Abstract
Sclerostin is most recognized for its role in controlling bone formation; however, it is also expressed in the heart, aorta, coronary, and peripheral arteries. Human studies have associated high circulating sclerostin levels with the presence of different cardiovascular diseases (CVD), surrogate CVD markers, and a high risk of cardiovascular events in some populations. However, this is still a matter of scientific debate, as the results have been very heterogeneous among studies. In the present review, the association between serum sclerostin levels and CVD and/or cardiovascular mortality was analyzed. For this purpose, a scoping review was performed in which articles measuring serum sclerostin levels and cardiovascular risk in patients were selected. Eleven articles answered the research question; of these articles, 8/11 evaluated the association between sclerostin and CVD, of which 4/8 found a positive association, 2/8 found a negative association, and 2/8 found no association between variables. Five (5/11) of the articles included in the study evaluated cardiovascular mortality, of which 3/5 found a positive association, 1/5 found a negative association, and 1/5 found no association between variables. In conclusion, we did not find sufficient results to be able to demonstrate an association between elevated sclerostin levels and the development of CVD and/or cardiovascular mortality in the general population due to heterogeneity in the results. However, there seems to be a tendency to consider increased sclerostin levels as a risk factor for both the development of cardiovascular events and cardiovascular mortality in specific populations. Further studies in this field will help to solve some of the inconsistencies found during this scoping review and allow for the future use of sclerostin measurement as a strategy in the prevention and diagnosis of CVD and/or cardiovascular mortality.
1. Introduction
Cardiovascular disease (CVD) is defined as a heterogeneous group of cardiac and circulatory system disorders that generally evolve silently and chronically until they reach a late stage [1]. Mortality rates of this pathological group are expected to increase in the near future due to the relationship between its prevalence and unhealthy lifestyles [2].
At present, 17.3 million people die each year from CVD; it is considered to be the leading cause of morbidity and mortality worldwide [3,4]. In Europe, it is the cause of death of more than 4 million people annually and is more frequent among men than women. However, 80% of these deaths are preventable [5].
In this context, cardiovascular prevention is an essential factor defined as a series of coordinated actions for the multitude, group, or individuals, with the aim to reduce or minimize the impact of CVD and related disabilities in the community.
The risk factors for CVD are classified as non-modifiable (age and sex, [men over 45 years of age and women over 55 years of age at menopause], family history with positive inheritance), and modifiable risk factors, generally associated with unhealthy lifestyle habits (smoking, sedentary lifestyle, arterial hypertension, obesity, dyslipidemia, and type 2 diabetes mellitus (T2DM), among others) [6]. According to the National Institute for Health and Care Excellence (NICE) Report, a population-based approach to reducing the risk of developing CVD would lead to the prevention of other diseases, such as cancer, T2DM, and Chronic Obstructive Pulmonary Disease (COPD) [7,8], in addition to the direct cost savings from avoided cardiac events, pharmacological treatment, or medical follow-up, and indirect cost savings from reduced sick leave due to incapacity [9].
Several risk scores, such as the Framingham Risk Score (FRS) [10], the Systematic Coronary Risk Evaluation (SCORE) [11], and the atherosclerotic cardiovascular disease (asCVD) score [12], have been developed to predict the future risk of CVD and CVD mortality. Likewise, the REGICOR score is the result of the validation of the Framingham equation in the Spanish population [13,14]. These scores were designed for risk prediction in the general population, and the FRS, in particular, is widely used in clinical settings [15].
Another tool that is becoming increasingly important for the correct stratification of cardiovascular risk is the determination of biomarkers. Recently, a relationship was observed between markers classically involved in bone metabolism (osteocalcin, osteopontin, osteoclastin, vitamin D, or sclerostin) and cardiovascular risk [16,17]. Sclerostin is a soluble glycoprotein encoded by the SOST gene on chromosome 17q12–q21. This protein is synthesized mainly by osteocytes and, to a lesser extent, other cell types, including osteoclast precursors and renal and vascular cells [16]. Sclerostin secretion leads to a reduction in bone formation as it suppresses osteoblast activation and inhibits bone turnover [18]. This process is carried out through the Wnt/β catenin signaling pathway in bone. The activation of this pathway in bone occurs through binding of Wnt proteins to Frizzled receptors and co-receptors of the low-density lipoprotein family (LDL) 5 and 6, producing β-catenin stabilization and activating gene transcription [17]. Activation of the Wnt pathway favors osteoblastic differentiation of mesenchymal stem cells and restricts chondrogenic and adipogenic differentiation. In addition, it also leads to osteoblast maturation processes, increased osteocyte survival, and inhibition of osteoclast genesis [5]. Sclerostin levels are generally higher among men; however, these levels tend to increase in women due to the decrease of estrogen levels during menopause [16]. Most studies that have determined sclerostin levels in the healthy population have indicated a mean value of approximately 40 ± 15 pmol/L, regardless of sex and age [19,20,21,22,23].
Determination of the sclerostin level is useful in the diagnosis of different pathologies, such as Van Buchem Dam disease [18], or to study the progression of chronic kidney disease (CKD) [24].
Regarding sclerostin at the vascular system, it seems clear that this protein has an important role at the vascular level. Different studies have shown that vascular smooth muscle cells (VSMCs) can induce a phenotypic transition to osteocyte-like cells capable of expressing typical osteocytic markers, including sclerostin, in a calcifying environment [25]. Thus, sclerostin expression has been found in atherosclerotic plaques and has been linked to vascular calcification in menopausal women [26], diabetic patients [27], and in those with abnormalities in the thickness of the arterial intima-media layers [28].
At the serum level, several studies have shown an association between serum sclerostin levels and the presence of cardiovascular events [29] or cardiovascular mortality [21]. These findings show that the action of sclerostin is not only restricted to the regulation of bone formation, it is also involved in vascular integrity, constituting an important modulator of Wnt signaling in CVD and acting as a potential serum marker of cardiovascular risk [23,25,30].
However, there is controversy regarding the role of sclerostin in vascular tissue. Some studies have reported a pathological role of this protein, mainly in CKD patients [31,32]. Sclerostin has also been described as a mediator between physical exercise and cardiovascular risk. Recent studies have demonstrated that mechanical loading associated with high-intensity interval training (HIIT) may improve bone status and atherosclerosis parameters (i.e., carotid intima-media thickness (cIMT)) through a decrease in the serum levels of Wnt signaling inhibitors, such as Dkk-1 and sclerostin, thus decreasing cardiovascular risk [33]. The most physically active individuals have the lowest serum sclerostin levels [33,34], which could be associated with a lower cardiovascular risk in this population. However, other studies have described a substantial but transient increase in sclerostin after physical activity [33,35,36], which returns to basal levels at 1 h post-exercise [37]. It is speculated that an exercise session results in an initial catabolic response (elevated sclerostin), which is subsequently followed by an anabolic response [37].
Therefore, in the literature, we found that short-term exercise causes an increase in sclerostin levels due to a physiological anabolic response [35], and that long-term exercise can decrease sclerostin levels, which is a cardioprotective action [34].
Conversely, other authors attribute a protective role to sclerostin, which may be involved in blocking the progression of vascular calcification through its inhibitory action on the Wnt signaling pathway, as observed in animal models [38] and in human studies [39,40].
In this context, there is no clear physiological link between sclerostin and CVD since, despite numerous studies, no consensus has yet been reached. Therefore, the association between higher sclerostin levels and predisposing factors to the development of CVD has prompted the scientific community to study it in large observational studies, to establish whether increased levels of this protein play a protective role in the survival of patients with CVD or, on the contrary, are involved in the pathogenesis of these complications.
The importance of understanding the pathophysiological mechanisms related to this protein and its effect on the vascular physiology could lie in advancements in the early diagnosis and prevention of CVD, reducing the high morbidity and mortality of the population at a higher risk [41].
In this study, we conducted a scoping review of the existing literature on the relationship between serum sclerostin levels and the development of cardiovascular events in humans. Clinical studies determining sclerostin values and their relationship with mortality and/or cardiovascular events were selected and critically analyzed.
2. Materials and Methods
This study is a scoping review based on the current scientific evidence on the relationship between elevated sclerostin serum levels and cardiovascular events and/or cardiovascular mortality.
2.1. Research Question
The design of a scoping review with systematic methodology has been followed. This type of review is a form of evidence synthesis that follows the process of a systematic review, in which some steps are simplified or omitted to produce information in a short period of time on the more specific elements related to the research objective [42].
To report the main findings of this review, we followed the verification guidelines established in the PRISMA (Preferred Reporting Items for Systematic Reviews) guide, in its 2020 update [43], adapting some items due to the chosen review design [42,43,44].
The main research question was as follows: Are elevated sclerostin values a risk exposure factor for CVD?
The PICO question was formulated for the development of the research question, focusing the search for original articles to demonstrate the main issue.
- P = articles studying sclerostin values and their relationship with cardiovascular mortality and/or cardiovascular events;
- I = elevated baseline sclerostin values as a factor of exposure;
- C = normal sclerostin values *;
- O = severe cardiovascular events: cardiovascular death, acute myocardial infarction, stroke, heart attack, myocardial infarction, stroke, angina pectoris;
- S = cohort, case-control, and cross-sectional studies.
- * Mean serum sclerostin levels in men and women were 40 ± 15 pmol/L, which was independent of age and sex [19,20,21,22,23].
2.2. Databases Consulted
The main databases consulted for the detailed search of the scientific evidence were PUBMED, SCOPUS, and Web of Science, where the following keywords were used: Sclerostin and Cardiovascular Disease. In PUBMED, the thesaurus developed by the National Library of Medicine (NLM), called Medical Subject Headings (MeSH), was used. In the SCOPUS and Web of Science databases, the thesaurus of Descriptors in Health Sciences (DeCS) was chosen. The search equation was formulated depending on the focused database and using the Boolean operator AND, resulting in the following search engine: (Sclerostin) AND “Cardiovascular Diseases” [Mesh] in PUBMED and “Sclerostin” AND “Cardiovascular diseases” in SCOPUS and Web of Science. Once the search string was obtained, the keywords were consulted separately in the different databases. The results obtained are shown in Table 1.

Table 1.
Primary search results.
2.3. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) freely published articles or those that can be accessed through the library of the University of Granada, (2) articles where the population studied were adults, (3) articles relating serum sclerostin levels to death from cardiovascular causes or severe cardiovascular events, and (4) written in English or Spanish language. The exclusion criteria were as follows: (1) articles performed in animal models, (2) articles in which a pediatric population was studied, (3) articles where serum sclerostin levels were not measured, and (4) articles that did not show a severe cardiovascular event or death from cardiovascular cause.
2.4. Selection of Studies
To carry out the study selection, a two-step review was applied. The titles and abstracts were screened independently by two authors (RST and BRG), and all publications reporting the measurement of sclerostin levels and CV risk and/or CV mortality and/or CV events were included. Secondly, the entire publication was reviewed to ensure that it fulfilled the rest of the inclusion criteria. Disagreements about inclusion or exclusion of articles were discussed until a consensus was reached.
2.5. Data Extraction
The variables assessed were author and date, country, age, number of participants, study design, follow-up, percentage of diabetes patients, and percentage of dialyzed patients. Sclerostin levels were expressed in pmol/L in four articles, in pg/mL in five articles, in ng/mL in one article, and in μg/L in another. Due to the diversity of units of measurement, the results were unified in ng/mL. To analyze the association between sclerostin levels and cardiovascular events, data related to CVD and cardiovascular mortality of the patients included in the selected studies were extracted.
2.6. Risk of Bias Assesment
The evaluation of the methodological quality of the selected articles was carried out with the Critical Appraisal Skills Programme Español (CASPe) tool. CASPe is the most used tool for quality appraisal in health-related qualitative evidence syntheses [45]. The CASPe checklist for assessing the quality of observational studies has 11 items designed to assist in the response to three questions to consider when assessing a cohort, case-control, or cross-sectional study: Section A: Are the results of the study valid; Section B: What are the results; Section C: Will the results help at the local level? The first two questions are screening questions and can be answered quickly. If the answer to both is “yes”, it is worth continuing with the remaining questions. You are asked to record a “yes”, “no” or “can’t say” to most of the questions.
3. Results
3.1. Selection and Description of Studies
On 21 December 2021, a bibliographic search was performed using the databases mentioned above (Section 2.2). The initial sample of data consisted of a total of 298 articles. A screening of the bibliography was carried out by means of a previous reading of the title and abstract, obtaining those articles that focused on the line of research to be dealt with.
After the initial selection of the articles, we proceeded to read them completely, discarding those whose characteristics did not meet the inclusion/exclusion criteria previously established. Finally, duplicate studies were eliminated. The final sample consisted of 11 articles with a total population of 2786 patients (Figure 1). The main patient information from the selected articles is summarized in Table 2.
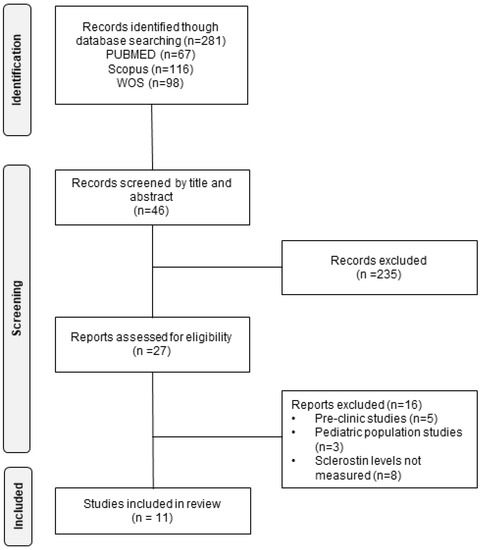
Figure 1.
Flow chart of the literature search performed for the selection of the studies included in the analysis.
Nine of the studies were longitudinal [21,40,46,47,48,49,50,51,52], and two were case-control [53,54]. The duration of follow-up ranged from 7 months [54] to 15.5 years [51]. The percentage of male patients ranged from 43.87% [40] to 68% [49] of the study population. The mean age of the patients ranged from a minimum of 52.5 years [47] to a maximum of 83 years [21]. The presence of diabetes was measured nine out of 11 studies [51,53], with the prevalence of diabetes ranging from 7.28% [52] to 57.7% [21]. The percentage of patients undergoing dialysis was measured in six out of 11 articles, in four of which all patients were undergoing dialysis [46,47,50,51] and two of which 54.6% [48] and 62.4% [49] were undergoing dialysis.

Table 2.
Characteristics of the studies included.
Table 2.
Characteristics of the studies included.
| Author and Date | Country | N | Male Patients (%) | Average Age (Years) | Diabetes (%) | Dialysis (%) | Follow-Up (Months) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Zou Y. et al., 2020 | China | 165 | 55.8 | 56.5 | 29.1 | 100 | 24.9 | [50] |
| Novo-Rodríguez C. et al., 2018 | Spain | 130 | 51.5 | 58.8 | 57.69 | N/C | 82 | [21] |
| Kalousová M. et al., 2019 | Czech Republic | 106 | 60.38 | 61 | 40.6 | 100 | 26.5 | [46] |
| Gong L. et al., 2018 | China | 98 | 49 | 52.5 | 21.4 | 100 | 72 | [47] |
| Wang X-R. et al., 2017 | China | 161 | 49.38 | 58.3 | 20.5 | 54.66 | 23 | [48] |
| Jørgensen HS. et al., 2018 | Denmark | 157 | 68 | 54 | 26 | 62.42 | 36 | [49] |
| He W. et al., 2020 | China | 310 | 43.87 | 76 | 35.48 | N/C | 36 | [40] |
| Klingenschmid G. et al., 2020 | Italy | 706 | 47.6 | 66.3 | N/C | N/C | 187.2 | [51] |
| Drechsler C. et al., 2015 | Netherlands | 673 | 57 | 63 | 7.28 | 100 | 48 | [52] |
| He XW. et al., 2016 | China | 186 | 61.3 | 71.7 | 20.43 | N/C | 7 | [54] |
| Mathold K. et al., 2018 | Sweden | 94 | 50 | 83 | N/C | N/C | N/C | [53] |
3.2. Methodological Quality
The statistical analyses used in each study are summarized in Table 3. The co-variates included in the analyses to adjust the dataset were also collected. Furthermore, after evaluating the methodological quality of the selected studies according to the CASPe tool, a moderate score (8–9/11) was found in four of the articles [46,48,53,54] and a high methodological score (10–11/11) in seven of the remaining articles [21,40,47,49,50,51,52] (see Table 4 and Table 5).
3.3. Association between Sclerostin Levels and Cardiovascular Events
Eight of the articles studied the relationship between sclerostin levels and the development of CVD. Four of the studies consisted of a total of 543 patients, including two cohorts Zou Y. et al., 2020 [50] (in the peritoneal dialysis population), Gong L. et al., 2018 [47], and two case-control studies by He X.W. et al., 2016 [54] and Mathold K. et al., 2018 [53], showed a positive significant relationship between sclerostin levels and the development of CVD (Table 4). On the other hand, two of the studies showed a negative association between sclerostin levels and the development of CVD (Table 4) (Wang X-R. et al., 2017 [48], He W. et al., 2020 [40]); the other two found no association between these variables (Table 4) (Jørgensen H.S. et al., 2018 [49] and Klingenschmid G. et al., 2020 [51]). Most studies analyzed the relationship between sclerostin levels as a continuous variable and the occurrence of cardiovascular events with the exception of the studies by Jørgensen H.S. et al. 2018 [49] and He X.W. et al., 2016 [54], which used average sclerostin values.
3.4. Association between Sclerostin Levels and Cardiovascular Mortality
Five articles prospectively studied the relationship between sclerostin levels and cardiovascular mortality [21,40,46,47,52]. In three longitudinal studies with a total of 334 patients, a significant positive relationship was found between both variables (Table 5). On the other hand, in the study by Drechsler C. et al., 2015 [52], an inverse relationship between sclerostin levels and cardiovascular mortality in patients with CKD undergoing dialysis was found (Table 5). Finally, in the study of He W. et al., 2020 [40], no relationship was found between sclerostin levels and cardiovascular mortality (Table 5).

Table 3.
Statistical analysis and co-variables used in each study.
Table 3.
Statistical analysis and co-variables used in each study.
| Author and Date | Type of Analysis | Binary CVD (Yes/No) | Co-Variates Included in the Model | Ref. |
|---|---|---|---|---|
| Drechsler C. et al., 2015 | Competing risk analysis | No | Age, sex, residual GFR, blood pressure, levels of serum albumin, haemoglobin, calcium, PTH, AP | [52] |
| Wang X.R. et al., 2017 | Univariate and Multivariate Cox regression | Yes | Diabetes, smoking habit, dialysis status | [48] |
| Mathold K. et al., 2018 | One-way ANOVA and ANCOVA | Yes | Age, sex, waist circumference, eGFR | [53] |
| Jørgensen HS. et al., 2018 | Univariate and Multivariate Cox regression | Yes | Age, sex, BMI, dialysis therapy | [49] |
| Gong L. et al., 2018 | Univariate and Multivariate Cox regression | Yes | Age, sex, diabetes mellitus, LVEF, NT-proBNP, Pi, PTH, OPG | [47] |
| Novo-Rodríguez C. et al., 2018 | Competing risk analysis | Yes | Age, diabetes, sex, prevalent CVD, pIMT, tobacco use, hypertension, eGFR | [21] |
| Kalousová M. et al., 2019 | Linear regression model and real survival curves | Yes | Age | [46] |
| He W. et al., 2020 | Univariate and Multivariate Cox regression | Yes | Cardiovascular risk factors (TG, TC, LDL-c, HDL-c, hs-CRP, HbA1c, creatine), osteoporosis, CAD severity, angiographic characteristics, use of medication | [40] |
| Klingenschmid G. et al., 2020 | Linear regression models | Yes | Age, sex | [51] |
| Zou Y. et al., 2020 | Univariate and multivariate Cox regression analysis | Yes | Age, sex, Framingham cardiovascular risk factors (smoking, diabetes status, BP, HDL-c), factors associated with mortality in patients with CKD (CRP, phosphate, BMI, and albumin), and confounding factors associated with mortality, new onset CVEs | [50] |
| He XW. et al., 2016 | Multiple logistic regression analysis | Yes | BMI, hypertension, diabetes mellitus, dyslipidaemia, smoking, drinking, homocysteine, hs-CRP | [54] |
GFR: glomerular filtration rate; BMI: body mass index; hs-CRP: hypersensitive C-reactive protein; CVEs: cardiovascular events; CKD: Chronic kidney disease; CRP: C-Reactive Protein; BP: blood pressure; PTH: parathyroid hormone; AP: alkaline phosphatase; TG: triglyceride; TC: total cholesterol; LDL-c: LDL-cholesterol; HDL-c: HDL-cholesterol; HbA1c: glycated hemoglobin; CAD: coronary artery disease.

Table 4.
Association between sclerostin levels and CVD.
Table 4.
Association between sclerostin levels and CVD.
| Author and Date | Country | N | Study Type | Population | Scl Level (ng/mL) | Cox Proportional HR Analysis CV Events | Scl-CVD | CASPe |
|---|---|---|---|---|---|---|---|---|
| Zou Y. et al., 2020 [50] | China | 165 | Longitudinal | HD; PD | 0.2509 | HD patients (HR = 1.164, p = 0.509) PD patients (HR = 3.819, p = 0.011) | + | 10/11 |
| Gong L. et al., 2018 [47] | China | 98 | Longitudinal | PD | 1.945 | UA (HR = 2.456, p = 0.013); MA (HR = 2.475, p = 0.026) | + | 10/11 |
| Wang X-R. et al., 2017 [48] | China | 161 | Longitudinal | Stage 3,4 and 5 CKD patients | 0.898 | MA (HR = 0.294, p = 0.001) | − | 9/11 |
| Jørgensen H.S. et al., 2018 [49] | Denmark | 157 | Longitudinal | Candidates for renal transplantation due to CKD | 0.259 | MA (HR = 0.99, p = 0.88) | NS | 10/11 |
| He W. et al., 2020 [40] | China | 310 | Longitudinal | Geriatric patients undergoing PCI | 0.179 | MA (HR = 0.456, p = 0.013) | − | 11/11 |
| Klingenschmid G. et al., 2020 [51] | Italy | 706 | Longitudinal | Bruneck area population | 1.07 | UA (HR = 0.95, p = 0.507); MA (HR = 0.92, p = 0.507) | NS | 11/11 |
| He X.W. et al., 2016 [54] | China | 186 | Case-control | LAA and SAO incident stroke patients | LAA: 0.15; SAO: 0.15 | ROC Curve Analysis AUC = 0.773, p < 0.001 | + | 9/11 |
| Mathold K. et al., 2018 [53] | Sweden | 94 | Case-control | Geriatric patients with N-CEIS vs. healthy controls. | Cases: 3; Controls: 1.9 | ANOVA 3.0 (1.0–7.3) vs. 2.0 (0.2–6.5), p < 0.001 | + | 8/11 |
Scl: Sclerostin; CV: Cardiovascular; CVD: Cardiovascular disease; CKD: Chronic Kidney Disease; PCI: Percutaneous Coronary Intervention; LAA: Large Artery Atherosclerosis; SAO: Small-Artery Occlusion; HD: Hemodialysis; PD: Peritoneal Dialysis; HR: Hazard Regression; ROC: Receiver Operating Characteristic; AUC: Area Under the Curve; N-CEIS: Non-Cardioembolic Ischemic Stroke; UA: Univariate Analysis; MA: Multivariate Analysis. CASPe: The Critical Appraisal Skills Programme Español tool; NS: non-significant.

Table 5.
Association between sclerostin levels and cardiovascular mortality.
Table 5.
Association between sclerostin levels and cardiovascular mortality.
| Author and Date | Country | N | Study Type | Population | Scl Level (ng/mL) | Cox Proportional HR Analysis Mortality | Scl-CV Mortality | CASPe |
|---|---|---|---|---|---|---|---|---|
| Novo-Rodríguez C. et al., 2018 [51] | Spain | 130 | Longitudinal | DP | 1.317 | HR = 1.318, p = 0.004 | + | 10/11 |
| Kalousová M. et al., 2019 [46] | Czech Republic | 106 | Longitudinal | HD | 1.90 | HR = 2.557, p = 0.04 | + | 9/11 |
| Gong L. et al., 2018 [47] | China | 98 | Longitudinal | PD | 1.945 | UA (HR = 4.362, p = 0.008); MA (HR = 3.484, p = 0.029) | + | 10/11 |
| He W. et al., 2020 [40] | China | 310 | Longitudinal | Geriatric patients undergoing PCI | 0.179 | N/C | NS | 11/11 |
| Drechsler C. et al., 2015 [52] | Netherlands | 673 | Longitudinal | CKD patients with HD and PD | 1.37 | Short term (HR = 0.30 (0.14–0.61); Long term (HR = 0.60 (0.37–0.97) | − | 10/11 |
Scl: Sclerostin; CV: Cardiovascular; DP: diabetic patients; HD: hemodialysis; PD: peritoneal dialysis; PCI: percutaneous coronary intervention; CKD: chronic kidney disease; UA: Univariate Analysis; MA: Multivariate Analysis. CASPe: The Critical Appraisal Skills Programme Español tool; NS: non-significant.
4. Discussion
The present scoping review evaluated the association between elevated serum sclerostin levels and the development of cardiovascular events and cardiovascular mortality. Eleven articles in which sclerostin levels were evaluated and related to the development of CVD and cardiovascular mortality were analyzed.
The relationship between serum sclerostin levels and CVD has been addressed by eight studies, six of which were longitudinal and two of which were case-control studies. The results of the case-control studies showed a direct relationship between circulating levels of sclerostin and the presence of cardiovascular events and cardiovascular risk. The longitudinal studies showed an equal proportion of positive results in terms of association between elevated sclerostin levels and cardiovascular risk (n = 2), negative results (n = 2), and results with no association between the study variables (n = 2). Regarding the relationship between circulating sclerostin and cardiovascular mortality, this aspect was evaluated in five longitudinal studies; three of them showed an independent association between elevated sclerostin levels and cardiovascular mortality, while one showed the opposite result, and one showed no association between the study variables. Although the results of the cross-sectional studies do not imply causality, the absence of significant results in some of the longitudinal studies does not imply the inexistence of a relationship, but rather suggests that there is insufficient evidence to validate the proposed hypothesis. There does seems to be a tendency towards increased sclerostin levels as a risk factor, both for development of cardiovascular events and cardiovascular mortality, since more than half of the analyzed studies showed a significant association between the study variables.
The heterogeneity of the results obtained is probably due to the comorbidities associated with the studied patients, which does not allow us to draw solid conclusions for the general population. However, the observed trend of increased sclerostin levels as a cardiovascular risk factor seems to be more pronounced in specific populations. Thus, the literature suggests a positive association between high sclerostin serum levels and the development of CVD in patients with incident stroke. Likewise, serum sclerostin levels of patients undergoing peritoneal dialysis and hemodialysis were positively associated with the development of CVD but not with cardiovascular mortality. On the other hand, studies analyzing a Brunek area population-based cohort did not find a significant relationship with our initial hypothesis, as well as those studying patients with CKD and patients undergoing percutaneous coronary intervention.
The pathophysiological process that determines both the development of CVD and cardiovascular mortality in relation to elevated sclerostin levels is the most uncertain aspect of the proposed hypothesis. Researchers, such as Brandenburg V.M. et al., 2013 [55], suggest that the cause of the cardiovascular events is increased cardiovascular calcification due to sclerostin elevation; this was also pointed out in six of the 11 reviewed articles including patients with renal pathology. Pelletier et al., 2013 [56] showed an inverse relationship between the serum concentration of sclerostin and the glomerular filtration rate in these patients. In addition, the levels of sclerostin are modified due to the presence of dialysis as a renal therapeutic measure. Kalousová et al., 2019 [46] found that mean sclerostin levels in hemodialysis patients were almost three times higher than in patients with adequate renal function. Some authors have debated the cause of this process, i.e., whether it is due to reduced renal clearance, increased skeletal production, or even extra-skeletal production is still a subject of debate [24]. The hypothesis put forward by many authors is the mediation of high sclerostin values as a fundamental cause of bone calcification in the progression of CKD [57]. In fact, evidence has suggested that increased sclerostin may have a positive association with aortic calcification, abnormal intima-media thickness, high arterial stiffness, and carotid plaques in T2DM patients [29] and in healthy subjects [58]. The upregulation of sclerostin in calcified tissues led researchers and clinicians to come up with a hypothesis that sclerostin may be related to the pathogenic mechanism of vascular calcification in renal disease patients.
However, the role of sclerostin at the vascular level has not yet been fully determined and other studies show opposite results. Although recent reviews point out the association between increased sclerostin levels and large carotid intima media thickness, severe vascular calcification, and high arterial stiffness, as well as with an increased prevalence of atherosclerotic plaques and the development of other cardiovascular events [58,59], the role of sclerostin as an inhibitor of mineralization could suggest that the increase in sclerostin could be a reflection of a compensatory mechanism to slow down vascular calcification. This fact puts the role of sclerostin at the vascular level as a focus of current research. In this context, a recent review reported a protective role of sclerostin against abdominal aortic aneurysms and atherosclerosis formation in preclinical models [58]. Human genetic studies reported that low arterial sclerostin expression was associated with a high risk of cardiovascular events [58]. However, it has not been possible to obtain a clear conclusion due to the diversity of the results obtained in several studies [21,40,46,47,48,49,50,51,52,53,54].
In this context, the anti-sclerostin monoclonal antibody Romosozumab is currently under investigation. This antibody promotes bone formation by inhibiting sclerostin activity. The results have shown an increase in bone mineralization and avoidance of fractures in those at high risk, especially in the postmenopausal population. However, serious cardiovascular adverse effects were reported during the study with Romosozumab, which agrees with the results obtained by Gay A. and Towler D.A. in 2017 [60]. However, other meta-analyses of randomized controlled clinical trials suggested that administration of Romosozumab did not significantly increase the risk of major adverse cardiovascular events [61,62]. Thus, there is a high level of controversy regarding the cardiovascular safety of this drug. In fact, the data available to date supports restricting the prescription recommendations detailed in the drug’s data sheet, stating that patients at high risk of cardiovascular disease and stroke should not be considered for treatment with Romosozumab.
Due to the heterogeneity in the relationship between sclerostin and cardiovascular risk shown in numerous studies, perhaps there is a spurious relationship between these variables. There could be a third confounding factor that affects the two initial variables. It is therefore possible that there is not a simple unilateral relationship between sclerostin and cardiovascular risk, since there could be other underlying factors that are not currently being evaluated, as occurs in other processes detailed in scientific literature [63].
Based on our results and the results of other studies, we encourage further research to obtain more robust results to elucidate the involvement of this protein in vascular tissue and its relationship with CV risk and mortality, considering its potential clinical importance in the prevention of CVD, survival rate, and the quality of life of patients.
Deepening the studies in this field will help to solve some of the inconsistencies found during this literature review and allow for the future use of sclerostin measurement as a strategy in the diagnosis and prevention of CVD and/or cardiovascular mortality.
4.1. Limitations
Due to the diversity of procedures used to analyze the sclerostin levels, certain difficulties arose when unifying the mean values, and it is advisable to establish uniform criteria that facilitate understanding for health care professionals. The differences in terms of sample size, type of population, underlying pathologies at an average age, medication, comorbidities, and duration of follow-up could contribute to the appearance of biases, which may make it difficult to obtain comparable results between groups.
On the other hand, the present review, despite presenting a systematic approach, did not comply with all the items recommended by the PRISMA guidelines; therefore, it may have certain methodological limitations.
Furthermore, a possible spurious relationship between sclerostin levels and the incidence of cardiovascular events cannot be ruled out. The main limitation of this study was the inconsistency of the results obtained, since we have found articles whose results agreed with this hypothesis, articles that deny it, and articles that did not find a statistical association between the outcome variables.
4.2. Perspectives
The search process through the different scientific databases, covering a wide spectrum of the current literature, as well as the methodological evaluation of the selected articles through CASPe, allowed us to objectively analyze the results obtained in these studies and weigh them for their methodological quality, which added robustness to the results shown.
In this context, it is hoped that the present literature review will be useful as an incentive for researchers to carry out further in-depth analysis of the potential of sclerostin.
5. Conclusions
Due to the heterogeneity of the results obtained in the present review, we cannot robustly demonstrate a relationship between elevated sclerostin serum levels and the development of CVD and/or cardiovascular mortality in the general population; however, there does appear to be a positive trend in specific populations. Because of the clinical importance of this hypothesis, we encourage further research along these lines.
Author Contributions
Study design: R.S.-d.l.T., B.R.-G. and B.G.-F.; study conduct: R.S.-d.l.T. and B.R.-G., data collection: R.S.-d.l.T. and B.R.-G.; data interpretation: B.R.-G., B.G.-F., M.M.-T., S.G.-S. and F.A.-V.; drafting of the manuscript: R.S.-d.l.T., B.R.-G., B.G.-F. and C.G.-F.; reviewing the manuscript and approving final version of manuscript: All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto de Salud Carlos III grants (PI18-00803, PI21/01069 and PI18-01235), co-funded by the European Regional Development Fund (FEDER) and by Junta de Andalucía grant (PI-0268-2019). In addition, C.G.-F. and B.R.-G. are funded by postdoctoral fellowships from Instituto de Salud Carlos III and Junta de Andalucía (CD20/00022 and RH-0069-2021, respectively) and S.G.-S. and R.S.-d.l.T. are funded by a predoctoral fellowship from Carlos III Health Institute and funded by the University of Granada grant with co-funding by FEDER, (FI19/00118 and 8110 respectively).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CASPe | Critical Appraisal Skills Programme Español |
| AsCVD | Atherosclerotic Cardiovascular Disease |
| CVD | Cardiovascular Disease |
| COPD | Chronic Obstructive Pulmonar Disease |
| CKD | Chronic Kidney Disease |
| FRS | Framingham Risk Score |
| LDL | Low-Density Lipoprotein |
| NICE | National Institute for Health and Care Excellence |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SCORE | Systematic Coronary Risk Evaluation |
| T2DM | Type 2 Diabetes Mellitus |
References
- Frančula-Zaninović, S.; Nola, I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018, 14, 153. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the Future of Cardiovascular Disease in the United States. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Masters, R.K.; Powers, D.A.; Link, B.G. Obesity and US Mortality Risk over the Adult Life Course. Am. J. Epidemiol. 2013, 177, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M.; et al. Global Burden of Stroke and Risk Factors in 188 Countries, during 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskina, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; de Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [PubMed]
- Impact of Using Different Score Tables for Estimating Cardiovascular Risk|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S1885585713002636?token=E4D89718FF1519D38114F2B73E0B791F465B406BFCAF2177AC15E960905FAA2D57701281638F4A8BF0A4FF438F3F5F0B&originRegion=eu-west-1&originCreation=20220623122228 (accessed on 23 June 2022).
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. Guidelines: Editor’s Choice: 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of 10 Societies and by Invited Experts)Developed with the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315. [Google Scholar] [CrossRef]
- Overview|Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/cg181 (accessed on 9 September 2022).
- Hessel, F.P. Overview of the Socio-Economic Consequences of Heart Failure. Cardiovasc. Diagn. Ther. 2021, 11, 254. [Google Scholar] [CrossRef]
- Wolf, P.A.; D’Agostino, R.B.; Belanger, A.J.; Kannel, W.B. Probability of Stroke: A Risk Profile from the Framingham Study. Stroke 1991, 22, 312–318. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; de Backer, G.; de Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of Ten-Year Risk of Fatal Cardiovascular Disease in Europe: The SCORE Project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J.; D’Agostino, R.; Sullivan, L.; Elosua, R.; Wilson, P.; Ordovas, J.; Solanas, P.; Cordón, F.; Ramos, R.; Sala, J.; et al. An Adaptation of the Framingham Coronary Heart Disease Risk Function to European Mediterranean Areas. J. Epidemiol. Community Health 2003, 57, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of 10 Societies and by Invited Experts): Developed with the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. J. Prev. Cardiol. 2016, 23, NP1–NP96. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-López, N.; Martínez-Arias, L.; Fernández-Villabrille, S.; Ruiz-Torres, M.P.; Dusso, A.; Cannata-Andía, J.B.; Naves-Díaz, M.; Panizo, S. Role of the RANK/RANKL/OPG and Wnt/β-Catenin Systems in CKD Bone and Cardiovascular Disorders. Calcif. Tissue Int. 2021, 108, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and Mechanism of Action of Sclerostin in Bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef]
- Mödder, U.I.; Hoey, K.A.; Amin, S.; McCready, L.K.; Achenbach, S.J.; Riggs, B.L.; Melton, L.J.; Khosla, S. Relation of Age, Gender, and Bone Mass to Circulating Sclerostin Levels in Women and Men. J. Bone Miner. Res. 2011, 26, 373–379. [Google Scholar] [CrossRef]
- Kanbay, M.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Unal, H.U.; Oguz, Y.; Sari, S.; et al. Serum Sclerostin and Adverse Outcomes in Nondialyzed Chronic Kidney Disease Patients. J. Clin. Endocrinol. Metab. 2014, 99, E1854–E1861. [Google Scholar] [CrossRef]
- Novo-Rodríguez, C.; García-Fontana, B.; de Dios Luna-Del Castillo, J.; Andújar-Vera, F.; Avila-Rubio, V.; García-Fontana, C.; Morales-Santana, S.; Rozas-Moreno, P.; Muñoz-Torres, M. Circulating Levels of Sclerostin Are Associated with Cardiovascular Mortality. PLoS ONE 2018, 13, e0199504. [Google Scholar] [CrossRef]
- Fassio, A.; Idolazzi, L.; Viapiana, O.; Benini, C.; Vantaggiato, E.; Bertoldo, F.; Rossini, M.; Gatti, D. In Psoriatic Arthritis Dkk-1 and PTH Are Lower than in Rheumatoid Arthritis and Healthy Controls. Clin. Rheumatol. 2017, 36, 2377–2381. [Google Scholar] [CrossRef]
- Behets, G.J.; Viaene, L.; Meijers, B.; Blocki, F.; Brandenburg, V.M.; Verhulst, A.; D’Haese, P.C.; Evenepoel, P. Circulating Levels of Sclerostin but Not DKK1 Associate with Laboratory Parameters of CKD-MBD. PLoS ONE 2017, 12, e0176411. [Google Scholar] [CrossRef] [PubMed]
- Bouquegneau, A.; Evenepoel, P.; Paquot, F.; Malaise, O.; Cavalier, E.; Delanaye, P. Sclerostin within the Chronic Kidney Disease Spectrum. Clin. Chim. Acta 2020, 502, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Winnier, D.; Mari, A.; Bruder, J.; Fourcaudot, M.; Pengou, Z.; Tripathy, D.; Jenkinson, C.; Folli, F. Sclerostin and Insulin Resistance in Prediabetes: Evidence of a Cross Talk Between Bone and Glucose Metabolism. Diabetes Care 2015, 38, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Matsui, S.; Yasui, T.; Kasai, K.; Keyama, K.; Kato, T.; Uemura, H.; Kuwahara, A.; Matsuzaki, T.; Irahara, M. Increase in Circulating Sclerostin at the Early Stage of Menopausal Transition in Japanese Women. Maturitas 2016, 83, 72–77. [Google Scholar] [CrossRef]
- Wędrychowicz, A.; Sztefko, K.; Starzyk, J.B. Sclerostin and Its Significance for Children and Adolescents with Type 1 Diabetes Mellitus (T1D). Bone 2019, 120, 387–392. [Google Scholar] [CrossRef]
- Mackey, R.H.; Venkitachalam, L.; Sutton-Tyrrell, K. Calcifications, Arterial Stiffness and Atherosclerosis. Adv. Cardiol. 2007, 44, 234–244. [Google Scholar] [CrossRef]
- Morales-Santana, S.; García-Fontana, B.; García-Martín, A.; Rozas-Moreno, P.; García-Salcedo, J.A.; Reyes-García, R.; Muñoz-Torres, M. Atherosclerotic Disease in Type 2 Diabetes Is Associated with an Increase in Sclerostin Levels. Diabetes Care 2013, 36, 1667–1674. [Google Scholar] [CrossRef]
- García-Martín, A.; Rozas-Moreno, P.; Reyes-García, R.; Morales-Santana, S.; García-Fontana, B.; García-Salcedo, J.A.; Muñoz-Torres, M. Circulating Levels of Sclerostin Are Increased in Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2012, 97, 234–241. [Google Scholar] [CrossRef]
- Kundakci Gelir, G.; Sengul, S.; Nergizoglu, G.; Ertürk, S.; Duman, N.; Kutlay, S. Is Sclerostin Level Associated with Cardiovascular Diseases in Hemodialysis Patients? Blood Purif. 2018, 46, 118–125. [Google Scholar] [CrossRef]
- Ji, Y.Q.; Guan, L.N.; Yu, S.X.; Yin, P.Y.; Shen, X.Q.; Sun, Z.W.; Liu, J.; Lv, W.; Yu, G.P.; Ren, C. Serum Sclerostin as a Potential Novel Biomarker for Heart Valve Calcification in Patients with Chronic Kidney Disease. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8822–8829. [Google Scholar] [CrossRef]
- Falk, B.; Klentrou, P. Elevation in Sclerostin After Exercise: Is It Affected by Age and Sex? Calcif. Tissue Int. 2018, 102, 380–381. [Google Scholar] [CrossRef]
- Cheung, A.M.; Giangregorio, L. Mechanical Stimuli and Bone Health: What Is the Evidence? Curr. Opin. Rheumatol. 2012, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Pickering, M.E.; Simon, M.; Sornay-Rendu, E.; Chikh, K.; Carlier, M.C.; Raby, A.L.; Szulc, P.; Confavreux, C.B. Serum Sclerostin Increases After Acute Physical Activity. Calcif. Tissue Int. 2017, 101, 170–173. [Google Scholar] [CrossRef]
- Kouvelioti, R.; Kurgan, N.; Falk, B.; Ward, W.E.; Josse, A.R.; Klentrou, P. Response of Sclerostin and Bone Turnover Markers to High Intensity Interval Exercise in Young Women: Does Impact Matter? BioMed Res. Int. 2018, 2018, 4864952. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.; Haddad, F.; Klentrou, P.; Ward, W.; Kish, K.; Mezil, Y.; Radom-Aizik, S. Differential Sclerostin and Parathyroid Hormone Response to Exercise in Boys and Men. Osteoporos. Int. 2016, 27, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- de Maré, A.; Opdebeeck, B.; Neven, E.; D’Haese, P.C.; Verhulst, A. Sclerostin Protects Against Vascular Calcification Development in Mice. J. Bone Miner. Res. 2022, 37, 687–699. [Google Scholar] [CrossRef]
- Ghardashi-Afousi, A.; Davoodi, M.; Hesamabadi, B.K.; Asvadi-Fard, M.; Bigi, M.A.B.; Izadi, M.R.; Gaeini, A.A. Improved Carotid Intima-Media Thickness-Induced High-Intensity Interval Training Associated with Decreased Serum Levels of Dkk-1 and Sclerostin in Type 2 Diabetes. J. Diabetes Complicat. 2020, 34, 107469. [Google Scholar] [CrossRef]
- He, W.; Li, C.; Chen, Q.; Xiang, T.; Wang, P.; Pang, J. Serum Sclerostin and Adverse Outcomes in Elderly Patients with Stable Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Aging Clin. Exp. Res. 2020, 32, 2065–2072. [Google Scholar] [CrossRef]
- Kanbay, M.; Solak, Y.; Siriopol, D.; Aslan, G.; Afsar, B.; Yazici, D.; Covic, A. Sclerostin, Cardiovascular Disease and Mortality: A Systematic Review and Meta-Analysis. Int. Urol. Nephrol. 2016, 48, 2029–2042. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; Clowes, M.; Preston, L.; Booth, A. Meeting the Review Family: Exploring Review Types and Associated Information Retrieval Requirements. Health Inf. Libr. J. 2019, 36, 202–222. [Google Scholar] [CrossRef] [PubMed]
- López, J.B.C. Lectura Crítica de la Evidencia Clínica; Elsevier: Amsterdam, The Netherlands, 2015; p. 184. [Google Scholar]
- Kalousová, M.; Dusilová-Sulková, S.; Kuběna, A.A.; Zakiyanov, O.; Tesař, V.; Zima, T. Sclerostin Levels Predict Cardiovascular Mortality in Long-Term Hemodialysis Patients: A Prospective Observational Cohort Study. Physiol. Res. 2019, 68, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zheng, D.; Yuan, J.; Cao, L.; Ni, Z.; Fang, W. Elevated Levels of Serum Sclerostin Are Linked to Adverse Cardiovascular Outcomes in Peritoneal Dialysis Patients. Int. Urol. Nephrol. 2018, 50, 955–961. [Google Scholar] [CrossRef]
- Wang, X.R.; Yuan, L.; Zhang, J.J.; Hao, L.; Wang, D.G. Serum Sclerostin Values Are Associated with Abdominal Aortic Calcification and Predict Cardiovascular Events in Patients with Chronic Kidney Disease Stages 3–5D. Nephrology 2017, 22, 286–292. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Winther, S.; Dupont, L.; Bøttcher, M.; Rejnmark, L.; Hauge, E.M.; Svensson, M.; Ivarsen, P. Sclerostin Is Not Associated with Cardiovascular Event or Fracture in Kidney Transplantation Candidates. Clin. Nephrol. 2018, 90, 18–26. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, M.; Wang, J.; Cui, L.; Jiang, Z.; Ding, J.; Li, M.; Zhou, H. Association of Sclerostin with Cardiovascular Events and Mortality in Dialysis Patients. Ren. Fail. 2020, 42, 282–288. [Google Scholar] [CrossRef]
- Klingenschmid, G.; Tschiderer, L.; Himmler, G.; Rungger, G.; Brugger, S.; Santer, P.; Willeit, J.; Kiechl, S.; Willeit, P. Associations of Serum Dickkopf-1 and Sclerostin With Cardiovascular Events: Results From the Prospective Bruneck Study. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2020, 9, e014816. [Google Scholar] [CrossRef]
- Drechsler, C.; Evenepoel, P.; Vervloet, M.G.; Wanne, C.; Ketteler, M.; Marx, N.; Floege, J.; Dekker, F.W.; Brandenburg, V.M. High Levels of Circulating Sclerostin Are Associated with Better Cardiovascular Survival in Incident Dialysis Patients: Results from the NECOSAD Study. Nephrol. Dial. Transplant. 2015, 30, 288–293. [Google Scholar] [CrossRef]
- Mathold, K.; Wanby, P.; Brudin, L.; Von, S.P.; Carlsson, M. Alterations in Bone Turnover Markers in Patients with Noncardio-Embolic Ischemic Stroke. PLoS ONE 2018, 13, e0207348. [Google Scholar] [CrossRef]
- He, X.W.; Wang, E.; Bao, Y.Y.; Wang, F.; Zhu, M.; Hu, X.F.; Jin, X.P. High Serum Levels of Sclerostin and Dickkopf-1 Are Associated with Acute Ischaemic Stroke. Atherosclerosis 2016, 253, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; Kramann, R.; Koos, R.; Krüger, T.; Schurgers, L.; Mühlenbruch, G.; Hübner, S.; Gladziwa, U.; Drechsler, C.; Ketteler, M. Relationship between Sclerostin and Cardiovascular Calcification in Hemodialysis Patients: A Cross-Sectional Study. BMC Nephrol 2013, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.; Dubourg, L.; Carlier, M.C.; Hadj-Aissa, A.; Fouque, D. The Relation between Renal Function and Serum Sclerostin in Adult Patients with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Guo, C.; Cai, J.; Tang, C.; Dong, Z. Serum Sclerostin in Vascular Calcification and Clinical Outcome in Chronic Kidney Disease. Diabetes Vasc. Dis. Res. 2018, 15, 99–105. [Google Scholar] [CrossRef]
- Golledge, J.; Thanigaimani, S. Role of Sclerostin in Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2022, 42, E187–E202. [Google Scholar] [CrossRef]
- Catalano, A.; Bellone, F.; Morabito, N.; Corica, F. Sclerostin and Vascular Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4779. [Google Scholar] [CrossRef]
- Gay, A.; Towler, D.A. Wnt Signaling in Cardiovascular Disease: Opportunities and Challenges. Curr. Opin. Lipidol. 2017, 28, 387–396. [Google Scholar] [CrossRef]
- Bovijn, J.; Krebs, K.; Chen, C.Y.; Boxall, R.; Censin, J.C.; Ferreira, T.; Pulit, S.L.; Glastonbury, C.A.; Laber, S.; Millwood, I.Y.; et al. Evaluating the Cardiovascular Safety of Sclerostin Inhibition Using Evidence from Meta-Analysis of Clinical Trials and Human Genetics. Sci. Transl. Med. 2020, 12, eaay6570. [Google Scholar] [CrossRef]
- Lv, F.; Cai, X.; Yang, W.; Gao, L.; Chen, L.; Wu, J.; Ji, L. Denosumab or Romosozumab Therapy and Risk of Cardiovascular Events in Patients with Primary Osteoporosis: Systematic Review and Meta-Analysis. Bone 2020, 130, 115121. [Google Scholar] [CrossRef]
- Kujawska, A.; Kujawski, S.; Kozakiewicz, M.; Hajec, W.; Kwiatkowska, M.; Skierkowska, N.; Husejko, J.; Newton, J.L.; Zalewski, P.; Kędziora-Kornatowska, K. Adipokines Level and Cognitive Function-Disturbance in Homeostasis in Older People with Poorly Managed Hypertension: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 6467. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).