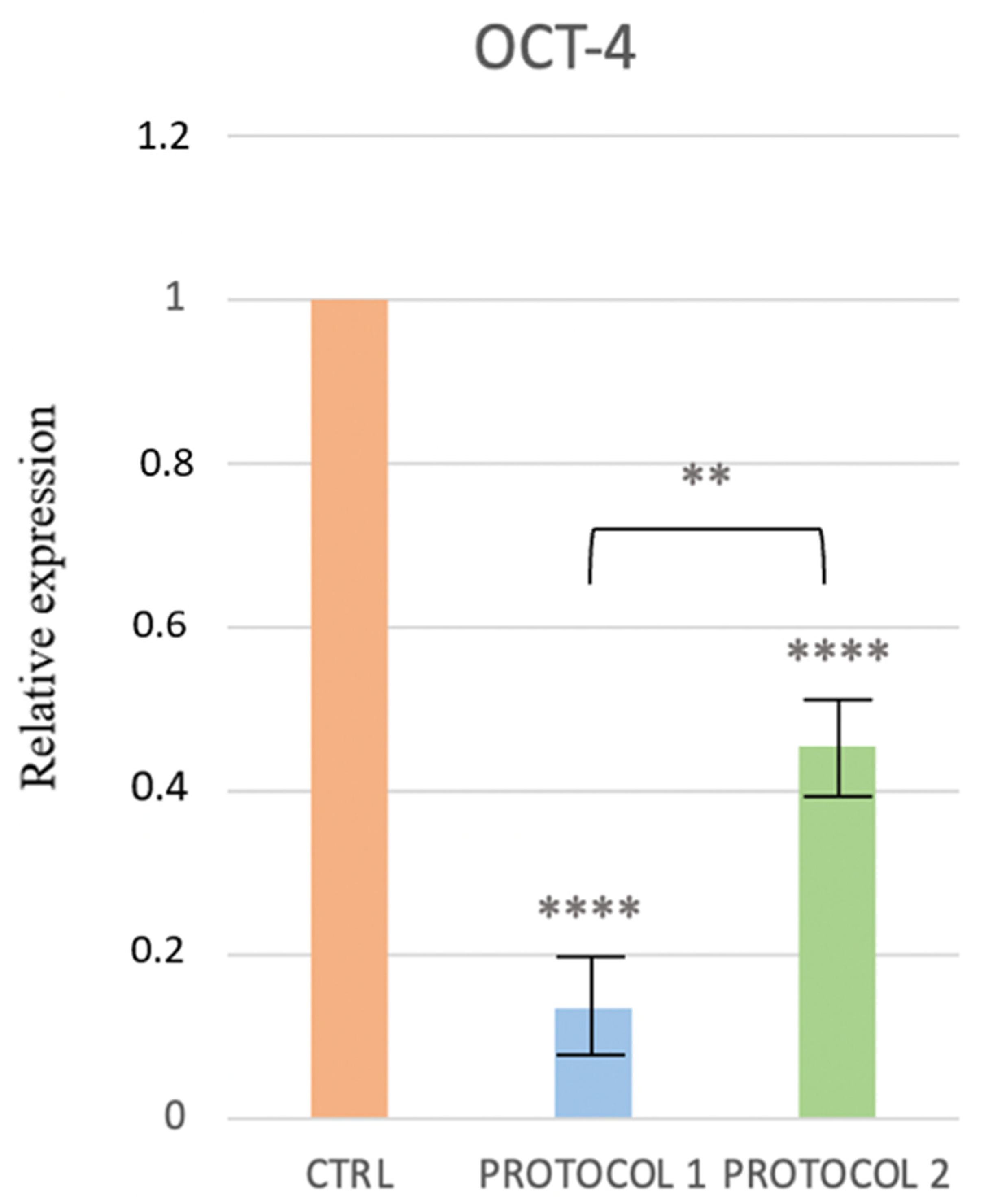
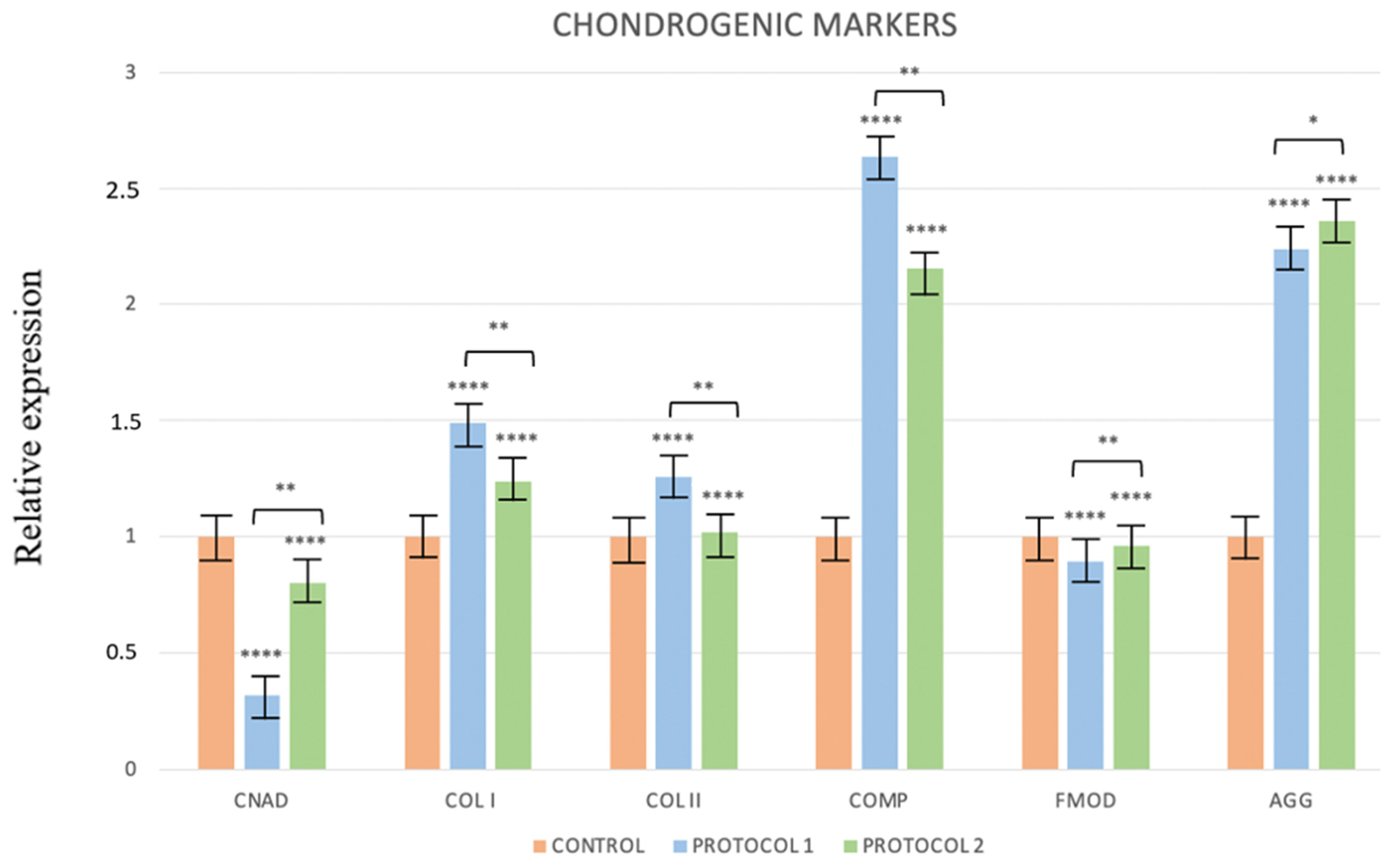
3.4. Real-Time PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fidanza, A.; Schettini, I.; Palozzi, G.; Mitrousias, V.; Logroscino, G.; Romanini, E.; Calvisi, V. What Is the Inpatient Cost of Hip Replacement? A Time-Driven Activity Based Costing Pilot Study in an Italian Public Hospital. J. Clin. Med. 2022, 11, 6928. [Google Scholar] [CrossRef]
- Presutti, M.; Goderecci, R.; Palumbo, P.; Giannetti, A.; Mazzoleni, M.G.; Randelli, F.M.N.; Angelozzi, M.; Calvisi, V.; Fidanza, A. A novel biplanar medial opening-wedge high tibial osteotomy: The Z-shaped technique. A case series at 7.2 years follow-up. J. Orthop. Traumatol. 2021, 22, 53. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone Substitutes in Orthopaedic Surgery: From Basic Science to Clinical Practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, A.; Antonucci, I.; Guelfi, M.; Pantalone, P.; Usuelli, F.G.; Stuppia, L.; Salini, V. Amniotic fluid stem cells: An ideal resource for therapeutic application in bone tissue engineering. Eur. Rev. Med. Pharm. Sci. 2016, 20, 2884–2890. [Google Scholar]
- Ross, R.; Glomset, J.; Kariya, B.; Harker, L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc. Natl. Acad. Sci. USA 1974, 71, 1207–1210. [Google Scholar] [CrossRef]
- Lang, S.; Loibl, M.; Herrmann, M. Platelet-Rich Plasma in Tissue Engineering: Hype and Hope. Eur. Surg. Res. 2018, 59, 265–275. [Google Scholar] [CrossRef]
- Venosa, M.; Calafiore, F.; Mazzoleni, M.; Romanini, E.; Cerciello, S.; Calvisi, V. Platelet-Rich Plasma and Adipose-Derived Mesenchymal Stem Cells in Association with Arthroscopic Microfracture of Knee Articular Cartilage Defects: A Pilot Randomized Controlled Trial. Adv. Orthop. 2022, 2022, 6048477. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.; Szyluk, K.; Iwanicka, J.; Balcerzyk, A.; Nowak, T.; Iwanicki, T.; Negru, M.; Kalita, M.; Francuz, T.; Garczorz, W.; et al. What Role Does PDGFA Gene Polymorphisms Play in Treating Tennis Elbow with PRP? A Prospective Cohort Study. J. Clin. Med. 2022, 11, 3504. [Google Scholar] [CrossRef]
- Szyluk, K.; Niemiec, P.; Sieron, D.; Lukoszek, D.; Marcin, G.; Lorek, A.; Christe, A. Shoulder Dislocation Incidence and Risk Factors—Rural vs. Urban Populations of Poland. Int. J. Environ. Res. Public Health 2022, 19, 11857. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Reffat, S.A.; Hassan, A.; Eskander, F. Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Ann. Vasc. Surg. 2017, 38, 206–211. [Google Scholar] [CrossRef]
- Gupta, S.; Paliczak, A.; Delgado, D. Evidence-based indications of platelet-rich plasma therapy. Expert Rev. Hematol. 2021, 14, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Szyluk, K.; Jarosz, A.; Balcerzyk-Matić, A.; Iwanicka, J.; Iwanicki, T.; Nowak, T.; Gierek, M.; Negru, M.; Kalita, M.; Górczyńska-Kosiorz, S.; et al. Polymorphic Variants of the PDGFRB Gene Influence Efficacy of PRP Therapy in Treating Tennis Elbow: A Prospective Cohort Study. J. Clin. Med. 2022, 11, 6362. [Google Scholar] [CrossRef] [PubMed]
- Ham, O.; Song, B.W.; Lee, S.Y.; Choi, E.; Cha, M.J.; Lee, C.Y.; Park, J.H.; Kime, K., II; Chang, W.; Lim, S.; et al. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling. Biomaterials 2012, 33, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, R.M.; Roche, M.W.; Alcerro, J.C.; Lavernia, C.J. The Current Status of Cell Based Therapies for Primary Knee Osteoarthritis. Orthop. Clin. 2019, 50, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, A.; Giannetti, A.; Vanni, D.; Verna, S.; Di Gregorio, P.; Salini, V. The Effect of Platelet-Rich Plasma on the Chondrogenic and Osteogenic Differentiation of Stem Cells. Annals. Stem Cells Regen. Med. 2018, 1, 1003. [Google Scholar]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef]
- Monckeberg, J.E.; Rafols, C.; Apablaza, F.; Gerhard, P.; Rosale, J. Intra-articular administration of peripheral blood stem cells with platelet-rich plasma regenerated articular cartilage and improved clinical outcomes for knee chondral lesions. Knee 2019, 26, 824–831. [Google Scholar] [CrossRef]
- Griffiths, M.J.D.; Bonnet, D.; Janes, S.M. Stem cells of the alveolar epithelium. Lancet 2005, 366, 249–260. [Google Scholar] [CrossRef]
- Tang, H.C.; Chen, W.C.; Chiang, C.W.; Chen, L.Y.; Chang, Y.C.; Chen, C.H. Differentiation effects of platelet-rich plasma concentrations on synovial fluid mesenchymal stem cells from pigs cultivated in alginate complex hydrogel. Int. J. Mol. Sci. 2015, 16, 18507–18521. [Google Scholar] [CrossRef]
- Yanasse, R.H.; De Lábio, R.W.; Marques, L.; Fukasawa, J.T.; Segato, R.; Kinoshita, A.; Matsumoto, M.A.; Felisbino, S.L.; Solano, B.; Santos, R.R.D.; et al. Xenotransplantation of Human Dental Pulp Stem Cells in Platelet-Rich Plasma for the Treatment of Full-Thickness Articular Cartilage Defects in a Rabbit Model. Exp. Ther. Med. 2019, 17, 4344–4356. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z. Platelet-rich Plasma-Derived Growth Factors Promote Osteogenic Differentiation of Rat Muscle Satellite Cells: In Vitro and in Vivo Studies. Cell Biol. Int. 2012, 36, 1195–1205. [Google Scholar] [CrossRef]
- Miao, Z.; Jin, J.; Chen, L.; Zhu, J.; Huang, W.; Zhao, J.; Qian, H.; Zhang, X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006, 30, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Gu, W.; Cui, J.; Yu, M.; Zhang, Y.; Tang, C.; Yang, P.; Xu, X. Platelet-rich Plasma Enhanced Umbilical Cord Mesenchymal Stem Cells-Based Bone Tissue Regeneration. Arch. Oral Biol. 2014, 59, 1146–1154. [Google Scholar] [CrossRef]
- Joo, M.W.; Chung, S.J.; Shin, S.H.; Chung, Y.G. The Effect of Autologous Platelet-Rich Plasma on Bone Regeneration by Autologous Mesenchymal Stem Cells Loaded Onto Allogeneic Cancellous Bone Granules. Cells Tissues Organs 2017, 203, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Barlian, A.; Judawisastra, H.; Alfarafisa, N.M.; Wibowo, U.A.; Rosadi, I. Chondrogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells Induced by L-ascorbic Acid and Platelet Rich Plasma on Silk Fibroin Scaffold. PeerJ 2018, 6, e5809. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Geeslin, A.G.; Goudie, E.B.; Petrigliano, F.A.; LaPrade, R.F. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): Platelet-Rich Plasma and Mesenchymal Stem Cells. J. Bone Jt. Surg. Am. 2017, 99, 809–819. [Google Scholar] [CrossRef]
- Gierek, M.; Kawecki, M.; Mikus, K.; Klama-Baryla, A.; Nowak, M. Biological dressings as a substitutes of the skin in the treatment of burn wounds. Pol. J. Surg. 2013, 85, 354–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Pipino, C.; Pierdomenico, L.; Di Tomo, P.; Di Giuseppe, F.; Cianci, E.; D'Alimonte, I.; Morabito, C.; Centurione, L.; Antonucci, I.; Mariggiò, M.A.; et al. Molecular and phenotypic characterization of human amniotic fluid-derived cells: A morphological and proteomic approach. Stem Cells Dev. 2015, 24, 1415–1428. [Google Scholar] [CrossRef]
- Hede, K.; Christensen, B.B.; Jensen, J.; Foldager, C.B.; Lind, M. Combined Bone Marrow Aspirate and Platelet-Rich Plasma for Cartilage Repair: Two-Year Clinical Results. Cartilage 2019, 13, 937S–947S. [Google Scholar] [CrossRef] [PubMed]
- Beigi, M.H.; Atefi, A.; Ghanaei, H.R.; Labbaf, S.; Ejeian, F.; Nasr-Esfahani, M.H. Activated Platelet-Rich Plasma Improves Cartilage Regeneration Using Adipose Stem Cells Encapsulated in a 3D Alginate Scaffold. J. Tissue Eng. Regen. Med. 2018, 12, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Prusa, A.R.; Hengstschlager, M. Amniotic fluid cells and human stem cell research: A new connection. Med. Sci. Monitor 2002, 8, RA253–RA257. [Google Scholar]
- Weibrich, G.; Kleis, W.K.G.; Hafner, G.; Hitzler, W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J. Cranio-Maxillofac. Surg. 2002, 30, 97–102. [Google Scholar] [CrossRef]
- Lucarelli, E.; Beccheroni, A.; Donati, D.; Sangiorgi, L.; Cenacchi, A.; Del Vento, A.M.; Meotti, C.; Bertoja, A.Z.; Giardino, R.; Fornasiri, P.M.; et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 2003, 24, 3095–3100. [Google Scholar] [CrossRef]
- Atashi, F.; Jaconi, M.E.; Pittet-Cuenod, B.; Modarressi, A. Autologous platelet-rich plasma: A biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng. Part C Methods 2015, 21, 253–262. [Google Scholar] [CrossRef]
- Loukogeorgakis, S.T.; De Coppi, P. Concise Review: Amniotic Fluid Stem Cells: The Known, the Unknown, and Potential Regenerative Medicine Applications. Stem Cells 2017, 35, 1663–1673. [Google Scholar] [CrossRef]
- Kunisaki, S.M.; Freedman, D.A.; Fauza, D.O. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J. Pediatr. Surg. 2006, 41, 675–682. [Google Scholar] [CrossRef]
- Steigman, S.A.; Ahmed, A.; Shanti, R.M.; Tuan, R.S.; Valim, C.; Fauza, D.O. Sternal repair with bone grafts engineered from amniotic mesenchymal stem cells. J. Pediatr. Surg. 2009, 44, 1120–1126. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Si, J.; Dai, J.; Shi, J.; Wang, X.; Guo, L.; Shen, G. Amniotic fluid-derived stem cells mixed with platelet rich plasma for restoration of rat alveolar bone defect. Acta Biochim. Biophys. Sin. 2017, 49, 197–207. [Google Scholar] [CrossRef]
- Ghaffarinovin, Z.; Soltaninia, O.; Mortazavi, Y.; Esmaeilzadeh, A.; Nadri, S. Repair of rat cranial bone defect by using amniotic fluid-derived mesenchymal stem cells in polycaprolactone fibrous scaffolds and platelet-rich plasma. BioImpacts BI 2021, 11, 209–217. [Google Scholar] [CrossRef] [PubMed]

| 1a | |
| PLURIPOTENCY MARKERS | |
| OCT-4 | Forward (5′ to 3′): CTT GCT GCA GAA GTG GGT GGA GGA |
| Reverse (5′ to 3′): CTG CAG TGT GGG TTT CGG GCA | |
| SOX-2 | Forward (5′ to 3′): TTG CTG CCT TAA GAC TAG GA |
| Reverse (5′ to 3′): CTG GGG CTC AAA CTT CTC TC | |
| 1b | |
| CHONDROGENIC MARKERS | |
| TYPE I COLLAGEN | Forward (5′ to 3′): CCA ATC ACC TGC GTA CAG AAC |
| Reverse (5′ to 3′): GGC ACG GAA ATT CCT CCG GTT GAT | |
| TYPE II COLLAGEN | Forward (5′ to 3′): CCA GGT CAA GAT GGT C |
| Reverse (5′ to 3′): CTT CAG CAC CTG TCT CAC CA | |
| CHONDROADHERIN | Forward (5′ to 3′): ACC TGG ACC ACA AGG TC |
| Reverse (5′ to 3′): GAA CTT CTC CAG GTT GT | |
| COMP | Forward (5′ to 3′): CAG GAC TTT GAT GCA GA |
| Reverse (5′ to 3′): AAG CTG GAG CTG TCC TGG TA | |
| FIBROMODULIN | Forward (5′ to 3′): ACC AGT GAT AAG GTG GGC AG |
| Reverse (5′ to 3′): AAG TAG TTA TCG GGG ACG GT | |
| AGGRECAN | Forward (5′ to 3′): GGC TTG AGC AGT TCA CCT TC |
| Reverse (5′ to 3′): CTC TTC TAC GGG GAC AGC AG | |
| 1c | |
| OSTEOGENIC MARKERS | |
| OSTEOPONTIN | Forward (5′ to 3′): AGG AGG CAG AGC ACA |
| Reverse (5′ to 3′): CTG GTA TGG CAC AGG TGA TG | |
| BONE SIALOPROTEIN | Forward (5′ to 3′): CTA TGG AGA GGA CGC CAC GCC T |
| Reverse (5′ to 3′): CAT AGC CAT CGT AGC CTT GTC CT | |
| 1d | |
| CONTROL | |
| GAPDH | Forward (5′ to 3′): CGC TCT CTG CTC CTG TT |
| Reverse (5′ to 3′): CCA TGG TGT CTG AGC GAT GT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetti, A.; Pantalone, A.; Antonucci, I.; Verna, S.; Di Gregorio, P.; Stuppia, L.; Calvisi, V.; Buda, R.; Salini, V. The Role of Platelet-Rich Plasma on the Chondrogenic and Osteogenic Differentiation of Human Amniotic-Fluid-Derived Stem Cells. Int. J. Environ. Res. Public Health 2022, 19, 15786. https://doi.org/10.3390/ijerph192315786
Giannetti A, Pantalone A, Antonucci I, Verna S, Di Gregorio P, Stuppia L, Calvisi V, Buda R, Salini V. The Role of Platelet-Rich Plasma on the Chondrogenic and Osteogenic Differentiation of Human Amniotic-Fluid-Derived Stem Cells. International Journal of Environmental Research and Public Health. 2022; 19(23):15786. https://doi.org/10.3390/ijerph192315786
Chicago/Turabian StyleGiannetti, Alessio, Andrea Pantalone, Ivana Antonucci, Sandra Verna, Patrizia Di Gregorio, Liborio Stuppia, Vittorio Calvisi, Roberto Buda, and Vincenzo Salini. 2022. "The Role of Platelet-Rich Plasma on the Chondrogenic and Osteogenic Differentiation of Human Amniotic-Fluid-Derived Stem Cells" International Journal of Environmental Research and Public Health 19, no. 23: 15786. https://doi.org/10.3390/ijerph192315786
APA StyleGiannetti, A., Pantalone, A., Antonucci, I., Verna, S., Di Gregorio, P., Stuppia, L., Calvisi, V., Buda, R., & Salini, V. (2022). The Role of Platelet-Rich Plasma on the Chondrogenic and Osteogenic Differentiation of Human Amniotic-Fluid-Derived Stem Cells. International Journal of Environmental Research and Public Health, 19(23), 15786. https://doi.org/10.3390/ijerph192315786









