Typical Sulfonamide Antibiotics Removal by Biochar-Amended River Coarse Sand during Groundwater Recharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Biochar
2.2. Material Characterization
2.3. Batch Experiments
2.4. Biochar-Amended SAT
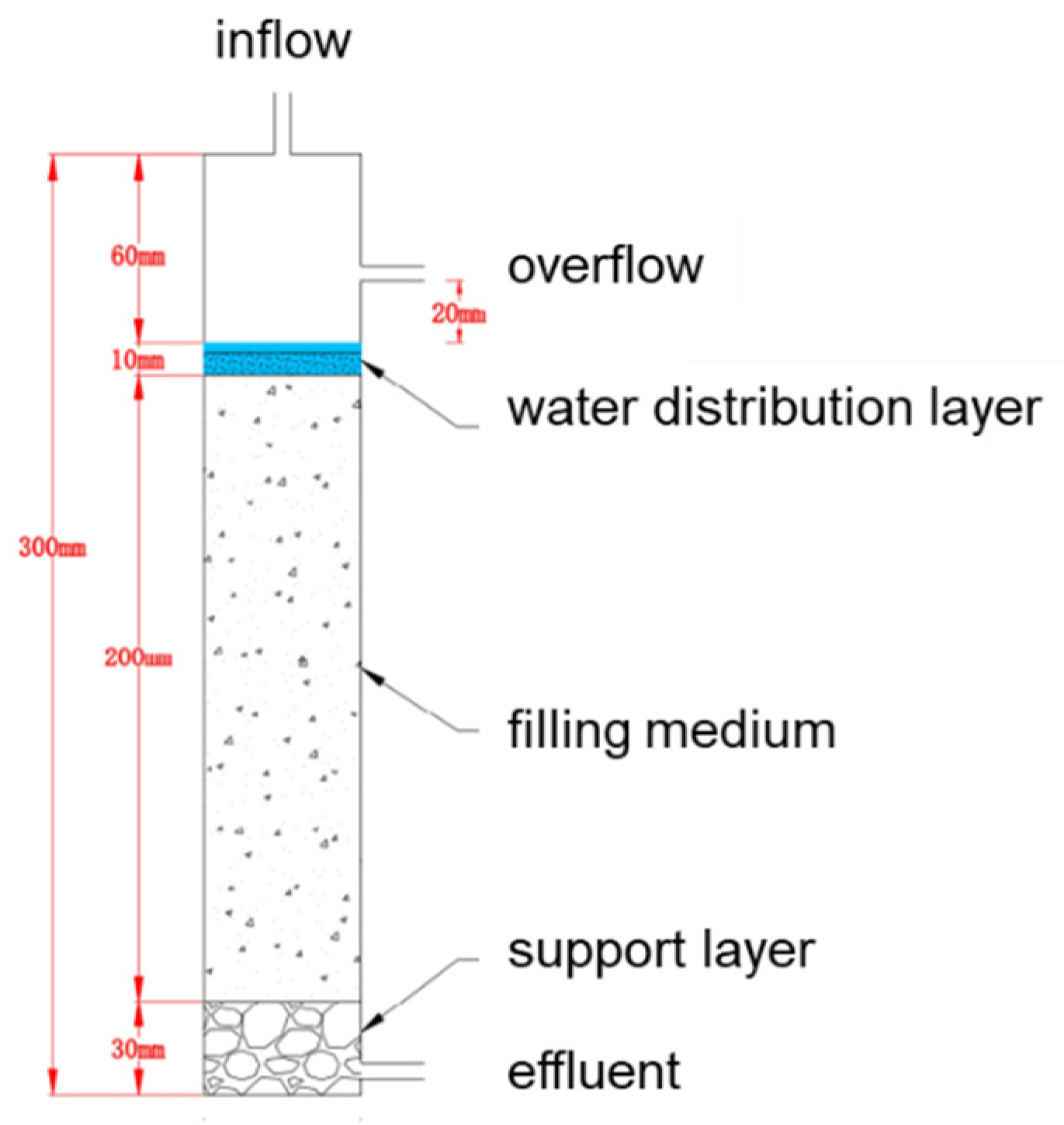
2.4.1. Packed Soil Column Set-Up
2.4.2. Packed Soil Column Experiments
3. Results and Discussion
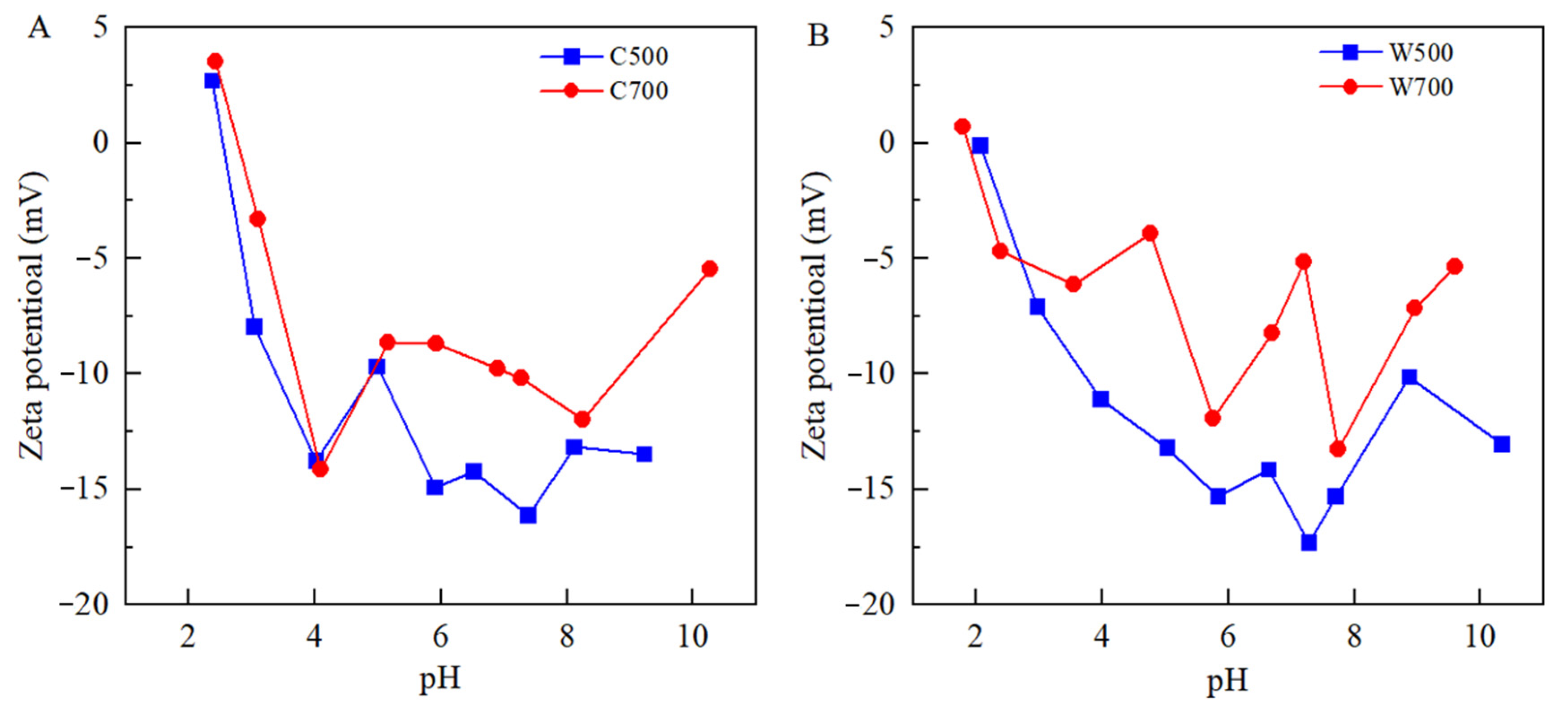
3.1. Characterization of Biochars
3.2. The Adsorption of SMX/TMP on Biochars
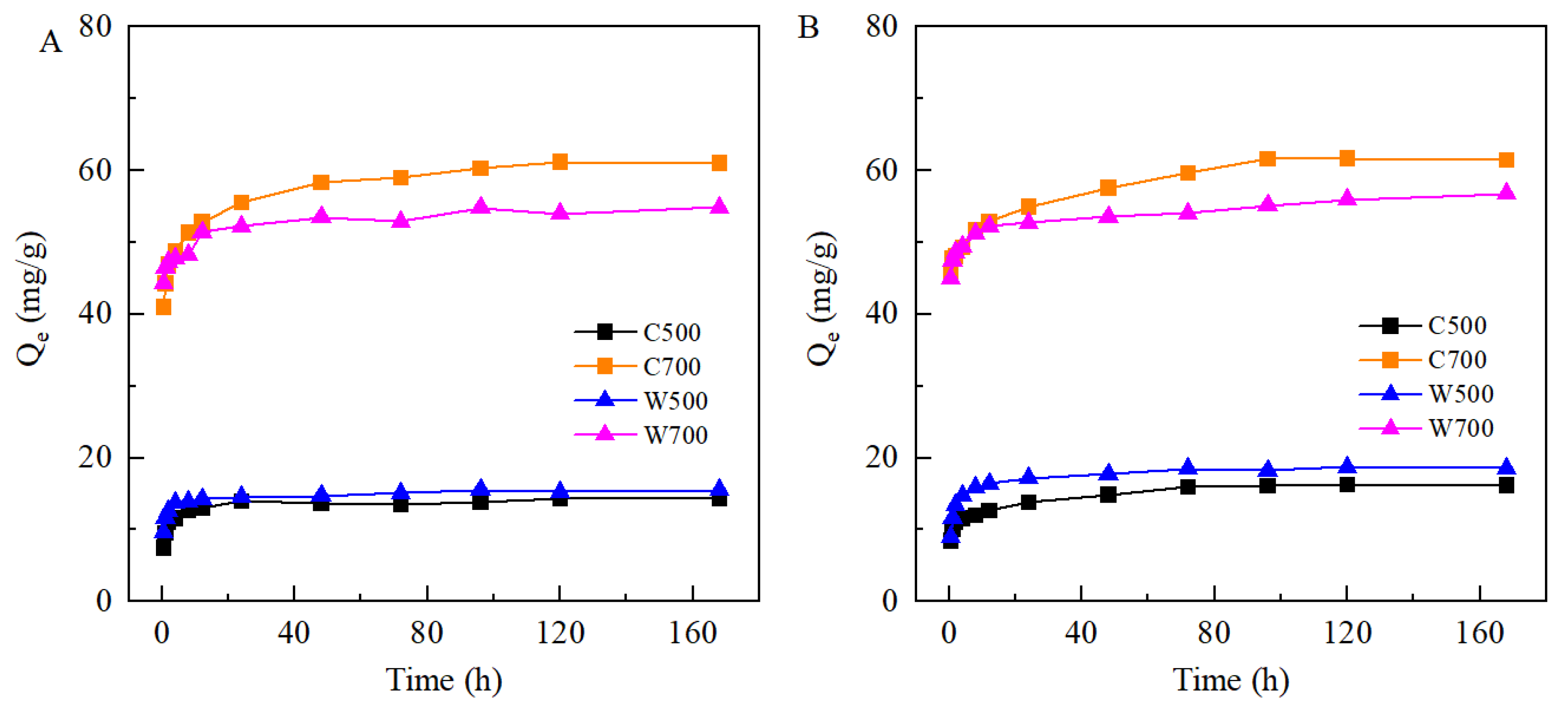
3.2.1. Kinetics of SMX and TMP Adsorption on Biochars
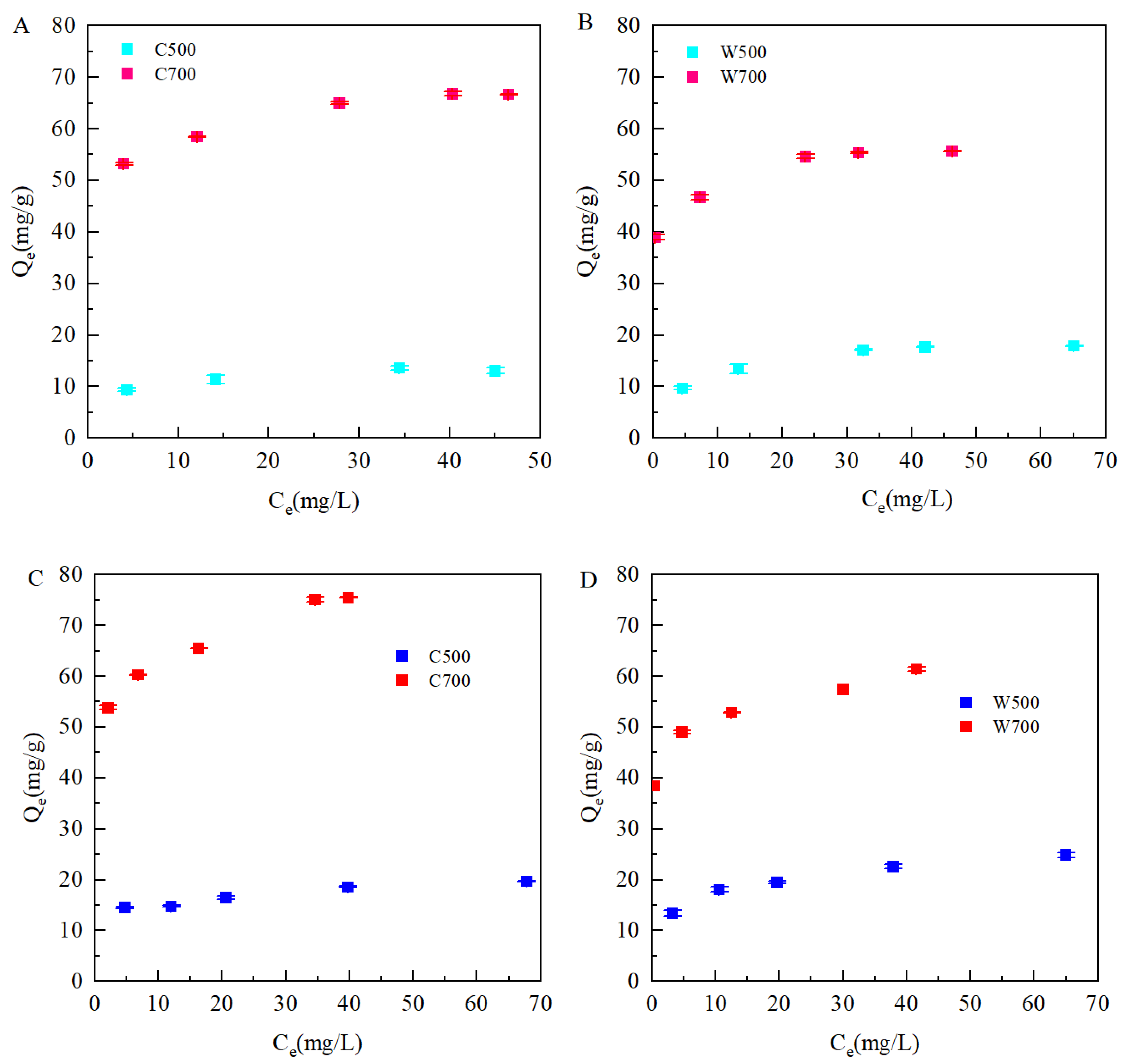
3.2.2. Adsorption Isotherm by Biochars
3.3. The Removal of SMX/TMP for Biochar-Amended SAT
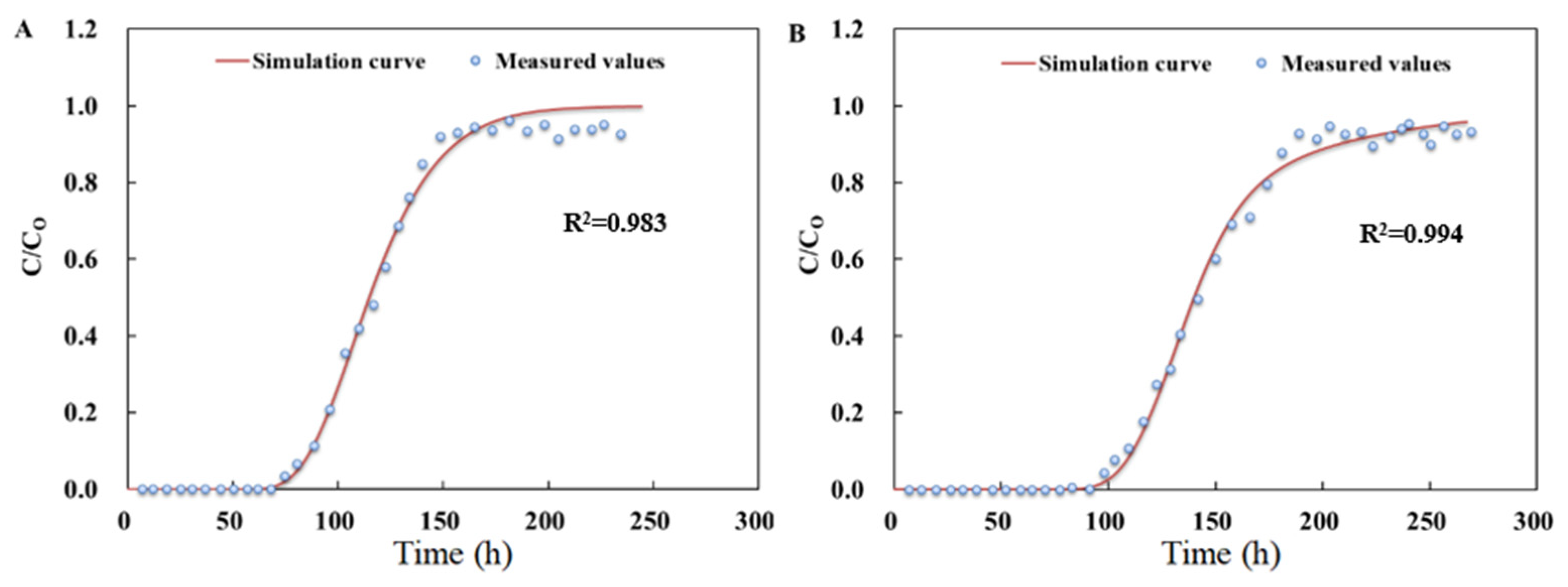
3.3.1. Parameters Fitting for Biochar-Amended SAT
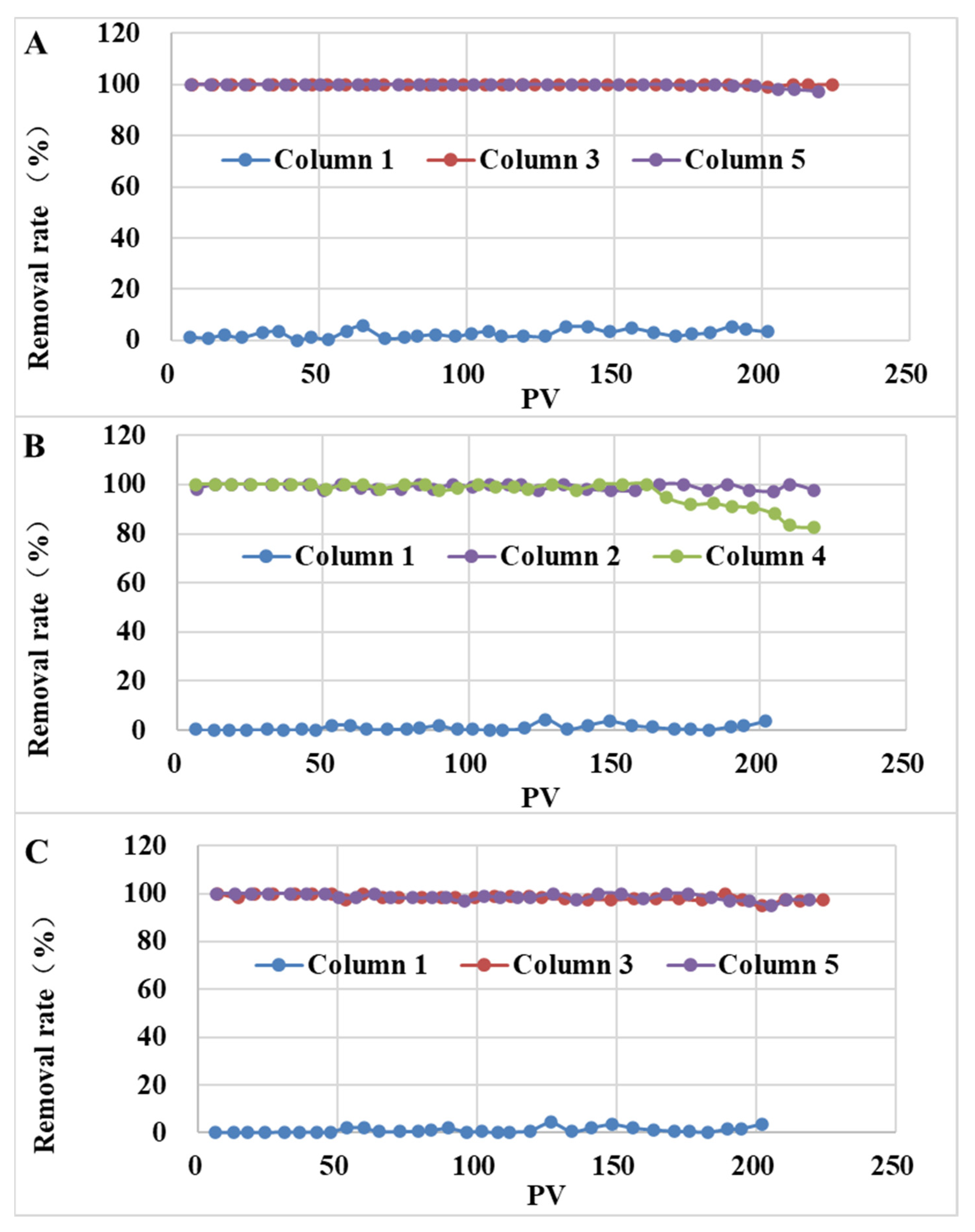
3.3.2. Removal of SMX and TMP for Soil Columns
3.3.3. The Adsorption Capacity of SMX in the Experimental Columns
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Wang, W. Correction to: Managing aquifer recharge with multi-source water to realize sustainable management of groundwater resources in Jinan, China. Environ. Sci. Pollut. Res. 2020, 28, 7598. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, M.; Wu, M.; Li, Z.; Liu, X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015, 518, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Huang, S.; Wang, Y.; Liu, H.; Li, M. Occurrence of antibiotics in the aquatic environment of Jianghan Plain, central China. Sci. Total Environ. 2014, 497, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Huwe, B. Effect of pH and soil structure on transport of sulfonamide antibiotics in agricultural soils. Environ. Pollut. 2016, 213, 561–570. [Google Scholar] [CrossRef]
- Wang, N.; Guo, X.Y.; Xu, J.; Hao, L.J.; Kong, D.Y.; Gao, S.X. Sorption and transport of five sulfonamide antibiotics in agricultural soil and soil-manure systems. J. Environ. Sci. Health Part B 2015, 50, 23–33. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Batista, A.P.S.; Pires, F.C.C.; Teixeira, A.C.S.C. Photochemical degradation of sulfadiazine, sulfamerazine and sulfamethazine: Relevance of concentration and heterocyclic aromatic groups to degradation kinetics. J. Photochem. Photobiol. A Chem. 2014, 286, 40–46. [Google Scholar] [CrossRef]
- Pérez-Moya, M.; Graells, M.; Castells, G.; Amigó, J.; Ortega, E.; Buhigas, G.; Pérez, L.M.; Mansilla, H.D. Characterization of the degradation performance of the sulfamethazine antibiotic by photo-Fenton process. Water Res. 2010, 44, 2533–2540. [Google Scholar] [CrossRef]
- Rudrashetti, A.P.; Jadeja, N.; Gandhi, D.; Juwarkar, A.A.; Sharma, A.; Kapley, A.; Pandey, R.A. Microbial population shift caused by sulfamethoxazole in engineered-Soil Aquifer Treatment (e-SAT) system. World J. Microbiol. Biotechnol. 2017, 33, 121. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Zhang, C.; Wang, Z.X.; Shao, L. Tailoring nanofiltration membrane performance for highly-efficient antibiotics removal by mussel-inspired modification. J. Membr. Sci. 2016, 499, 326–334. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Belhaj, D. Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment. Bioresour. Technol. 2017, 238, 306–312. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Li, B.; Li, Q.; Han, X.; Jiang, Y.; Jupa, R.; Wang, C.; Li, T. Simultaneous remediation of sediments contaminated with sulfamethoxazole and cadmium using magnesium-modified biochar derived from Thalia dealbata. Sci. Total Environ. 2018, 659, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Razali, Y.S.; Ismail, N.; Rafatullah, M.; Tajarudin, H.A. Selective adsorption and recovery of volatile fatty acids from fermented landfill leachate by activated carbon process. Sci. Total. Environ. 2019, 707, 134533. [Google Scholar] [CrossRef]
- Halim, A.A.; Aziz, H.A.; Johari, M.A.M.; Ariffin, K.S.; Bashir, M.J. Semi-Aerobic Landfill Leachate Treatment Using Carbon–Minerals Composite Adsorbent. Environ. Eng. Sci. 2012, 29, 306–312. [Google Scholar] [CrossRef]
- Daud, Z.; Detho, A.; Rosli, M.A.; Abubakar, M.H.; Samo, K.A.; Rais, N.F.M.; Halim, A.A.; Tajarudin, H.A. Ammoniacal nitrogen and COD removal from stabilized landfill leachate using granular activated carbon and green mussel (Perna viridis) shell powder as a composite adsorbent. Desalination Water Treat. 2020, 192, 111–117. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zheng, T.; Li, Y.; Liu, X. Removal of tylosin and copper from aqueous solution by biochar stabilized nano-hydroxyapatite. Chemosphere 2019, 235, 136–142. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Sun, K.; Kang, M.; Ro, K.S.; Libra, J.A.; Zhao, Y.; Xing, B. Variation in sorption of propiconazole with biochars: The effect of temperature, mineral, molecular structure, and nano-porosity. Chemosphere 2016, 142, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, Y.; Jing, M.; Chen, J. Sorption and transport of sulfonamides in soils amended with wheat straw-derived biochar: Effects of water pH, coexistence copper ion, and dissolved organic matter. J. Soils Sediments 2015, 17, 771–779. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Zhao, J.; Herbert, S.; Xing, B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Xing, B.; Liu, W.; Tao, S.; Lin, X.; Zhang, Y.; Yuan, H.; Dai, H.; Zhang, X.; Xiao, Y. Two-compartment sorption of phenanthrene on eight soils with various organic carbon contents. J. Environ. Sci. Health Part B 2006, 41, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total. Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. II Liquids. J. Am. Chem. Soc. 1968, 40, 1361–1402. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, M.; Che, Q.; Li, Y.; Liu, X. Synergistic removal of tylosin/sulfamethoxazole and copper by nano-hydroxyapatite modified biochar. Bioresour. Technol. 2019, 294, 122163. [Google Scholar] [CrossRef]
- Nasir, H.M.; Wee, S.Y.; Aris, A.Z.; Abdullah, L.C.; Ismail, I. Processing of natural fibre and method improvement for removal of endocrine-disrupting compounds. Chemosphere 2021, 291, 132726. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Yin, H.; Zhao, K.; Zhao, H.; An, T. Adsorption and desorption mechanism of aromatic VOCs onto porous carbon adsorbents for emission control and resource recovery: Recent progress and challenges. Environ. Sci. Nano 2021, 9, 81–104. [Google Scholar] [CrossRef]
- Lian, F.; Sun, B.; Chen, X.; Zhu, L.; Liu, Z.; Xing, B. Effect of humic acid (HA) on sulfonamide sorption by biochars. Environ. Pollut. 2015, 204, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Wang, Y.; Li, R.-X.; Cao, H.-L.; Li, Y.-F.; Lü, J. Impacts of temperatures and phosphoric-acid modification to the physicochemical properties of biochar for excellent sulfadiazine adsorption. Biochar 2022, 4, 14. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, X.; Peng, D. Iron and manganese oxides modified maize straw to remove tylosin from aqueous solutions. Chemosphere 2018, 205, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, B.; Jährig, J.; Reemtsma, T.; Jekel, M. Long term laboratory column experiments to simulate bank filtration: Factors controlling removal of sulfamethoxazole. Water Res. 2011, 45, 211–220. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Microbial degradation of sulfamethoxazole in the environment. Appl. Microbiol. Biotechnol. 2018, 102, 3573–3582. [Google Scholar] [CrossRef]
| Biochars | C/% | H/% | O/% | N/% | Ash Content/% | O/C | H/C | (O+N)/C | Yield/% |
|---|---|---|---|---|---|---|---|---|---|
| W500 | 63.50 | 2.16 | 17.75 | 0.89 | 15.70 | 0.28 | 0.03 | 0.29 | 23.79 |
| W700 | 57.28 | 1.04 | 11.07 | 0.59 | 30.03 | 0.19 | 0.02 | 0.20 | 16.48 |
| C500 | 67.44 | 2.68 | 24.13 | 1.62 | 4.13 | 0.36 | 0.04 | 0.38 | 22.64 |
| C700 | 72.67 | 1.92 | 17.73 | 1.03 | 6.65 | 0.24 | 0.03 | 0.26 | 15.01 |
| Biochar | SSA a (m2/g) | Smic b (m2/g) | ESSA c (m2/g) | Vt d (cm3/g) | Vmic e (cm3/g) | PS f (nm) |
|---|---|---|---|---|---|---|
| W500 | 461.00 | 393.90 | 67.09 | 0.28 | 0.18 | 2.43 |
| W700 | 532.80 | 410.40 | 122.40 | 0.37 | 0.19 | 2.77 |
| C500 | 369.00 | 175.58 | 193.46 | 0.30 | 0.09 | 3.24 |
| C700 | 598.60 | 453.74 | 144.90 | 0.42 | 0.22 | 2.80 |
| Dual-Chamber First-Order Kinetics Model | |||||||
|---|---|---|---|---|---|---|---|
| Qe (mg/g) | f1 | f2 | k1 (h−1) | k2 (h−1) | R2 | ||
| SMX | W500 | 15.21 | 0.82 | 0.18 | 2.83 | 0.09 | 0.96 |
| C500 | 13.97 | 0.72 | 0.28 | 2.46 | 0.13 | 0.98 | |
| W700 | 54.17 | 0.85 | 0.15 | 6.28 | 0.06 | 0.96 | |
| C700 | 60.39 | 0.75 | 0.25 | 4.34 | 0.05 | 0.98 | |
| TMP | W500 | 18.29 | 0.67 | 0.33 | 2.60 | 0.10 | 0.99 |
| C500 | 16.38 | 0.67 | 0.33 | 2.51 | 0.03 | 0.99 | |
| W700 | 55.54 | 0.87 | 0.13 | 5.17 | 0.04 | 0.94 | |
| C700 | 61.68 | 0.78 | 0.23 | 6.23 | 0.03 | 0.99 | |
| Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|
| KL | Qm | R2 | Kf | n | R2 | ||
| SMX | W500 | 0.23 | 19.27 | 0.98 | 8.87 | 0.18 | 0.87 |
| W700 | 7.87 | 54.73 | 0.73 | 42.41 | 0.07 | 0.92 | |
| C500 | 0.43 | 14.33 | 0.97 | 8.09 | 0.13 | 0.93 | |
| C700 | 0.71 | 67.62 | 0.80 | 46.28 | 0.10 | 0.98 | |
| TMP | W500 | 0.34 | 25.63 | 0.90 | 10.75 | 0.21 | 0.96 |
| W700 | 1.01 | 59.3 | 0.83 | 41.58 | 0.1 | 0.98 | |
| C500 | 0.55 | 20.1 | 0.92 | 11.29 | 0.14 | 0.88 | |
| C700 | 1.01 | 73.38 | 0.82 | 48.18 | 0.12 | 0.98 | |
| No. | Filling Medium | Pore Volume (mL) | Porosity | Effective Pore Volume (mL) | Specific Tetention (%) | Column Volume (mL) |
|---|---|---|---|---|---|---|
| 1 | MCS a | 157 | 0.40 | 23.1 | 42.5 | 392.5 |
| 2 | MCS + C500 | 140 | 0.357 | 21.6 | 35.7 | 392.5 |
| 3 | MCS + C700 | 141 | 0.359 | 20.8 | 35.9 | 392.5 |
| 4 | MCS + W500 | 142 | 0.362 | 19.4 | 36.2 | 392.5 |
| 5 | MCS + W700 | 143 | 0.364 | 22.1 | 36.4 | 392.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Yu, H.; Hou, X.; Liu, X.; Bi, E.; Wang, W.; Li, M. Typical Sulfonamide Antibiotics Removal by Biochar-Amended River Coarse Sand during Groundwater Recharge. Int. J. Environ. Res. Public Health 2022, 19, 16957. https://doi.org/10.3390/ijerph192416957
Liu R, Yu H, Hou X, Liu X, Bi E, Wang W, Li M. Typical Sulfonamide Antibiotics Removal by Biochar-Amended River Coarse Sand during Groundwater Recharge. International Journal of Environmental Research and Public Health. 2022; 19(24):16957. https://doi.org/10.3390/ijerph192416957
Chicago/Turabian StyleLiu, Rui, Hechun Yu, Xiaoshu Hou, Xiang Liu, Erping Bi, Wenjing Wang, and Miao Li. 2022. "Typical Sulfonamide Antibiotics Removal by Biochar-Amended River Coarse Sand during Groundwater Recharge" International Journal of Environmental Research and Public Health 19, no. 24: 16957. https://doi.org/10.3390/ijerph192416957
APA StyleLiu, R., Yu, H., Hou, X., Liu, X., Bi, E., Wang, W., & Li, M. (2022). Typical Sulfonamide Antibiotics Removal by Biochar-Amended River Coarse Sand during Groundwater Recharge. International Journal of Environmental Research and Public Health, 19(24), 16957. https://doi.org/10.3390/ijerph192416957






