AMF Inoculum Enhances Crop Yields of Zea mays L. ‘Chenghai No. 618’ and Glycine max L. ‘Zhonghuang No. 17’ without Disturbing Native Fugal Communities in Coal Mine Dump
Abstract
:1. Introduction
2. Materials and Methods
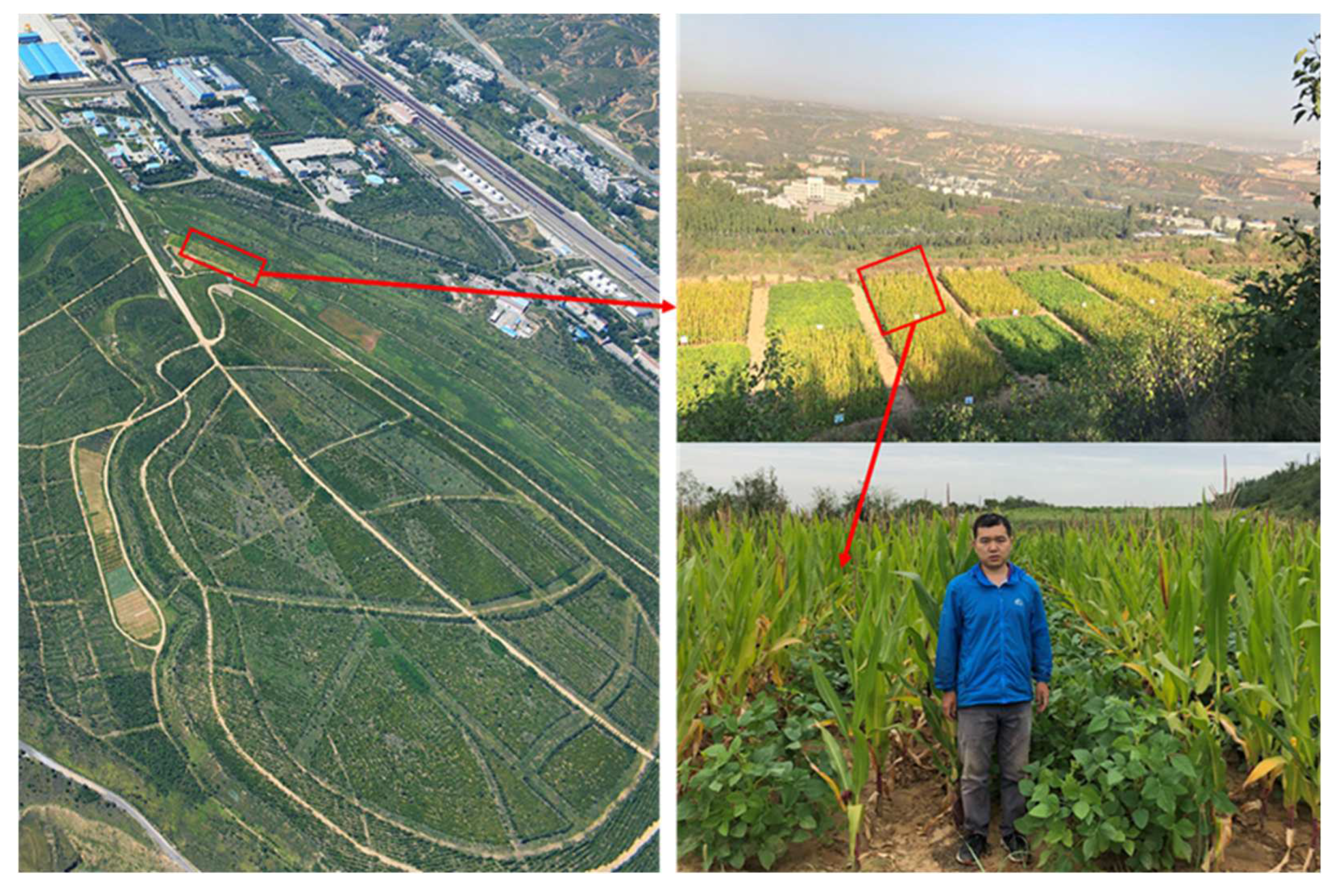
2.1. Study Area
2.2. Experimental Design
2.3. Soil Sampling
2.4. Edaphic Variables
2.5. Molecular Identification of AMF
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of AMF Treatment and Cropping Pattern on the Yield of Maize and Soybean
3.2. Effect of AMF Treatment and Cropping Pattern on Edaphic Variables
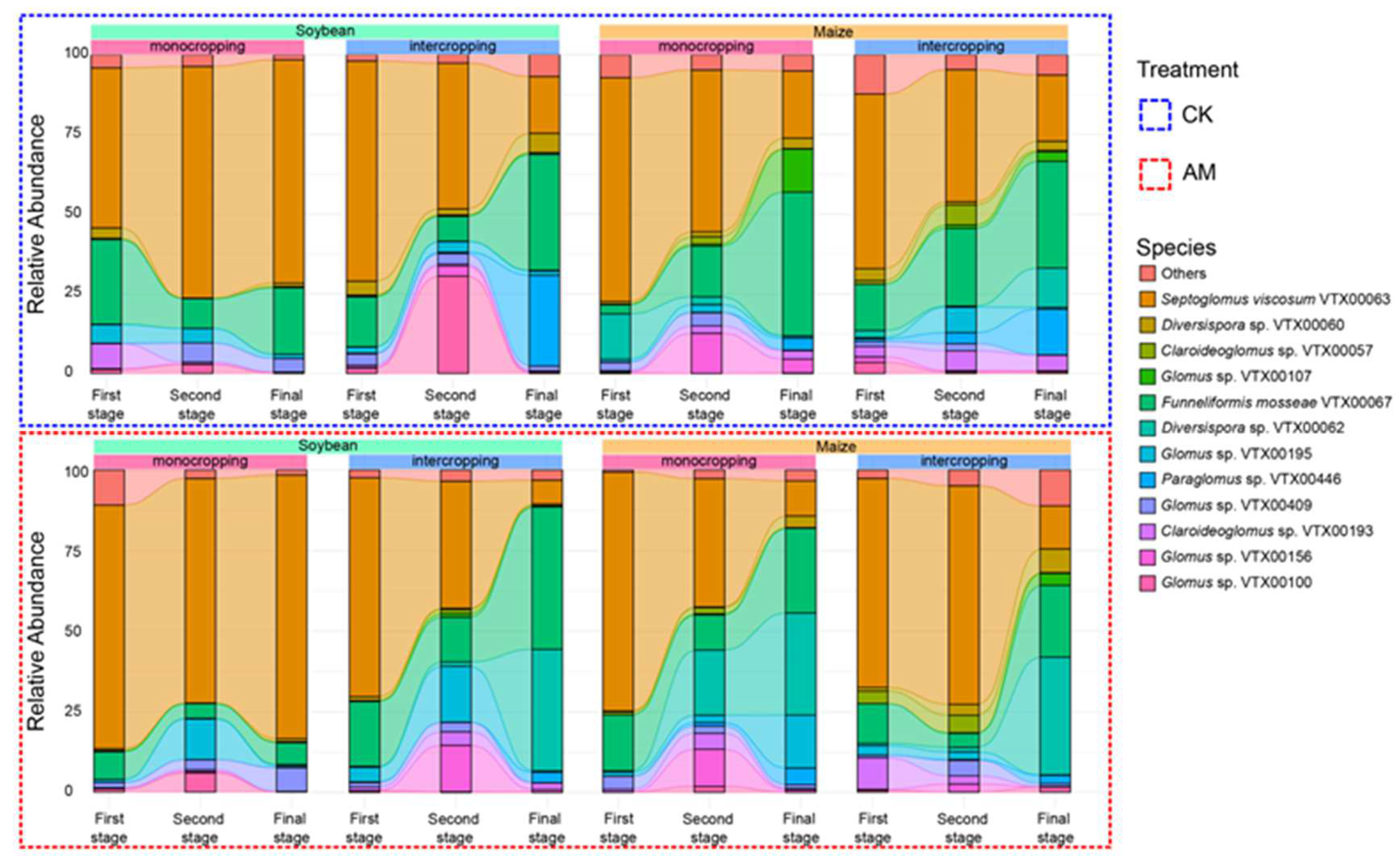
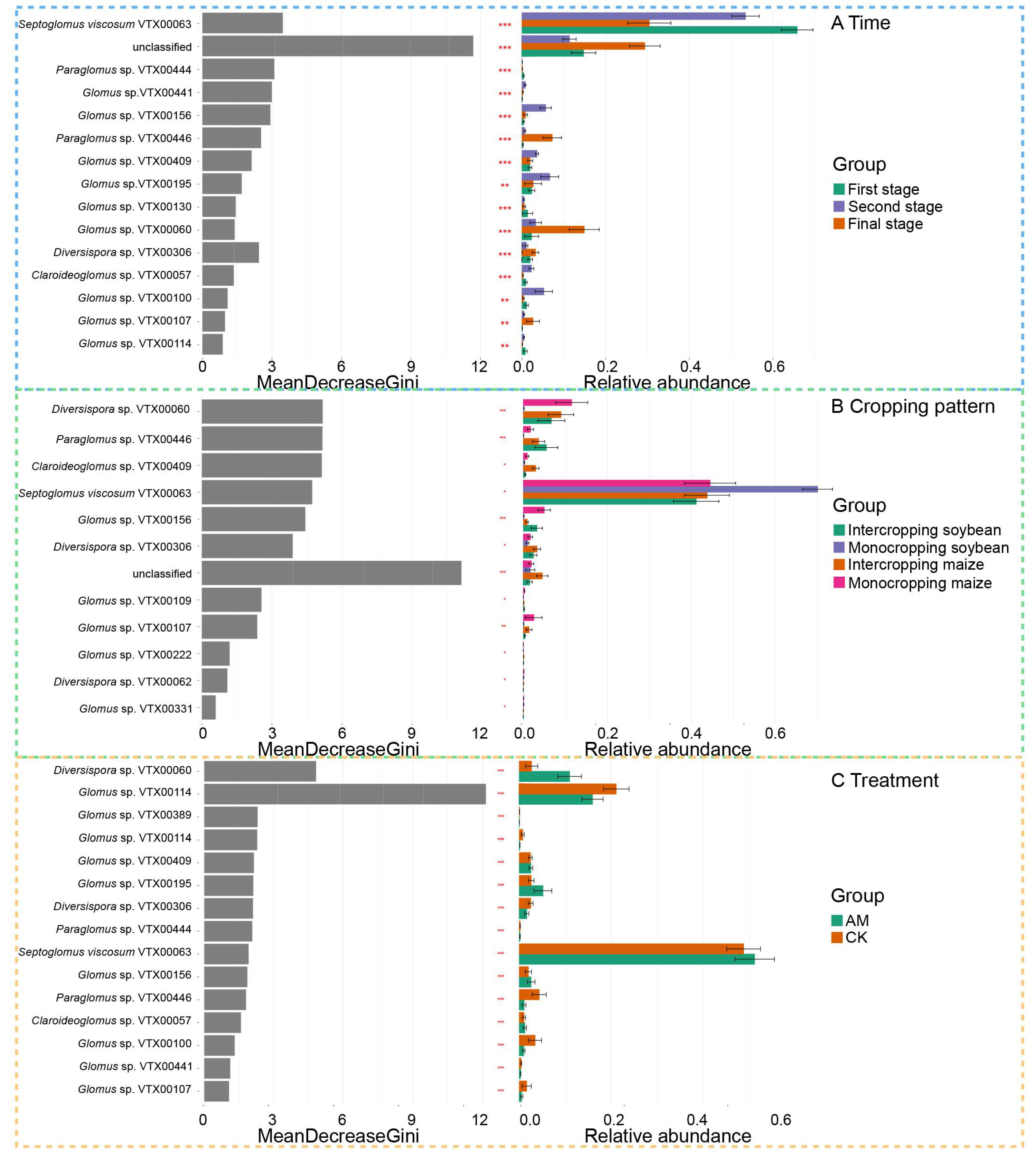
3.3. Effect of AMF Treatment and Cropping Pattern on the Native AMF
3.4. Effect of AMF Treatment and Cropping Pattern on the Diversity of AMF Communities
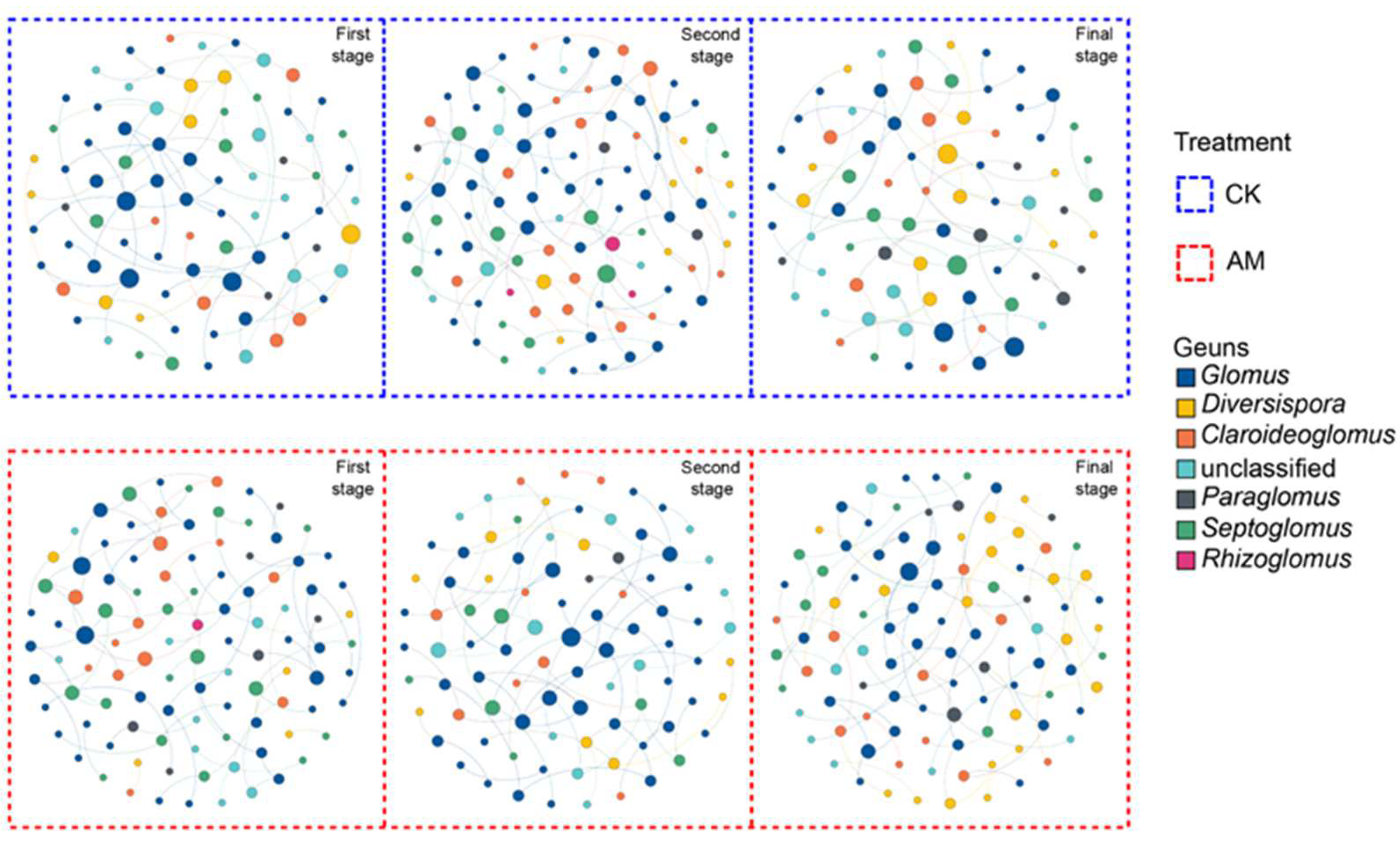
3.5. Network Analyses
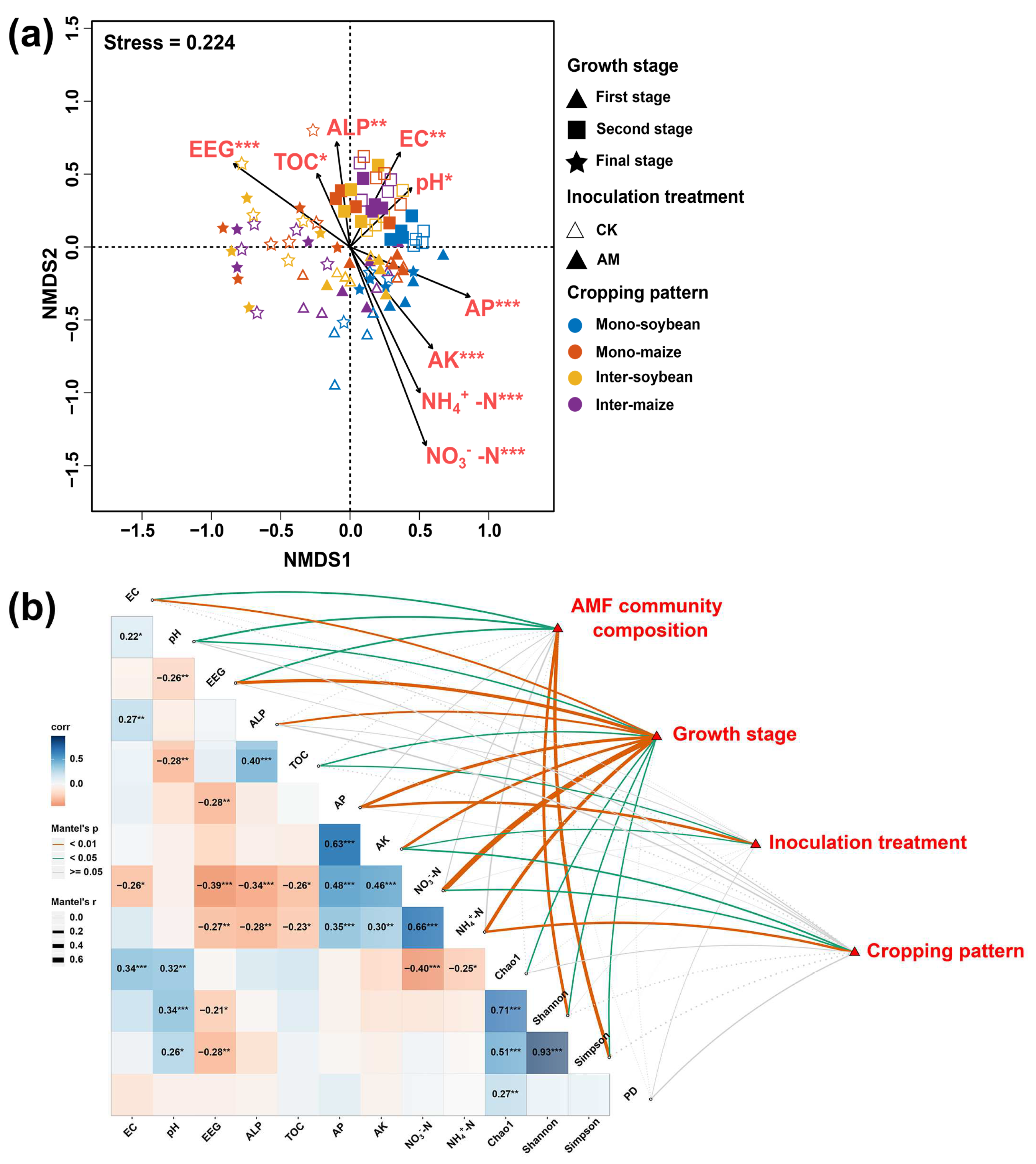
3.6. Correlation Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Xiao, W.; Zhao, Y.; Zhang, W.; Li, S.; Sun, H. Drivers of spatio-temporal ecological vulnerability in an arid, coal mining region in Western China. Ecol. Indic. 2019, 106, 105475. [Google Scholar] [CrossRef]
- Ramani, R.V. Surface mining technology: Progress and prospects. Procedia Eng. 2012, 46, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Wang, K.; Du, S.; Ma, S.; Zhang, J.; Xie, L. Shifts in arbuscular mycorrhizal fungal community composition and edaphic variables during reclamation chronosequence of an open-cast coal mining dump. Catena 2021, 203, 105301. [Google Scholar] [CrossRef]
- Wang, K.; Bi, Y.; Cao, Y.; Peng, S.; Christie, P.; Ma, S.; Zhang, J.; Xie, L. Shifts in composition and function of soil fungal communities and edaphic properties during the reclamation chronosequence of an open-cast coal mining dump. Sci. Total Environ. 2021, 767, 144465. [Google Scholar] [CrossRef]
- D’hondt, K.; Kostic, T.; McDowell, R.; Eudes, F.; Singh, B.K.; Sarkar, S.; Markakis, M.; Schelkle, B.; Maguin, E.; Sessitsch, A. Microbiome innovations for a sustainable future. Nat. Microbiol. 2021, 6, 138–142. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Roy, J.; van der Heijden, M.G.; Yang, G. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Li, S.; Bi, Y.; Kong, W.; Yu, H.; Lang, Q.; Miao, Y. Effects of arbuscular mycorrhizal fungi on ecological restoration in coal mining areas. Russ. J. Ecol. 2015, 46, 431–437. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Bi, Y.-L.; Jiang, B.; Zhakypbek, Y.; Peng, S.-P.; Liu, W.-W.; Liu, H. Arbuscular mycorrhizal fungi enhance soil carbon sequestration in the coalfields, northwest China. Sci. Rep. 2016, 6, 1–11. [Google Scholar]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Thomsen, C.N.; Hart, M.M. Using invasion theory to predict the fate of arbuscular mycorrhizal fungal inoculants. Biol. Invasions 2018, 20, 2695–2706. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field: Is the reward greater than the risk? Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbruggen, E.; van der Heijden, M.G.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Loján, P.; Senés-Guerrero, C.; Suárez, J.P.; Kromann, P.; Schüßler, A.; Declerck, S. Potato field-inoculation in Ecuador with Rhizophagus irregularis: No impact on growth performance and associated arbuscular mycorrhizal fungal communities. Symbiosis 2017, 73, 45–56. [Google Scholar] [CrossRef]
- Sýkorová, Z.; Börstler, B.; Zvolenská, S.; Fehrer, J.; Gryndler, M.; Vosátka, M.; Redecker, D. Long-term tracing of Rhizophagus irregularis isolate BEG140 inoculated on Phalaris arundinacea in a coal mine spoil bank, using mitochondrial large subunit rDNA markers. Mycorrhiza 2012, 22, 69–80. [Google Scholar] [CrossRef]
- Bender, S.F.; Schlaeppi, K.; Held, A.; Van der Heijden, M.G. Establishment success and crop growth effects of an arbuscular mycorrhizal fungus inoculated into Swiss corn fields. Agric. Ecosyst. Environ. 2019, 273, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Symanczik, S.; Courty, P.-E.; Boller, T.; Wiemken, A.; Al-Yahya’ei, M.N. Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non-native AMF species. Mycorrhiza 2015, 25, 639–647. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Abbott, L.K. Unknown risks to soil biodiversity from commercial fungal inoculants. Nat. Ecol. Evol. 2017, 1, 115. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Blackburn, T.M.; Carlton, J.T.; Dick, J.T.; Hulme, P.E.; Iacarella, J.C.; Jeschke, J.M.; Liebhold, A.M.; Lockwood, J.L.; MacIsaac, H.J. Invasion science: A horizon scan of emerging challenges and opportunities. Trends Ecol. Evol. 2017, 32, 464–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Shrestha, S.; Kunwar, S.; Tseng, T.-M. Crop diversification for improved weed management: A review. Agriculture 2021, 11, 461. [Google Scholar] [CrossRef]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Brahmachari, K.; Shankar, T.; Bhadra, P.; Palai, J.B. Intercropping—A low input agricultural strategy for food and environmental security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Hao, W.-Y.; Ren, L.-X.; Ran, W.; Shen, Q.-R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Broadbent, A.A.; Snell, H.S.; Michas, A.; Pritchard, W.J.; Newbold, L.; Cordero, I.; Goodall, T.; Schallhart, N.; Kaufmann, R.; Griffiths, R.I. Climate change alters temporal dynamics of alpine soil microbial functioning and biogeochemical cycling via earlier snowmelt. ISME J. 2021, 15, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Carini, P.; Delgado-Baquerizo, M.; Hinckley, E.-L.S.; Holland-Moritz, H.; Brewer, T.E.; Rue, G.; Vanderburgh, C.; McKnight, D.; Fierer, N. Effects of spatial variability and relic DNA removal on the detection of temporal dynamics in soil microbial communities. MBio 2020, 11, e02776-19. [Google Scholar] [CrossRef] [Green Version]
- Babalola, B.J.; Li, J.; Willing, C.E.; Zheng, Y.; Wang, Y.L.; Gan, H.Y.; Li, X.C.; Wang, C.; Adams, C.A.; Gao, C. Nitrogen fertilization disrupts the temporal dynamics of arbuscular mycorrhizal fungal hyphae but not spore density and community composition in a wheat field. New Phytol. 2022, 234, 2057–2072. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hu, J.; Wang, J.; Yang, J.; Lin, X. Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 2016, 98, 140–149. [Google Scholar] [CrossRef]
- Davison, J.; Öpik, M.; Zobel, M.; Vasar, M.; Metsis, M.; Moora, M. Communities of arbuscular mycorrhizal fungi detected in forest soil are spatially heterogeneous but do not vary throughout the growing season. PLoS ONE 2012, 7, e41938. [Google Scholar] [CrossRef] [PubMed]
- García de León, D.; Moora, M.; Öpik, M.; Neuenkamp, L.; Gerz, M.; Jairus, T.; Vasar, M.; Bueno, C.G.; Davison, J.; Zobel, M. Symbiont dynamics during ecosystem succession: Co-occurring plant and arbuscular mycorrhizal fungal communities. FEMS Microbiol. Ecol. 2016, 92, fiw097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, C.; Kohout, P.; Janoušková, M.; Püschel, D.; Frouz, J.; Rydlová, J. Plant communities rather than soil properties structure arbuscular mycorrhizal fungal communities along primary succession on a mine spoil. Front. Microbiol. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, J.; Reichel, R.; Brüggemann, N.; Hempel, S.; Rillig, M.C. Succession of arbuscular mycorrhizal fungi along a 52-year agricultural recultivation chronosequence. FEMS Microbiol. Ecol. 2017, 93, fix102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarafdar, J.; Marschner, H. Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol. Biochem. 1994, 26, 387–395. [Google Scholar] [CrossRef]
- Janos, D.P.; Garamszegi, S.; Beltran, B. Glomalin extraction and measurement. Soil Biol. Biochem. 2008, 40, 728–739. [Google Scholar] [CrossRef]
- Rillig, M.C.; Caldwell, B.A.; Wösten, H.A.; Sollins, P. Role of proteins in soil carbon and nitrogen storage: Controls on persistence. Biogeochemistry 2007, 85, 25–44. [Google Scholar] [CrossRef]
- Schwarzott, D.; Schüßler, A. A simple and reliable method for SSU rRNA gene DNA extraction, amplification, and cloning from single AM fungal spores. Mycorrhiza 2001, 10, 203–207. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl. Sci. 2005, 51, 179–181. [Google Scholar] [CrossRef]
- Simon, L.; Lalonde, M.; Bruns, T. Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl. Environ. Microbiol. 1992, 58, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Öpik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.; Reier, Ü.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Brunson, J.C. Ggalluvial: Layered grammar for alluvial plots. J. Open Source Softw. 2020, 5, 2017. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. Community Ecol. Package Version 2013, 2, 1–295. [Google Scholar]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Kurtz, Z.D.; Müller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and compositionally robust inference of microbial ecological networks. PLoS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, E.; Bedini, S. Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2014, 68, 429–439. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Barto, E.K.; Powell, J.R.; Rillig, M.C. Mycorrhizal responsiveness trends in annual crop plants and their wild relatives—A meta-analysis on studies from 1981 to 2010. Plant Soil 2012, 355, 231–250. [Google Scholar] [CrossRef]
- Martignoni, M.M.; Garnier, J.; Hart, M.M.; Tyson, R.C. Investigating the impact of the mycorrhizal inoculum on the resident fungal community and on plant growth. Ecol. Model. 2020, 438, 109321. [Google Scholar] [CrossRef]
- Koide, R.T. Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol. 2000, 147, 233–235. [Google Scholar] [CrossRef]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef]
- Jansa, J.; Thonar, C.; Frossard, E. Enhancement of Symbiotic Benefits Through Manipulation of the Mycorrhizal Community Composition. In Proceedings of the Positive Plant Microbial Interactions in Relation to Plant Performance and Ecosystem Function, Grantham, Lincolnshire, UK, 15–16 December 2009; pp. 9–15. [Google Scholar]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Raverkar, K.; Bhattacharya, S. Arbuscular mycorrhizae: Status and potential. In Bioresources for Sustainable Plant Nutrient Management; Chandra, R., Raverkar, K.P., Eds.; Satish Ser. Publ. House: New Delhi, India, 2014; Chapter 10. [Google Scholar]
- Kim, S.J.; Eo, J.-K.; Lee, E.-H.; Park, H.; Eom, A.-H. Effects of arbuscular mycorrhizal fungi and soil conditions on crop plant growth. Mycobiology 2017, 45, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Malusà, E.; Pinzari, F.; Canfora, L. Efficacy of biofertilizers: Challenges to improve crop production. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: Berlin/Heidelberg, Germany, 2016; pp. 17–40. [Google Scholar]
- Hart, M.M.; Klironomos, J.N. Diversity of arbuscular mycorrhizal fungi and ecosystem functioning. In Mycorrhizal Ecology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 225–242. [Google Scholar]
- Pau, S.; Cordell, S.; Ostertag, R.; Inman, F.; Sack, L. Climatic sensitivity of species’ vegetative and reproductive phenology in a Hawaiian montane wet forest. Biotropica 2020, 52, 825–835. [Google Scholar] [CrossRef]
- Aguilar-Trigueros, C.A.; Hempel, S.; Powell, J.R.; Cornwell, W.K.; Rillig, M.C. Bridging reproductive and microbial ecology: A case study in arbuscular mycorrhizal fungi. ISME J. 2019, 13, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [Green Version]
- Torrecillas, E.; Alguacil, M.; Roldán, A. Host preferences of arbuscular mycorrhizal fungi colonizing annual herbaceous plant species in semiarid Mediterranean prairies. Appl. Environ. Microbiol. 2012, 78, 6180–6186. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.G.; Zhang, B.; Yuan, Z.; Fu, Z.; Yuan, Y.; Zhu, L.; Ma, S.; Zhang, J. Arbuscular mycorrhizal fungi associated with tree species in a planted forest of eastern China. Forests 2019, 10, 424. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Sheng, L.; Zhao, D.; Sheng, J.; Wang, X.; Liao, H. Allocation of nitrogen and carbon is regulated by nodulation and mycorrhizal networks in soybean/maize intercropping system. Front. Plant Sci. 2016, 7, 1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, E.S. Grain yield, symbiotic N2 fixation and interspecific competition for inorganic N in pea-barley intercrops. Plant Soil 1996, 182, 25–38. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, W.; Sun, J.; Müller, C.; Lei, B. Effects of wheat/faba bean intercropping on soil nitrogen transformation processes. J. Soils Sediments 2019, 19, 1724–1734. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.; Jin, J.; Liu, J.; Zhang, Q.; Liu, X. Bacterial communities in soybean rhizosphere in response to soil type, soybean genotype, and their growth stage. Soil Biol. Biochem. 2009, 41, 919–925. [Google Scholar] [CrossRef]
- Gottshall, C.B.; Cooper, M.; Emery, S.M. Activity, diversity and function of arbuscular mycorrhizae vary with changes in agricultural management intensity. Agric. Ecosyst. Environ. 2017, 241, 142–149. [Google Scholar]
- Mommer, L.; Van Ruijven, J.; De Caluwe, H.; Smit-Tiekstra, A.E.; Wagemaker, C.A.; Joop Ouborg, N.; Bögemann, G.M.; Van Der Weerden, G.M.; Berendse, F.; De Kroon, H. Unveiling below-ground species abundance in a biodiversity experiment: A test of vertical niche differentiation among grassland species. J. Ecol. 2010, 98, 1117–1127. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, N.; Zhang, J.; Hu, Y.; Cai, D.; Guo, J.; Wu, D.; Sun, G. Soil physicochemical properties and the rhizosphere soil fungal community in a mulberry (Morus alba L.)/Alfalfa (Medicago sativa L.) intercropping system. Forests 2019, 10, 167. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P.; Crowley, D.; Yang, C.H. Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 2004, 261, 199–208. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W. Effects of different 3-year cropping systems on soil microbial communities and Rhizoctonia diseases of potato. Phytopathology 2006, 96, 68–79. [Google Scholar] [CrossRef] [PubMed]



| Source of Variation | Growth Stage (G) | Inoculation Treatment (I) | Cropping Pattern (C) | G × I | G × C | I × C | G × I × C |
|---|---|---|---|---|---|---|---|
| EC | <0.001 | ≥0.05 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| pH | <0.01 | <0.05 | ≥0.05 | <0.01 | <0.001 | ≥0.05 | <0.001 |
| EEG | <0.001 | ≥0.05 | <0.001 | <0.01 | <0.001 | ≥0.05 | <0.05 |
| ALP | <0.001 | ≥0.05 | <0.001 | ≥0.05 | <0.001 | <0.01 | <0.05 |
| TOC | <0.05 | <0.01 | <0.05 | ≥0.05 | <0.001 | <0.001 | <0.001 |
| AP | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| AK | ≥0.05 | <0.001 | <0.001 | ≥0.05 | ≥0.05 | <0.001 | ≥0.05 |
| NO3−-N | <0.001 | <0.05 | <0.001 | <0.01 | <0.001 | <0.01 | <0.001 |
| NH4+-N | <0.001 | ≥0.05 | <0.001 | <0.01 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Bi, Y.; Zhang, J.; Ma, S. AMF Inoculum Enhances Crop Yields of Zea mays L. ‘Chenghai No. 618’ and Glycine max L. ‘Zhonghuang No. 17’ without Disturbing Native Fugal Communities in Coal Mine Dump. Int. J. Environ. Res. Public Health 2022, 19, 17058. https://doi.org/10.3390/ijerph192417058
Wang K, Bi Y, Zhang J, Ma S. AMF Inoculum Enhances Crop Yields of Zea mays L. ‘Chenghai No. 618’ and Glycine max L. ‘Zhonghuang No. 17’ without Disturbing Native Fugal Communities in Coal Mine Dump. International Journal of Environmental Research and Public Health. 2022; 19(24):17058. https://doi.org/10.3390/ijerph192417058
Chicago/Turabian StyleWang, Kun, Yinli Bi, Jiayu Zhang, and Shaopeng Ma. 2022. "AMF Inoculum Enhances Crop Yields of Zea mays L. ‘Chenghai No. 618’ and Glycine max L. ‘Zhonghuang No. 17’ without Disturbing Native Fugal Communities in Coal Mine Dump" International Journal of Environmental Research and Public Health 19, no. 24: 17058. https://doi.org/10.3390/ijerph192417058
APA StyleWang, K., Bi, Y., Zhang, J., & Ma, S. (2022). AMF Inoculum Enhances Crop Yields of Zea mays L. ‘Chenghai No. 618’ and Glycine max L. ‘Zhonghuang No. 17’ without Disturbing Native Fugal Communities in Coal Mine Dump. International Journal of Environmental Research and Public Health, 19(24), 17058. https://doi.org/10.3390/ijerph192417058






