Epidemiological Characteristics and Spatio-Temporal Distribution of Hepatitis A in Spain in the Context of the 2016/2017 European Outbreak
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Sources
2.3. Hepatitis A Outbreaks
2.4. Hepatitis A Cases
2.4.1. Temporal Trends
2.4.2. Space–Time Cluster Analysis
2.5. Statistical Analyses
3. Results
3.1. Outbreaks Related to MSM
3.2. Temporal Trends and Male-to-Female Ratio
3.3. Space–Time Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa-Mattioli, M.; Napoli, A.D.; Ferré, V.; Billaudel, S.; Perez-Bercoff, R.; Cristina, J. Genetic variability of hepatitis A virus. J. Gen. Virol. 2003, 84 Pt 12, 3191–3201. [Google Scholar] [CrossRef]
- Averhoff, F.; Khudfyakov, Y.; Vellozi, C. Hepatitis A virus. In Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 2243–2261. [Google Scholar]
- WHO. WHO Position Paper on Hepatitis A Vaccines—June 2012. Wkly. Epidemiol. Rec. 2012, 87, 261–276. [Google Scholar]
- European Centre for Disease Prevention and Control; Hepatitis, A. In ECDC. Annual Epidemiological Report for 2016. Stockholm: ECDC. 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-2016-hepatitis-A.pdf (accessed on 9 June 2022).
- Theeten, H.; Van Herck, K.; Van Der Meeren, O.; Crasta, P.; Van Damme, P.; Hens, N. Long-term antibody persistence after vaccination with a 2-dose HavrixTM (inactivated hepatitis A vaccine): 20 years of observed data, and long-term model-based predictions. Vaccine 2015, 33, 5723–5727. [Google Scholar] [CrossRef] [Green Version]
- Petrecz, M.; Acosta, C.J.; Klopfer, S.O.; Kuter, B.J.; Goveia, M.G.; Stek, J.E.; Schödel, F.P.; Lee, A.W. Safety and immunogenicity of VAQTA® in children 12-to-23 months of age with and without administration of other US pediatric vaccines. Hum. Vaccines Immunother. 2019, 15, 426–432. [Google Scholar] [CrossRef]
- Grupo de trabajo vacunación en población adulta y grupos de riesgo de la Ponencia de Programa y Registro de Vacunaciones. Vacunación en grupos de riesgo de todas las edades y en determinadas situaciones. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad, Consumo y Bienestar Social, julio. 2018. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/riesgo/Vac_GruposRiesgo_todasEdades.htm (accessed on 12 October 2021).
- European Centre for Disease Prevention and Control. Hepatitis A outbreaks in the EU/EEA mostly affecting men who have sex with men—second update, 19 May 2017. Stockholm: ECDC. 2017. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-19-May-2017_UPDATE_2-HepatitisA-in-mostly-MSM.pdf (accessed on 19 July 2022).
- Rodríguez-Tajes, S.; Perpiñán, E.; Caballol, B.; Lens, S.; Mariño, Z.; Costa, J.; Vilella, A.; Pérez-Del-Pulgar, S.; Forns, X.; Koutsoudakis, G. Hepatitis A outbreak in Barcelona among men who have sex with men (MSM), January-June 2017: A hospital perspective. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 588–593. [Google Scholar] [CrossRef]
- Ibáñez-Tomás, E.; Gasch-Gallén, À. Sexual practices and the risk of Hepatitis A in men who have sex with men in Spain. J. Nurs. Manag. 2021, 29, 32–42. [Google Scholar] [CrossRef]
- Ndumbi, P.; Freidl, G.S.; Williams, C.J.; Mårdh, O.; Varela, C.; Avellón, A.; Friesema, I.; Vennema, H.; Beebeejaun, K.; Ngui, S.L.; et al. Hepatitis A outbreak disproportionately affecting men who have sex with men (MSM) in the European Union and European Economic Area, June 2016 to May 2017. Euro Surveill. 2018, 23, 1700641. [Google Scholar] [CrossRef] [Green Version]
- Áreas urbanas en España, 2021. Ministerio de Transportes, Movilidad y Agenda Urbana, DG de Vivienda y Suelo. Available online: http://atlasau.fomento.gob.es/#c=home (accessed on 26 April 2022).
- España. Real Decreto 2210/1995, de 28 de Diciembre, por el que se crea la Red Nacional de Vigilancia Epidemiológica. (Boletín Oficial del Estado núm. 21, de 24 de enero de 1996). Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-1996-1502 (accessed on 9 October 2022).
- Red Nacional de Vigilancia Epidemiológica (RENAVE). Protocolo de Vigilancia de la Hepatitis A. Protocolos de la Red Nacional de Vigilancia Epidemiológica. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/PROTOCOLOS/Protocolo%20de%20Vigilancia%20de%20Hepatitis%20A.pdf (accessed on 11 January 2022).
- National Statistics Institute (INE), Ministry of Economic Affairs and Digital Transformation, Spain. Available online: https://www.ine.es (accessed on 8 February 2022).
- Kulldorff, M. A spatial scan statistic. Commun. Stat Theory Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Friesema, I.H.; Sonder, G.J.; Petrignani, M.W.; Meiberg, A.E.; van Rijckevorsel, G.G.; Ruijs, W.L.; Vennema, H. Spillover of a hepatitis A outbreak among men who have sex with men (MSM) to the general population, the Netherlands, 2017. Euro Surveill. 2018, 23, 1800265. [Google Scholar] [CrossRef] [Green Version]
- Aulicino, G.; Faccini, M.; Lamberti, A.; Senatori, S.; Ciconali, G.; Gandolfi, C.; Galli, C.; Tagliacarne, C.; Castaldi, S.; Romanò, L. Hepatitis A epidemic in men who have sex with men (MSM) in Milan, Italy. Acta Biomed. 2020, 91, 106–110. [Google Scholar] [CrossRef]
- Tortajada, C.; Saunas Working Group; de Olalla, P.G.; Diez, E.; Pinto, R.M.; Bosch, A.; Perez, U.; Sanz, M.; A Caylà, J. Hepatitis A among men who have sex with men in Barcelona, 1989–2010: Insufficient control and need for new approaches. BMC Infect. Dis. 2012, 12, 11. [Google Scholar] [CrossRef]
- Simms, I.; Field, N.; Jenkins, C.; Childs, T.; Gilbart, V.L.; Dallman, T.J.; Mook, P.; Crook, P.D.; Hughes, G. Intensified shigellosis epidemic associated with sexual transmission in men who have sex with men--Shigella flexneri and S. sonnei in England, 2004 to end of February 2015. Euro Surveill. 2015, 20, 21097. [Google Scholar] [CrossRef] [Green Version]
- Savage, E.J.; Hughes, G.; Ison, C.; Lowndes, C.M. European Surveillance of Sexually Transmitted Infections network. Syphilis and gonorrhoea in men who have sex with men: A European overview. Euro Surveill. 2009, 14, 19417. [Google Scholar] [CrossRef] [Green Version]
- Plunkett, J.; Mandal, S.; Balogun, K.; Beebeejaun, K.; Ngui, S.L.; Ramsay, M.; Edelstein, M. Hepatitis A outbreak among men who have sex with men (MSM) in England, 2016-2018: The contribution of past and current vaccination policy and practice. Vaccine X 2019, 1, 100014. [Google Scholar] [CrossRef]
- Zimmermann, R.; Faber, M.; Dudareva, S.; Ingiliz, P.; Jessen, H.; Koch, J.; Marcus, U.; Michaelis, K.; Rieck, T.; Ruscher, C.; et al. Hepatitis A outbreak among MSM in Berlin due to low vaccination coverage: Epidemiology, management, and successful interventions. Int. J. Infect. Dis. 2021, 103, 146–153. [Google Scholar] [CrossRef]
- Alberts, C.J.; Boyd, A.; Bruisten, S.M.; Heijman, T.; Hogewoning, A.; van Rooijen, M.; Siedenburg, E.; Sonder, G.J. Hepatitis A incidence, seroprevalence, and vaccination decision among MSM in Amsterdam, the Netherlands. Vaccine 2019, 37, 2849–2856. [Google Scholar] [CrossRef]
- Regan, D.G.; Wood, J.G.; Benevent, C.; Ali, H.; Smith, L.W.; Robertson, P.W.; Ferson, M.J.; Fairley, C.K.; Donovan, B.; Law, M.G. Estimating the critical immunity threshold for preventing hepatitis A outbreaks in men who have sex with men. Epidemiol. Infect. 2016, 144, 1528–1537. [Google Scholar] [CrossRef] [Green Version]
- Freidl, G.S.; Sonder, G.J.; Bovée, L.P.; Friesema, I.H.; van Rijckevorsel, G.G.; Ruijs, W.L.; van Schie, F.; Siedenburg, E.C.; Yang, J.-Y.; Vennema, H. Hepatitis A outbreak among men who have sex with men (MSM) predominantly linked with the EuroPride, the Netherlands, July 2016 to February 2017. Euro Surveill. 2017, 22, 30468. [Google Scholar] [CrossRef] [Green Version]
- Suárez Gaiche, N.; Purriños-Hermida, M.J.; Pousa Ortega, A. Hepatitis A outbreak in Galicia during 2016–2018. Rev. Esp. Salud. Publica 2020, 94, e202001003. [Google Scholar] [CrossRef]
- Galdeano Osuna, M.C.; Baca Fuentes, M.; Jiménez Narvajo, B.; Porras-Povedano, M. Study of the prevention and control campaign for the hepatitis A outbreak in men who have sex with men in Seville (2016–2018). Vacunas 2022, 208–214. [Google Scholar] [CrossRef]
- Lanini, S.; Minosse, C.; Vairo, F.; Garbuglia, A.; Di Bari, V.; Agresta, A.; Rezza, G.; Puro, V.; Pendenza, A.; Loffredo, M.R.; et al. A large ongoing outbreak of hepatitis A predominantly affecting young males in Lazio, Italy; August 2016–March 2017. PLoS ONE 2017, 12, e0185428. [Google Scholar] [CrossRef] [PubMed]
- Pardhan-Ali, A.; Berke, O.; Wilson, J.; Edge, V.L.; Furgal, C.; Reid-Smith, R.; Santos, M.; A McEwen, S. A spatial and temporal analysis of notifiable gastrointestinal illness in the Northwest Territories, Canada, 1991–2008. Int. J. Health Geogr. 2012, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, G.J.; Gorton, R. An evaluation of SaTScan for the prospective detection of space-time Campylobacter clusters in the North East of England. Epidemiol. Infect. 2013, 141, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Gleason, J.A.; Ross, K.M.; Greeley, R.D. Analysis of population-level determinants of legionellosis: Spatial and geovisual methods for enhancing classification of high-risk areas. Int. J. Health Geogr. 2017, 16, 45. [Google Scholar] [CrossRef] [Green Version]
- Kammerer, J.S.; Shang, N.; Althomsons, S.P.; Haddad, M.B.; Grant, J.; Navin, T.R. Using statistical methods and genotyping to detect tuberculosis outbreaks. Int. J. Health Geogr. 2013, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Stelling, J.; Yih, W.K.; Galas, M.; Kulldorff, M.; Pichel, M.; Terragno, R.; Tuduri, E.; Espetxe, S.; Binsztein, N.; O’Brien, T.F.; et al. Automated use of WHONET and SaTScan to detect outbreaks of Shigella spp. using antimicrobial resistance phenotypes. Epidemiol. Infect. 2010, 138, 873–883. [Google Scholar] [CrossRef]




| OUTBREAKS AMONG MSM | ||||
|---|---|---|---|---|
| Variables | Total | 2016 | 2017 | |
| Total outbreaks, n (%) | 26 | 2 (7.69%) | 24 (92.31%) | |
| Duration of outbreak in days, median (IQR *) | 28 (18–34) | 36 (18–54) | 28 (16–34) | |
| Cases per outbreak, median (p25–p75) | 2 (2–2) | 2 (2–2) | 2 (2–2) | |
| Settings, n (%) | Household | 22 (84.62%) | 1 (50.00%) | 21 (87.50%) |
| Other | 4 (15.38%) | 1 (50.00%) | 3 (12.50%) | |
| CASES IN OUTBREAKS AMONG MSM ^ | ||||
| Variables | Total | 2016 | 2017 | |
| Total number of cases | 120 | 2 | 118 | |
| Male sex, n (%) | 118 (98.33%) | 2 (100%) | 116 (98.31%) | |
| Age groups, n (%) | 0–14 years | 1 (0.83%) | 0 | 1 (0.85%) |
| 15–44 years | 106 (88.33%) | 2 (100%) | 104 (88.14%) | |
| 45–64 years | 13 (10.83%) | 0 | 13 (11.02%) | |
| ≥65 years | 0 | 0 | 0 | |
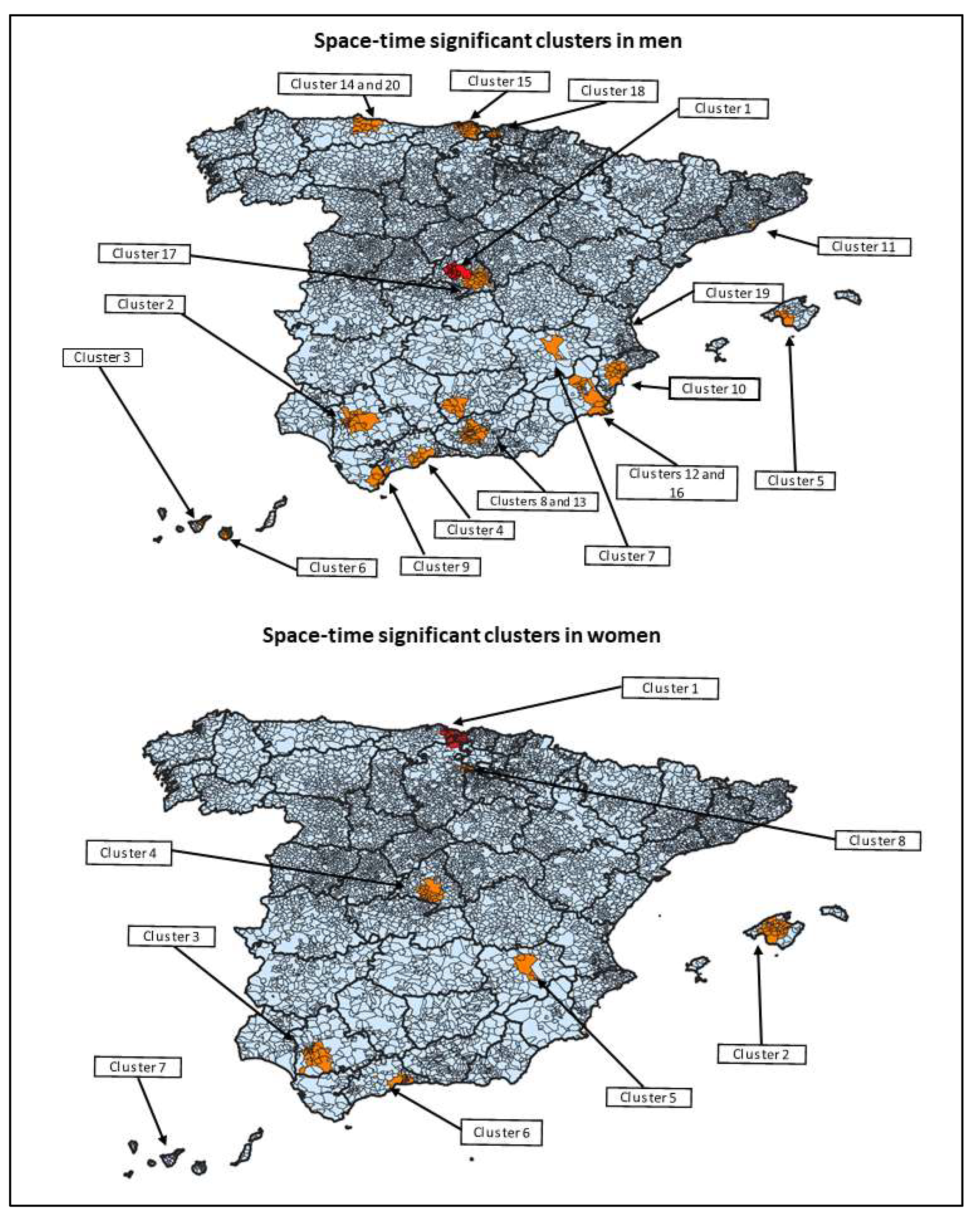
| Cluster | Start Date | End Date | Duration (Days) | Observed | Expected | Relative Risk | p-Value | Total Number of Municipalities (N Classified as Large Urban Area) |
|---|---|---|---|---|---|---|---|---|
| STATISTICALLY SIGNIFICANT SPACE-TIME CLUSTERS IN MEN | ||||||||
| 1 | 1 February 2017 | 21 April 2017 | 79 | 257 | 36.98 | 7.36 | <0.001 | 17 (14) |
| 2 | 31 December 2016 | 15 March 2017 | 74 | 113 | 12.12 | 9.57 | <0.001 | 31 (20) |
| 3 | 1 May 2017 | 17 July 2017 | 77 | 69 | 5.88 | 11.92 | <0.001 | 16 (9) |
| 4 | 20 February 2017 | 10 May 2017 | 79 | 80 | 9.90 | 8.22 | <0.001 | 13 (8) |
| 5 | 6 December 2017 | 6 December 2017 | 0 | 12 | 0.06 | 187.12 | <0.001 | 8 (6) |
| 6 | 5 May 2017 | 17 July 2017 | 73 | 41 | 6.96 | 5.94 | <0.001 | 13 (8) |
| 7 | 7 March 2017 | 16 March 2017 | 9 | 12 | 0.21 | 56.39 | <0.001 | 1 (1) |
| 8 | 1 August 2016 | 13 October 2016 | 73 | 22 | 1.71 | 12.96 | <0.001 | 12 (4) |
| 9 | 24 March 2017 | 5 June 2017 | 73 | 25 | 2.49 | 10.08 | <0.001 | 9 (4) |
| 10 | 29 March 2017 | 14 June 2017 | 77 | 42 | 8.50 | 4.99 | <0.001 | 19 (8) |
| 11 | 21 April 2017 | 6 July 2017 | 76 | 55 | 14.72 | 3.77 | <0.001 | 1 (1) |
| 12 | 28 April 2017 | 16 July 2017 | 79 | 40 | 8.04 | 5.01 | <0.001 | 8 (3) |
| 13 | 16 February 2017 | 4 May 2017 | 77 | 31 | 5.14 | 6.06 | <0.001 | 47 (27) |
| 14 | 15 February 2017 | 3 May 2017 | 77 | 28 | 4.28 | 6.58 | <0.001 | 13 (9) |
| 15 | 8 January 2017 | 28 March 2017 | 79 | 23 | 2.78 | 8.32 | <0.001 | 35 (3) |
| 16 | 17 April 2017 | 25 April 2017 | 8 | 8 | 0.12 | 69.24 | <0.001 | 10 (2) |
| 17 | 21 March 2017 | 2 June 2017 | 73 | 38 | 9.84 | 3.89 | <0.001 | 35 (13) |
| 18 | 20 April 2017 | 6 July 2017 | 77 | 22 | 3.36 | 6.57 | 0.001 | 20 (12) |
| 19 | 4 October 2017 | 4 October 2017 | 0 | 3 | 0.00 | 2791.78 | 0.004 | 1 (1) |
| 20 | 7 June 2017 | 23 August 2017 | 77 | 16 | 1.87 | 8.59 | 0.007 | 3 (3) |
| STATISTICALLY SIGNIFICANT SPACE-TIME CLUSTERS IN WOMEN | ||||||||
| 1 | 2 June 2017 | 23 June 2017 | 21 | 13 | 0.10 | 132.21 | <0.001 | 31 (7) |
| 2 | 6 December 2017 | 20 December 2017 | 14 | 13 | 0.21 | 63.78 | <0.001 | 33 (6) |
| 3 | 24 January 2017 | 10 March 2017 | 45 | 20 | 1.36 | 15.13 | <0.001 | 28 (24) |
| 4 | 13 April 2017 | 7 June 2017 | 55 | 34 | 5.83 | 6.07 | <0.001 | 27 (21) |
| 5 | 3 March 2017 | 21 March 2017 | 18 | 8 | 0.07 | 110.72 | <0.001 | 4 (1) |
| 6 | 15 March 2017 | 22 May 2017 | 68 | 15 | 1.05 | 14.61 | <0.001 | 15 (4) |
| 7 | 16 June 2017 | 1 September 2017 | 77 | 13 | 0.86 | 15.33 | <0.001 | 12 (6) |
| 8 | 22 November 2017 | 27 December 2017 | 35 | 5 | 0.02 | 205.91 | 0.0013 | 9 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Vadillo, M.; Peñuelas, M.; Domínguez, Á.; Godoy, P.; Gómez-Barroso, D.; Soldevila, N.; Izquierdo, C.; Martínez, A.; Torner, N.; Avellón, A.; et al. Epidemiological Characteristics and Spatio-Temporal Distribution of Hepatitis A in Spain in the Context of the 2016/2017 European Outbreak. Int. J. Environ. Res. Public Health 2022, 19, 16775. https://doi.org/10.3390/ijerph192416775
Guerrero-Vadillo M, Peñuelas M, Domínguez Á, Godoy P, Gómez-Barroso D, Soldevila N, Izquierdo C, Martínez A, Torner N, Avellón A, et al. Epidemiological Characteristics and Spatio-Temporal Distribution of Hepatitis A in Spain in the Context of the 2016/2017 European Outbreak. International Journal of Environmental Research and Public Health. 2022; 19(24):16775. https://doi.org/10.3390/ijerph192416775
Chicago/Turabian StyleGuerrero-Vadillo, María, Marina Peñuelas, Ángela Domínguez, Pere Godoy, Diana Gómez-Barroso, Nuria Soldevila, Conchita Izquierdo, Ana Martínez, Nuria Torner, Ana Avellón, and et al. 2022. "Epidemiological Characteristics and Spatio-Temporal Distribution of Hepatitis A in Spain in the Context of the 2016/2017 European Outbreak" International Journal of Environmental Research and Public Health 19, no. 24: 16775. https://doi.org/10.3390/ijerph192416775








