Uncovering Evidence: Associations between Environmental Contaminants and Disparities in Women’s Health
Abstract
1. What Are Environmental Contaminants?
1.1. Persistent Organic Pollutants, Endocrine Disrupting Chemicals, and Heavy Metals
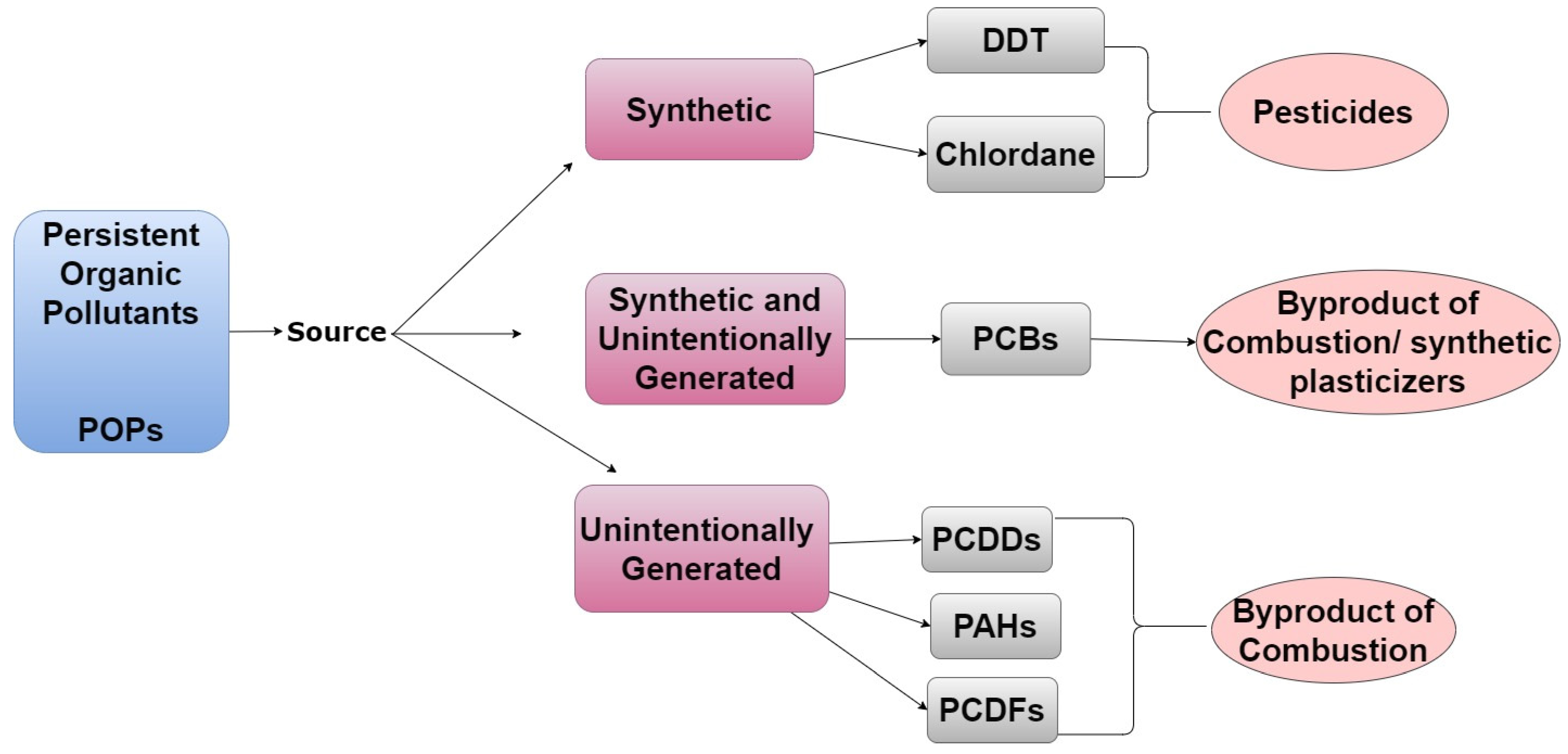
1.1.1. Examples of POPs
1.1.2. Examples of EDCs
1.1.3. Examples of Heavy Metals
1.2. Risk Factors Associated with Human Exposure to Environmental Contaminants
1.2.1. Socioeconomic Status, Occupation, and Geographic Locale
1.2.2. Diet
1.2.3. Use of Personal Care Products
2. Scope of Review
3. Breast Cancer
4. Endometriosis and Endometriosis-Related Infertility
5. Polycystic Ovarian Syndrome
6. Uterine Fibroids
7. Premature Birth
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloyd-Smith, M.; Sheffield-Brotherton, B. Children’s environmental health: Intergenerational equity in action—A civil society perspective. Ann. N. Y. Acad. Sci. 2008, 1140, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Agency, E.P. Toxic Substance Control Act Inventory. 2021. Available online: https://www.epa.gov/tsca-inventory (accessed on 1 August 2021).
- Cagnetta, G.; Hassan, M.M.; Huang, J.; Yu, G.; Weber, R. Dioxins reformation and destruction in secondary copper smelting fly ash under ball milling. Sci. Rep. 2016, 6, 22925. [Google Scholar] [CrossRef] [PubMed]
- Muto, H.; Takizawa, Y. Dioxins in cigarette smoke. Arch. Environ. Health 1989, 44, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Rumph, J.T.; Stephens, V.R.; Archibong, A.E.; Osteen, K.G.; Bruner-Tran, K.L. Environmental Endocrine Disruptors and Endometriosis. Adv. Anat. Embryol. Cell Biol. 2020, 232, 57–78. [Google Scholar]
- Bonefeld-Jørgensen, E.C.; Ghisari, M.; Wielsøe, M.; Bjerregaard-Olesen, C.; Kjeldsen, L.S.; Long, M. Biomonitoring and hormone-disrupting effect biomarkers of persistent organic pollutants in vitro and ex vivo. Basic Clin. Pharmacol. Toxicol. 2014, 115, 118–128. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP technical report on the toxicology and carcinogenesis studies of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (Gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2006, 529, 4–168. [Google Scholar]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Ye, S.; Chung, H.W.; Jeong, K.; Sung, Y.A.; Lee, H.; Park, S.Y.; Kim, H.; Ha, E.H. Blood cadmium and volume of uterine fibroids in premenopausal women. Ann. Occup. Environ. Med. 2017, 29, 22. [Google Scholar] [CrossRef]
- Jackson, L.W.; Zullo, M.D.; Goldberg, J.M. The association between heavy metals, endometriosis and uterine myomas among premenopausal women: National Health and Nutrition Examination Survey 1999–2002. Hum. Reprod. 2008, 23, 679–687. [Google Scholar] [CrossRef]
- Johnstone, E.B.; Louis, G.M.; Parsons, P.J.; Steuerwald, A.J.; Palmer, C.D.; Chen, Z.; Sun, L.; Hammoud, A.O.; Dorais, J.; Peterson, C.M. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod. Toxicol. 2014, 49, 27–32. [Google Scholar] [CrossRef]
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S.; et al. Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology 2003, 144, 2425–2436. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Jaga, K. What are the implications of the interaction between DDT and estrogen receptors in the body? Med. Hypotheses 2000, 54, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kelce, W.R.; Stone, C.R.; Laws, S.C.; Gray, L.E.; Kemppainen, J.A.; Wilson, E.M. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature 1995, 375, 581–585. [Google Scholar] [CrossRef]
- Corsini, E.; Luebke, R.W.; Germolec, D.R.; DeWitt, J.C. Perfluorinated compounds: Emerging POPs with potential immunotoxicity. Toxicol. Lett. 2014, 230, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zlatnik, M.G. Endocrine-Disrupting Chemicals and Reproductive Health. J. Midwifery Women’s Health 2016, 61, 442–455. [Google Scholar] [CrossRef]
- Faroon, O.; Ruiz, P. Polychlorinated biphenyls: New evidence from the last decade. Toxicol. Ind. Health 2016, 32, 1825–1847. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, K.; Gadupudi, G.S.; Lehmler, H.J.; Ludewig, G.; Duffel, M.W.; Robertson, L.W. Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs). Environ. Sci. Pollut. Res. Int. 2018, 25, 16277–16290. [Google Scholar] [CrossRef] [PubMed]
- Hutzinger, O.; Choudhry, G.G.; Chittim, B.G.; Johnston, L.E. Formation of polychlorinated dibenzofurans and dioxins during combustion, electrical equipment fires and PCB incineration. Environ. Health Perspect. 1985, 60, 3–9. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, E.N.; Hajeb, P.; Selamat, J.; Abdull Razis, A.F. Polycyclic Aromatic Hydrocarbons (PAHs) and their Bioaccessibility in Meat: A Tool for Assessing Human Cancer Risk. Asian Pac. J. Cancer Prev. 2016, 17, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Rathee, M.; Malik, P.; Singh, J. Bisphenol A in dental sealants and its estrogen like effect. Indian J. Endocrinol. Metab. 2012, 16, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Hartle, J.C.; Navas-Acien, A.; Lawrence, R.S. The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environ. Res. 2016, 150, 375–382. [Google Scholar] [CrossRef]
- Kendig, E.L.; Buesing, D.R.; Christie, S.M.; Cookman, C.J.; Gear, R.B.; Hugo, E.R.; Kendziorski, J.A.; Ungi, K.R.; Williams, K.; Belcher, S.M. Estrogen-like disruptive effects of dietary exposure to bisphenol A or 17α-ethinyl estradiol in CD1 mice. Int. J. Toxicol. 2012, 31, 537–550. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and hormone-associated cancers: Current progress and perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef]
- McDonough, C.M.; Xu, H.S.; Guo, T.L. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: Insights from a multi-species comparison. Crit. Rev. Toxicol. 2021, 51, 283–300. [Google Scholar] [CrossRef]
- Parlett, L.E.; Calafat, A.M.; Swan, S.H. Women’s exposure to phthalates in relation to use of personal care products. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 197–206. [Google Scholar] [CrossRef]
- Schechter, T.; Finkelstein, Y.; Koren, G. Pregnant “DES daughters” and their offspring. Can. Fam. Physician Med. Fam. Can. 2005, 51, 493–494. [Google Scholar]
- Jukes, T.H. Diethylstilbestrol in beef production: What is the risk to consumers? Prev. Med. 1976, 5, 438–453. [Google Scholar] [CrossRef]
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 1971, 284, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public Health 2009, 99 (Suppl. S3), S559–S566. [Google Scholar] [CrossRef] [PubMed]
- Wirbisky, S.E.; Freeman, J.L. Atrazine Exposure and Reproductive Dysfunction through the Hypothalamus-Pituitary-Gonadal (HPG) Axis. Toxics 2015, 3, 414–450. [Google Scholar] [CrossRef] [PubMed]
- Nessa, F.; Khan, S.A.; Abu Shawish, K.Y.I. Lead, Cadmium and Nickel Contents of Some Medicinal Agents. Indian J. Pharm. Sci. 2016, 78, 111–119. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Liu, X.; Liu, X.; Li, X.; Ren, Y.; Wang, J.; Dong, L. Partitioning and geochemical fractions of heavy metals from geogenic and anthropogenic sources in various soil particle size fractions. Geoderma 2018, 312, 104–113. [Google Scholar] [CrossRef]
- Kim, C.-H.; Yoo, D.-C.; Kwon, Y.-M.; Han, W.-S.; Kim, G.-S.; Park, M.-J.; Kim, Y.S.; Choi, D. A study on characteristics of atmospheric heavy metals in subway station. Toxicol. Res. 2010, 26, 157–162. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Rauh, V.A.; Galvez, M.P. Environmental justice and the health of children. Mt. Sinai J. Med. 2010, 77, 178–187. [Google Scholar] [CrossRef]
- Pasetto, R.; Mattioli, B.; Marsili, D. Environmental Justice in Industrially Contaminated Sites: A Review of Scientific Evidence in the WHO European Region. Int. J. Environ. Res. Public Health 2019, 16, 998. [Google Scholar] [CrossRef]
- Maantay, J. Mapping environmental injustices: Pitfalls and potential of geographic information systems in assessing environmental health and equity. Environ. Health Perspect. 2002, 110 (Suppl. S2), 161–171. [Google Scholar] [CrossRef]
- Mikati, I.; Benson, A.F.; Luben, T.J.; Sacks, J.D.; Richmond-Bryant, J. Disparities in Distribution of Particulate Matter Emission Sources by Race and Poverty Status. Am. J. Public Health 2018, 108, 480–485. [Google Scholar] [CrossRef]
- Li, Z.; Konisky, D.M.; Zirogiannis, N. Racial, ethnic, and income disparities in air pollution: A study of excess emissions in Texas. PLoS ONE 2019, 14, e0220696. [Google Scholar] [CrossRef] [PubMed]
- Kaushiva, A.; Kresovich, J.; Erdal, S.; Rauscher, G. Abstract D096: Disparities in toxic heavy metal burden and breast cancer risk: Findings from the Metropolitan Chicago Breast Cancer Registry. Cancer Epidemiol. Biomark. Prev. 2020, 29 (Suppl. S2), D096. [Google Scholar]
- Geretto, M.; Ferrari, M.; De Angelis, R.; Crociata, F.; Sebastiani, N.; Pulliero, A.; Au, W.; Izzotti, A. Occupational Exposures and Environmental Health Hazards of Military Personnel. Int. J. Environ. Res. Public Health 2021, 18, 5395. [Google Scholar] [CrossRef] [PubMed]
- Filippidou, E.-C.; Tsacheva, N. 007. Occupational exposure to chemical agents and its impact on the respiratory system. J. Thorac. Dis. 2015, 7 (Suppl. S1), AB007. [Google Scholar]
- Ash, M.; Boyce, J.K. Racial disparities in pollution exposure and employment at US industrial facilities. Proc. Natl. Acad. Sci. USA 2018, 115, 10636–10641. [Google Scholar] [CrossRef] [PubMed]
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Mannocci, A.; Ciarlo, I.; D’Egidio, V.; Del Cimmuto, A.; de Giusti, M.; Villari, P.; La Torre, G. Socioeconomic Deprivation Status and Air Pollution by PM10 and NO2: An Assessment at Municipal Level of 11 Years in Italy. J. Environ. Public Health 2019, 2019, 2058467. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Franchini, M. Health Effects of Ambient Air Pollution in Developing Countries. Int. J. Environ. Res. Public Health 2017, 14, 1048. [Google Scholar] [CrossRef]
- Bede-Ojimadu, O.; Orisakwe, O.E. Exposure to Wood Smoke and Associated Health Effects in Sub-Saharan Africa: A Systematic Review. Ann. Glob. Health 2020, 86, 32. [Google Scholar] [CrossRef]
- Weinstein, J.R.; Asteria-Peñaloza, R.; Diaz-Artiga, A.; Davila, G.; Hammond, S.K.; Ryde, I.T.; Meyer, J.N.; Benowitz, N.; Thompson, L.M. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds among recently pregnant rural Guatemalan women cooking and heating with solid fuels. Int. J. Hyg. Environ. Health 2017, 220, 726–735. [Google Scholar] [CrossRef]
- Chiang, T.A.; Wu, P.F.; Ko, Y.C. Identification of carcinogens in cooking oil fumes. Environ. Res. 1999, 81, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Viau, C.; Hakizimana, G.; Bouchard, M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int. Arch. Occup. Environ. Health 2000, 73, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bharat, G.K.; Chakraborty, P.; Martiník, J.; Audy, O.; Kukučka, P.; Přibylová, P.; Kukreti, P.K.; Sharma, A.; Kalina, J.; et al. A comprehensive assessment of endocrine-disrupting chemicals in an Indian food basket: Levels, dietary intakes, and comparison with European data. Environ. Pollut. 2021, 288, 117750. [Google Scholar] [CrossRef] [PubMed]
- Law, A.Y.S.; Wei, X.; Zhang, X.; Mak, N.K.; Cheung, K.C.; Wong, M.H.; Giesy, J.P.; Wong, C.K.C. Biological analysis of endocrine-disrupting chemicals in animal meats from the Pearl River Delta, China. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Muncke, J. Exposure to endocrine disrupting compounds via the food chain: Is packaging a relevant source? Sci. Total Environ. 2009, 407, 4549–4559. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef]
- Zota, A.R.; Shamasunder, B. The environmental injustice of beauty: Framing chemical exposures from beauty products as a health disparities concern. Am. J. Obstet. Gynecol. 2017, 217, 418.e1–418.e6. [Google Scholar] [CrossRef]
- Branch, F.; Woodruff, T.J.; Mitro, S.D.; Zota, A.R. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environ. Health 2015, 14, 57. [Google Scholar] [CrossRef]
- James-Todd, T.; Senie, R.; Terry, M.B. Racial/ethnic differences in hormonally-active hair product use: A plausible risk factor for health disparities. J. Immigr. Minor. Health 2012, 14, 506–511. [Google Scholar] [CrossRef]
- Tiwary, C.M. Premature sexual development in children following the use of estrogen- or placenta-containing hair products. Clin. Pediatr. 1998, 37, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, C.M.; Ward, J.A. Use of hair products containing hormone or placenta by US military personnel. J. Pediatr. Endocrinol. Metab. 2003, 16, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- James-Todd, T.; Connolly, L.; Preston, E.V.; Quinn, M.R.; Plotan, M.; Xie, Y.; Gandi, B.; Mahalingaiah, S. Hormonal activity in commonly used Black hair care products: Evaluating hormone disruption as a plausible contribution to health disparities. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Becerra, M.; Jagai, J.S.; Ard, K.; Sargis, R.M. Disparities in Environmental Exposures to Endocrine-Disrupting Chemicals and Diabetes Risk in Vulnerable Populations. Diabetes Care 2018, 41, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Millikan, R.; DeVoto, E.; Duell, E.J.; Tse, C.-K.; Savitz, D.A.; Beach, J.; Edmiston, S.; Jackson, S.; Newman, B. Dichlorodiphenyldichloroethene, Polychlorinated Biphenyls, and Breast Cancer among African-American and White Women in North Carolina. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1233–1240. [Google Scholar]
- Rogan, W.J.; Gladen, B.C.; McKinney, J.D.; Carreras, N.; Hardy, P.; Thullen, J.; Tingelstad, J.; Tully, M. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: Effects of maternal factors and previous lactation. Am. J. Public Health 1986, 76, 172–177. [Google Scholar] [CrossRef]
- Acheampong, T.; Kehm, R.D.; Terry, M.B.; Argov, E.L.; Tehranifar, P. Incidence Trends of Breast Cancer Molecular Subtypes by Age and Race/Ethnicity in the US From 2010 to 2016. JAMA Netw. Open 2020, 3, e2013226. [Google Scholar] [CrossRef]
- Lamb, C.A.; Vanzulli, S.I.; Lanari, C. Hormone receptors in breast cancer: More than estrogen receptors. Medicina 2019, 79, 540–545. [Google Scholar]
- Miranda, F.; Prazeres, H.; Mendes, F.; Martins, D.; Schmitt, F. Resistance to endocrine therapy in HR + and/or HER2 + breast cancer: The most promising predictive biomarkers. Mol. Biol. Rep. 2021, 49, 717–733. [Google Scholar] [CrossRef]
- Ryu, W.J.; Sohn, J.H. Molecular Targets and Promising Therapeutics of Triple-Negative Breast Cancer. Pharmaceuticals 2021, 14, 1008. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; Fonseca, D.D.; Alo, R.A.; Payton, M.; Tchounwou, P.B. Health and Racial Disparity in Breast Cancer. Adv. Exp. Med. Biol. 2019, 1152, 31–49. [Google Scholar] [PubMed]
- Biancolella, M.; Ouédraogo, N.L.M.; Zongo, N.; Zohoncon, T.M.; Testa, B.; Rizzacasa, B.; Latini, A.; Conte, C.; Compaore, T.R.; Ouedraogo, C.M.R.; et al. Breast cancer in West Africa: Molecular analysis of BRCA genes in early-onset breast cancer patients in Burkina Faso. Hum. Genom. 2021, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.V.; Liu, D.; Shen, Y.; Olamigoke, O.O.; Lakomy, D.S.; Barrera, A.M.G.; Stecklein, S.R.; Sawakuchi, G.O.; Bright, S.J.; Bedrosian, I.; et al. Outcomes After Breast Radiation Therapy in a Diverse Patient Cohort With a Germline BRCA1/2 Mutation. Int. J. Radiat. Oncol. Biol. Phys. 2021, 112, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Beverly, L.N.; Flanders, W.D.; Go, R.C.; Soong, S.J. A comparison of estrogen and progesterone receptors in black and white breast cancer patients. Am. J. Public Health 1987, 77, 351–353. [Google Scholar] [CrossRef]
- Gabriel, C.A.; Mitra, N.; Demichele, A.; Rebbeck, T. Association of progesterone receptor gene (PGR) variants and breast cancer risk in African American women. Breast Cancer Res. Treat. 2013, 139, 833–843. [Google Scholar] [CrossRef][Green Version]
- Roberts, M.R.; Sucheston-Campbell, L.E.; Zirpoli, G.R.; Higgins, M.; Freudenheim, J.L.; Bandera, E.V.; Ambrosone, C.B.; Yao, S. Single nucleotide variants in metastasis-related genes are associated with breast cancer risk, by lymph node involvement and estrogen receptor status, in women with European and African ancestry. Mol. Carcinog. 2017, 56, 1000–1009. [Google Scholar] [CrossRef]
- Koual, M.; Tomkiewicz, C.; Guerrera, I.C.; Sherr, D.; Barouki, R.; Coumoul, X. Aggressiveness and Metastatic Potential of Breast Cancer Cells Co-Cultured with Preadipocytes and Exposed to an Environmental Pollutant Dioxin: An In Vitro and In Vivo Zebrafish Study. Environ. Health Perspect. 2021, 129, 37002. [Google Scholar] [CrossRef]
- Lamba, J.K.; Lin, Y.S.; Schuetz, E.G.; Thummel, K.E. Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug Deliv. Rev. 2002, 54, 1271–1294. [Google Scholar] [CrossRef]
- Reynolds, P.; Hurley, S.E.; Petreas, M.; Goldberg, D.E.; Smith, D.; Gilliss, D.; Mahoney, M.E.; Jeffrey, S.S. Adipose levels of dioxins and risk of breast cancer. Cancer Causes Control 2005, 16, 525–535. [Google Scholar] [CrossRef]
- Muscat, J.E.; Britton, J.A.; Djordjevic, M.V.; Citron, M.L.; Kemeny, M.; Busch-Devereaux, E.; Pittman, B.; Stellman, S.D. Adipose concentrations of organochlorine compounds and breast cancer recurrence in Long Island, New York. Cancer Epidemiol. Prev. Biomark. 2003, 12, 1474–1478. [Google Scholar]
- Parada, H., Jr.; Sun, X.; Tse, C.K.; Engel, L.S.; Hoh, E.; Olshan, A.F.; Troester, M.A. Plasma levels of polychlorinated biphenyls (PCBs) and breast cancer mortality: The Carolina Breast Cancer Study. Int. J. Hyg. Environ. Health 2020, 227, 113522. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk, A.; Lyndin, M.; Sikora, V.; Lyndina, Y.; Romaniuk, S.; Sikora, K. Heavy metals effect on breast cancer progression. J. Occup. Med. Toxicol. 2017, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Weinberg, C.R.; O’Meara, E.S.; Sandler, D.P.; Sprague, B.L. Airborne metals and polycyclic aromatic hydrocarbons in relation to mammographic breast density. Breast Cancer Res. 2019, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Brosens, I.; Benagiano, G. Endometriosis, a modern syndrome. Indian J. Med. Res. 2011, 133, 581–593. [Google Scholar]
- Chatman, D.L. Endometriosis in the black woman. Am. J. Obstet. Gynecol. 1976, 125, 987–989. [Google Scholar] [CrossRef]
- Bougie, O.; Yap, M.I.; Sikora, L.; Flaxman, T.; Singh, S. Influence of race/ethnicity on prevalence and presentation of endometriosis: A systematic review and meta-analysis. BJOG 2019, 126, 1104–1115. [Google Scholar] [CrossRef]
- Stillman, R.J.; Miller, L.C. Diethylstilbestrol exposure in utero and endometriosis in infertile females. Fertil. Steril. 1984, 41, 369–372. [Google Scholar] [CrossRef]
- Homberger, E.; Reggiani, G.; Sambeth, J.; Wipf, H.K. The Seveso accident: Its nature, extent and consequences. Ann. Occup. Hyg. 1979, 22, 327–367. [Google Scholar]
- Eskenazi, B.; Mocarelli, P.; Warner, M.; Samuels, S.; Vercellini, P.; Olive, D.; Needham, L.L.; Patterson, D.G., Jr.; Brambilla, P.; Gavoni, N.; et al. Serum dioxin concentrations and endometriosis: A cohort study in Seveso, Italy. Environ. Health. Perspect. 2002, 110, 629–634. [Google Scholar] [CrossRef]
- Warner, M.; Mocarelli, P.; Brambilla, P.; Wesselink, A.; Patterson, D.G., Jr.; Turner, W.E.; Eskenazi, B. Serum TCDD and TEQ concentrations among Seveso women, 20 years after the explosion. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Gnecco, J.; Ding, T.; Glore, D.R.; Pensabene, V.; Osteen, K.G. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: Translating lessons from murine models. Reprod. Toxicol. 2017, 68, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Potera, C. Women’s Health: Endometriosis and PCB Exposure. Environ. Health Perspect. 2006, 114, A404. [Google Scholar] [CrossRef]
- Rier, S.E.; Martin, D.C.; Bowman, R.E.; Dmowski, W.P.; Becker, J.L. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1993, 21, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.-L.; Wei, H.-J.; Ho, H.-Y.; Liao, K.-W.; Chien, L.-C. Relationship between risk factors for infertility in women and lead, cadmium, and arsenic blood levels: A cross-sectional study from Taiwan. BMC Public Health 2015, 15, 1220. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, A.K.; Bethea, T.N.; McClean, M.; Weuve, J.; Williams, P.L.; Hauser, R.; Sjödin, A.; Brasky, T.M.; Baird, D.D.; Wise, L.A. Predictors of plasma polychlorinated biphenyl concentrations among reproductive-aged black women. Int. J. Hyg. Environ. Health 2019, 222, 1001–1010. [Google Scholar] [CrossRef]
- de Abreu, L.G.; Romão, G.S.; Dos Reis, R.M.; Ferriani, R.A.; De Sá, M.F.; De Moura, M.D. Reduced aromatase activity in granulosa cells of women with endometriosis undergoing assisted reproduction techniques. Gynecol. Endocrinol. 2006, 22, 432–436. [Google Scholar] [CrossRef]
- Toya, M.; Saito, H.; Ohta, N.; Saito, T.; Kaneko, T.; Hiroi, M. Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil. Steril. 2000, 73, 344–350. [Google Scholar] [CrossRef]
- Ono, Y.J.; Tanabe, A.; Nakamura, Y.; Yamamoto, H.; Hayashi, A.; Tanaka, T.; Sasaki, H.; Hayashi, M.; Terai, Y.; Ohmichi, M. A low-testosterone state associated with endometrioma leads to the apoptosis of granulosa cells. PLoS ONE 2014, 9, e115618. [Google Scholar] [CrossRef]
- Rotenberg, O.; Kuo, D.Y.S.; Goldberg, G.L. Use of aromatase inhibitors in menopausal deep endometriosis: A case report and literature review. Climacteric 2021, Oct 25, 5. [Google Scholar] [CrossRef]
- Shaw, N.D.; Srouji, S.S.; Welt, C.K.; Cox, K.H.; Fox, J.H.; Adams, J.M.; Sluss, P.M.; Hall, J.E. Evidence that increased ovarian aromatase activity and expression account for higher estradiol levels in African American compared with Caucasian women. J. Clin. Endocrinol. Metab. 2014, 99, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Economos, K.; Miller, D.; Simpson, E.R. CYP19 (aromatase cytochrome P450) gene expression in human malignant endometrial tumors. J. Clin. Endocrinol. Metab. 1994, 79, 1831–1834. [Google Scholar] [PubMed]
- Kim, M.; Kim, S.H.; Kim, H.J.; Whang, D.H.; Yun, S.C.; Lee, S.R.; Chae, H.D.; Kang, B.M. Plasma levels of polychlorinated biphenyl, genetic polymorphisms, and the risk of advanced stage endometriosis. Gynecol. Endocrinol. 2020, 36, 636–640. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.S.; Kazmi, S.Z.; Hann, H.J.; Kang, T.; Cha, J.; Choi, S.; Swan, H.; Kim, H.; Lee, Y.S.; et al. Familial risk for endometriosis and its interaction with smoking, age at menarche and body mass index: A population-based cohort study among siblings. BJOG 2021, 128, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Woods, K.S.; Reyna, R.; Key, T.J.; Knochenhauer, E.S.; Yildiz, B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 2004, 89, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, G.; Gainder, S.; Suri, V.; Sachdeva, N.; Chopra, S. Comparison of the Different PCOS Phenotypes Based on Clinical Metabolic, and Hormonal Profile, and their Response to Clomiphene. Indian J. Endocrinol. Metab. 2019, 23, 326–331. [Google Scholar] [PubMed]
- Ladson, G.; Dodson, W.C.; Sweet, S.D.; Archibong, A.E.; Kunselman, A.R.; Demers, L.M.; Williams, N.I.; Coney, P.; Legro, R.S. Racial influence on the polycystic ovary syndrome phenotype: A black and white case-control study. Fertil. Steril. 2011, 96, 224–229.e2. [Google Scholar] [CrossRef]
- Ding, T.; Hardiman, P.J.; Petersen, I.; Wang, F.F.; Qu, F.; Baio, G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: A systematic review and meta-analysis. Oncotarget 2017, 8, 96351–96358. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Quiñones, M.J.; Azziz, R.; Rotter, J.I.; Hsueh, W.A.; Yang, H. Polycystic ovary syndrome in Mexican-Americans: Prevalence and association with the severity of insulin resistance. Fertil. Steril. 2005, 84, 766–769. [Google Scholar] [CrossRef]
- Wang, S.; Alvero, R. Racial and ethnic differences in physiology and clinical symptoms of polycystic ovary syndrome. Semin. Reprod. Med. 2013, 31, 365–369. [Google Scholar] [CrossRef]
- Maya, J.; Siegel, J.; Cheng, T.Q.; Rousseau-Pierre, T. Prevalence and risk factors of polycystic ovarian syndrome among an ethnically diverse overweight/obese adolescent population. Int. J. Adolesc. Med. Health 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Engmann, L.; Jin, S.; Sun, F.; Legro, R.S.; Polotsky, A.J.; Hansen, K.R.; Coutifaris, C.; Diamond, M.P.; Eisenberg, E.; Zhang, H.; et al. Racial and ethnic differences in the polycystic ovary syndrome metabolic phenotype. Am. J. Obstet. Gynecol. 2017, 216, 493.e1–493.e13. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tsutsumi, O.; Ikezuki, Y.; Takai, Y.; Taketani, Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J. 2004, 51, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, A.; Rachoń, D.; Owczarek, K.; Kubica, P.; Kowalewska, A.; Kudłak, B.; Wasik, A.; Namieśnik, J. Serum bisphenol A concentrations correlate with serum testosterone levels in women with polycystic ovary syndrome. Reprod. Toxicol. 2018, 82, 32–37. [Google Scholar] [CrossRef]
- Vagi, S.J.; Azziz-Baumgartner, E.; Sjödin, A.; Calafat, A.M.; Dumesic, D.; Gonzalez, L.; Kato, K.; Silva, M.J.; Ye, X.; Azziz, R. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: A case-control study. BMC Endocr. Disord. 2014, 14, 86. [Google Scholar] [CrossRef]
- Nilsson, E.; Klukovich, R.; Sadler-Riggleman, I.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Environmental toxicant induced epigenetic transgenerational inheritance of ovarian pathology and granulosa cell epigenome and transcriptome alterations: Ancestral origins of polycystic ovarian syndrome and primary ovarian insufiency. Epigenetics 2018, 13, 875–895. [Google Scholar] [CrossRef]
- Mimouni, N.E.H.; Paiva, I.; Barbotin, A.L.; Timzoura, F.E.; Plassard, D.; Le Gras, S.; Ternier, G.; Pigny, P.; Catteau-Jonard, S.; Simon, V.; et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021, 33, 513–530.e8. [Google Scholar] [CrossRef]
- Kurdoglu, Z.; Kurdoglu, M.; Demir, H.; Sahin, H.G. Serum trace elements and heavy metals in polycystic ovary syndrome. Hum. Exp. Toxicol. 2012, 31, 452–456. [Google Scholar] [CrossRef]
- Kirmizi, D.A.; Baser, E.; Turksoy, V.A.; Kara, M.; Yalvac, E.S.; Gocmen, A.Y. Are Heavy Metal Exposure and Trace Element Levels Related to Metabolic and Endocrine Problems in Polycystic Ovary Syndrome? Biol. Trace Elem. Res. 2020, 198, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, J.; Lawrence, A.; Dykstra, M.J.; Fannin, R.; Gerrish, K.; Tucker, C.J.; Scappini, E.; Dixon, D. Prolonged cadmium exposure alters benign uterine fibroid cell behavior, extracellular matrix components, and TGFB signaling. FASEB J. 2021, 35, e21738. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, H.; Shen, Y.; Ren, M.; Xu, X. The influence of phenolic environmental estrogen on the transcriptome of uterine leiomyoma cells: A whole transcriptome profiling-based analysis. Ecotoxicol. Environ. Saf. 2021, 211, 111945. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Prim. 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Naftalin, J.; Imrie, R.; Hoo, W.L. Natural History of Uterine Fibroids: A Radiological Perspective. Curr. Obstet. Gynecol. Rep. 2018, 7, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Mavrelos, D.; Ben-Nagi, J.; Holland, T.; Hoo, W.; Naftalin, J.; Jurkovic, D. The natural history of fibroids. Ultrasound Obstet. Gynecol. 2010, 35, 238–242. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Englund, K.; Masironi, B.; Eriksson, H.; Sahlin, L. Different expression of estrogen receptors alpha and beta in human myometrium and leiomyoma during the proliferative phase of the menstrual cycle and after GnRHa treatment. Gynecol. Endocrinol. 2001, 15, 443–452. [Google Scholar] [CrossRef]
- Benassayag, C.; Leroy, M.J.; Rigourd, V.; Robert, B.; Honoré, J.C.; Mignot, T.M.; Vacher-Lavenu, M.C.; Chapron, C.; Ferré, F.L. Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: Pregnancy and leiomyoma. Am. J. Physiol. 1999, 276, E1112-8. [Google Scholar]
- Brandon, D.D.; Erickson, T.E.; Keenan, E.J.; Strawn, E.Y.; Novy, M.J.; Burry, K.A.; Warner, C.; Clinton, G.M. Estrogen receptor gene expression in human uterine leiomyomata. J. Clin. Endocrinol. Metab. 1995, 80, 1876–1881. [Google Scholar]
- Wise, L.A.; Palmer, J.R.; Reich, D.; Cozier, Y.C.; Rosenberg, L. Hair relaxer use and risk of uterine leiomyomata in African-American women. Am. J. Epidemiol. 2012, 175, 432–440. [Google Scholar] [CrossRef]
- Helm, J.S.; Nishioka, M.; Brody, J.G.; Rudel, R.A.; Dodson, R.E. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ. Res. 2018, 165, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Mahalingaiah, S.; Hart, J.E.; Wise, L.A.; Terry, K.L.; Boynton-Jarrett, R.; Missmer, S.A. Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the Nurses’ Health Study II. Am. J. Epidemiol. 2014, 179, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Newbold, R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod. Toxicol. 2005, 20, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Chen, Z.; Kannan, K.; Peterson, C.M.; Pollack, A.Z.; Sun, L.; Buck Louis, G.M. Persistent organic pollutants (POPs) and fibroids: Results from the ENDO study. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 278–285. [Google Scholar] [CrossRef]
- Zota, A.R.; Geller, R.J.; Calafat, A.M.; Marfori, C.Q.; Baccarelli, A.A.; Moawad, G.N. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: A preliminary study. Fertil. Steril. 2019, 111, 112–121. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Leung, C.K.M.; Leung, A.O.W.; Wu, S.C.; Zheng, J.S.; Wong, M.H. Persistent organic pollutants and heavy metals in adipose tissues of patients with uterine leiomyomas and the association of these pollutants with seafood diet, BMI, and age. Environ. Sci. Pollut. Res. 2010, 17, 229–240. [Google Scholar] [CrossRef]
- Howson, C.P.; Kinney, M.V.; McDougall, L.; Lawn, J.E. Born Too Soon Preterm Birth Action, Group.. Born too soon: Preterm birth matters. Reprod. Health 2013, 10, S1. [Google Scholar] [CrossRef]
- Stillerman, K.P.; Mattison, D.R.; Giudice, L.C.; Woodruff, T.J. Environmental exposures and adverse pregnancy outcomes: A review of the science. Reprod. Sci. 2008, 15, 631–650. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Wei, Y.; Chen, J.; Kang, L.; Long, C.; Wu, S.; Shen, L.; Wei, G. Maternal exposure to endocrine disrupting chemicals (EDCs) and preterm birth: A systematic review, meta-analysis, and meta-regression analysis. Environ. Pollut. 2021, 292, 118264. [Google Scholar] [CrossRef]
- Henson, M.C.; Chedrese, P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. 2004, 229, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Candela, S.; Bonvicini, L.; Ranzi, A.; Baldacchini, F.; Broccoli, S.; Cordioli, M.; Carretta, E.; Luberto, F.; Angelini, P.; Evangelista, A.; et al. Exposure to emissions from municipal solid waste incinerators and miscarriages: A multisite study of the MONITER Project. Environ. Int. 2015, 78, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, A.B.; Deen, L.; Hougaard, K.S.; Petersen, K.U.; Meyer, H.W.; Pedersen, E.B.; Ebbehøj, N.E.; Heitmann, B.L.; Bonde, J.P.; Tøttenborg, S.S. Maternal exposure to airborne polychlorinated biphenyls (PCBs) and risk of adverse birth outcomes. Eur. J. Epidemiol. 2021, 36, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Kadhel, P.; Monfort, C.; Costet, N.; Rouget, F.; Thomé, J.P.; Multigner, L.; Cordier, S. Chlordecone exposure, length of gestation, and risk of preterm birth. Am. J. Epidemiol. 2014, 179, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Minichilli, F.; Linzalone, N.; Coi, A.; Maurello, M.T.; Sallese, D.; Bianchi, F. Adverse reproductive outcomes associated with exposure to a municipal solid waste incinerator. Ann. Dell’Ist. Super. Sanita 2016, 52, 576–581. [Google Scholar]
- Ghosh, R.E.; Freni-Sterrantino, A.; Douglas, P.; Parkes, B.; Fecht, D.; de Hoogh, K.; Fuller, G.; Gulliver, J.; Font, A.; Smith, R.B.; et al. Fetal growth, stillbirth, infant mortality and other birth outcomes near UK municipal waste incinerators; retrospective population based cohort and case-control study. Environ. Int. 2019, 122, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Preston, E.V.; Fruh, V.; Quinn, M.R.; Hacker, M.R.; Wylie, B.J.; O’Brien, K.; Mahalingaiah, S.; James-Todd, T. Endocrine disrupting chemical-associated hair product use during pregnancy and gestational age at delivery: A pilot study. Environ. Health 2021, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Cardona, B.; Zota, A.R.; Robinson Flint, J.; Navarro, S.; Shamasunder, B. Personal care product use among diverse women in California: Taking Stock Study. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 487–502. [Google Scholar] [CrossRef]
- Scott, L.L.; Staskal, D.F.; Williams, E.S.; Luksemburg, W.J.; Urban, J.D.; Nguyen, L.M.; Haws, L.C.; Birnbaum, L.S.; Paustenbach, D.J.; Harris, M.A. Levels of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in southern Mississippi catfish and estimation of potential health risks. Chemosphere 2009, 74, 1002–1010. [Google Scholar] [CrossRef]
- Weintraub, M.; Birnbaum, L.S. Catfish consumption as a contributor to elevated PCB levels in a non-Hispanic black subpopulation. Environ. Res. 2008, 107, 412–417. [Google Scholar] [CrossRef]
- Pope, R.; Wu, J.; Boone, C. Spatial patterns of air pollutants and social groups: A distributive environmental justice study in the phoenix metropolitan region of USA. Environ. Manag. 2016, 58, 753–766. [Google Scholar] [CrossRef]
- Bravo, M.A.; Anthopolos, R.; Bell, M.L.; Miranda, M.L. Racial isolation and exposure to airborne particulate matter and ozone in understudied US populations: Environmental justice applications of downscaled numerical model output. Environ. Int. 2016, 92–93, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Duffin, E. Distribution of Race and Ethnicity among the U.S. Military 2019. 2021. Available online: https://www.statista.com/statistics/214869/share-of-active-duty-enlisted-women-and-men-in-the-us-military/ (accessed on 1 August 2021).
- Lindler, L.E. Enhancing the Department of Defense’s Capability to Identify Environmental Exposures into the 21st Century. Mil. Med. 2015, 180 (Suppl. S10), 5–9. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.A.; Hisle-Gorman, E.; Gorman, G.H.; Dobson, N.R. Lower Preterm Birth Rates but Persistent Racial Disparities in an Open-Access Health Care System. Mil. Med. 2018, 183, e570–e575. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Mokshagundam, S.; Barlow, A.; Ding, T.; Osteen, K.G. Paternal Environmental Toxicant Exposure and Risk of Adverse Pregnancy Outcomes. Curr. Obstet. Gynecol. Rep. 2019, 8, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Maltepe, E.; Fisher, S.J. Placenta: The forgotten organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Morgan, T.K. Role of the Placenta in Preterm Birth: A Review. Am. J. Perinatol. 2016, 33, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Menon, R. Human fetal membranes at term: Dead tissue or signalers of parturition? Placenta 2016, 44, 1–5. [Google Scholar] [CrossRef]
- Wang, X.; Miller, D.C.; Harman, R.; Antczak, D.F.; Clark, A.G. Paternally expressed genes predominate in the placenta. Proc. Natl. Acad. Sci. USA 2013, 110, 10705–10710. [Google Scholar] [CrossRef]
- Barton, S.C.; Adams, C.A.; Norris, M.L.; Surani, M.A. Development of gynogenetic and parthenogenetic inner cell mass and trophectoderm tissues in reconstituted blastocysts in the mouse. J. Embryol. Exp. Morphol. 1985, 90, 267–285. [Google Scholar] [CrossRef]
- Ding, T.; Mokshagundam, S.; Rinaudo, P.F.; Osteen, K.G.; Bruner-Tran, K.L. Paternal developmental toxicant exposure is associated with epigenetic modulation of sperm and placental Pgr and Igf2 in a mouse model. Biol. Reprod. 2018, 99, 864–876. [Google Scholar] [CrossRef]
- Palomar, L.; DeFranco, E.A.; Lee, K.A.; Allsworth, J.E.; Muglia, L.J. Paternal race is a risk factor for preterm birth. Am. J. Obstet. Gynecol. 2007, 197, 152.e1–152.e7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Robledo, C.A.; Yeung, E.; Mendola, P.; Sundaram, R.; Maisog, J.; Sweeney, A.M.; Barr, D.B.; Louis, G.M. Preconception maternal and paternal exposure to persistent organic pollutants and birth size: The life study. Environ. Health Perspect. 2015, 123, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Sherf, Y.; Sheiner, E.; Vardi, I.S.; Sergienko, R.; Klein, J.; Bilenko, N. Recurrence of Preterm Delivery in Women with a Family History of Preterm Delivery. Am. J. Perinatol. 2017, 34, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Witherspoon, D.J.; Fraser, A.; Clark, E.A.; Rogers, A.; Stoddard, G.J.; Manuck, T.A.; Chen, K.; Esplin, M.S.; Smith, K.R.; et al. The heritability of gestational age in a two-million member cohort: Implications for spontaneous preterm birth. Hum. Genet. 2015, 134, 803–808. [Google Scholar] [CrossRef] [PubMed]
- DeFranco, E.; Hall, E.; Hossain, M.; Chen, A.; Haynes, E.N.; Jones, D.; Ren, S.; Lu, L.; Muglia, L. Air Pollution and Stillbirth Risk: Exposure to Airborne Particulate Matter during Pregnancy Is Associated with Fetal Death. PLoS ONE 2015, 10, e0120594. [Google Scholar] [CrossRef]
- DeFranco, E.; Moravec, W.; Xu, F.; Hall, E.; Hossain, M.; Haynes, E.N.; Muglia, L.; Chen, A. Exposure to airborne particulate matter during pregnancy is associated with preterm birth: A population-based cohort study. Environ. Health 2016, 15, 6. [Google Scholar] [CrossRef]
- Candela, S.; Ranzi, A.; Bonvicini, L.; Baldacchini, F.; Marzaroli, P.; Evangelista, A.; Luberto, F.; Carretta, E.; Angelini, P.; Sterrantino, A.F.; et al. Air pollution from incinerators and reproductive outcomes: A multisite study. Epidemiology 2013, 24, 863–870. [Google Scholar] [CrossRef]
- Elias, D.; Gimenez, L.; Poletta, F.; Campaña, H.; Gili, J.; Ratowiecki, J.; Pawluk, M.; Rittler, M.; Santos, M.R.; Uranga, R.; et al. Preterm birth and genitourinary tract infections: Assessing gene-environment interaction. Pediatr. Res. 2021, 90, 678–683. [Google Scholar] [CrossRef]
- Mustafa, M.D.; Banerjee, B.D.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K. Gene–Environment interaction in preterm delivery with special reference to organochlorine pesticides. Mol. Hum. Reprod. 2012, 19, 35–42. [Google Scholar] [CrossRef]



| Women’s Health Condition | Associated EDCs/POPs | Associated Heavy Metals | References |
|---|---|---|---|
| Breast Cancer | 1,2,3,7,8-PeDCC; 1,2,3,4,7,8-HxCDF; PCB-74; PCB-99; PCB-118 | Cadmium; lead; nickel | Reynolds et al. [80]; Muscat et al. [81]; Parada et al. [82]; Kaushiva et al. [43] |
| Endometriosis | PCB-138; PCB-153; PCB-180; TCDD; DES | Cadmium | Stillman et al. [90]; Homberger et al. [91]; Potera et al. [95]; Lei et al. [97]; Jackson et al. [11]. |
| Polycystic Ovarian Syndrome | BPA; perfluorooctanoate, perfluorooctane; PCB-153; PCB-170; PCB-180; PCB-183; PCB-196; PCB-203 | Cadmium; copper; lead; mercury; zinc | Takeuchi et al. [116]; Kandaraki [117]; Konieczna et al. [118]; Vagi et al. [119]; Kurdoglu et al. [122]; Kirmizi et al. [123] |
| Uterine Fibroids | DES; DDT; DDE; PCB-126; PCB-180; PCB-191; MiBP; MBzP; MEP | Arsenic; cadmium; lead; mercury | Mahalingaiah et al. [134]; Baird et al. [135]; Trabert et al. [136]; Zota et al. [137]; Qin et al. [138] |
| Endometriosis + Uterine Fibroids | PCB-99; PCB-138; PCB-146; PCB-153; PCB-196; PCB-206 | Unknown | Trabert et al. [136] |
| Preterm Birth | TCDD; BAP; unspecified PCBs (low chlorine content); Chlordecone | Cadmium; lead; chromium; copper; magnesium | Wu et al. [141]; Candela et al. [143]; Kofoed et al. [144]; Kadhel et al. [145]; Santoro et al. [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumph, J.T.; Stephens, V.R.; Martin, J.L.; Brown, L.K.; Thomas, P.L.; Cooley, A.; Osteen, K.G.; Bruner-Tran, K.L. Uncovering Evidence: Associations between Environmental Contaminants and Disparities in Women’s Health. Int. J. Environ. Res. Public Health 2022, 19, 1257. https://doi.org/10.3390/ijerph19031257
Rumph JT, Stephens VR, Martin JL, Brown LK, Thomas PL, Cooley A, Osteen KG, Bruner-Tran KL. Uncovering Evidence: Associations between Environmental Contaminants and Disparities in Women’s Health. International Journal of Environmental Research and Public Health. 2022; 19(3):1257. https://doi.org/10.3390/ijerph19031257
Chicago/Turabian StyleRumph, Jelonia T., Victoria R. Stephens, Joanie L. Martin, LaKendria K. Brown, Portia L. Thomas, Ayorinde Cooley, Kevin G. Osteen, and Kaylon L. Bruner-Tran. 2022. "Uncovering Evidence: Associations between Environmental Contaminants and Disparities in Women’s Health" International Journal of Environmental Research and Public Health 19, no. 3: 1257. https://doi.org/10.3390/ijerph19031257
APA StyleRumph, J. T., Stephens, V. R., Martin, J. L., Brown, L. K., Thomas, P. L., Cooley, A., Osteen, K. G., & Bruner-Tran, K. L. (2022). Uncovering Evidence: Associations between Environmental Contaminants and Disparities in Women’s Health. International Journal of Environmental Research and Public Health, 19(3), 1257. https://doi.org/10.3390/ijerph19031257







