Emission Characteristics of NOx and SO2 during the Combustion of Antibiotic Mycelial Residue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. TGA-MS and XPS Instruments
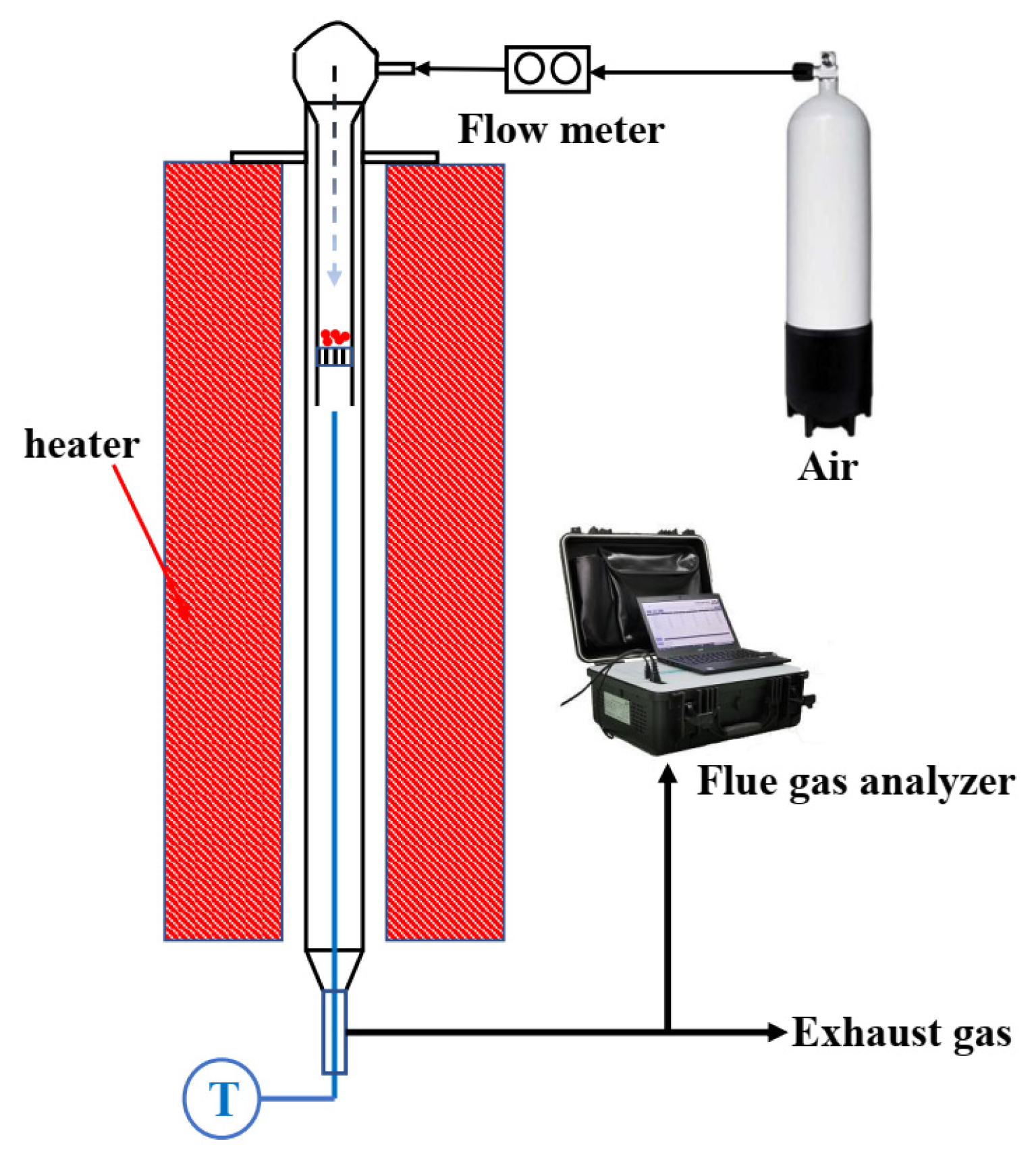
2.3. Fixed Bed Combustor
2.4. Gas Emission Calculation
3. Results and Discussion
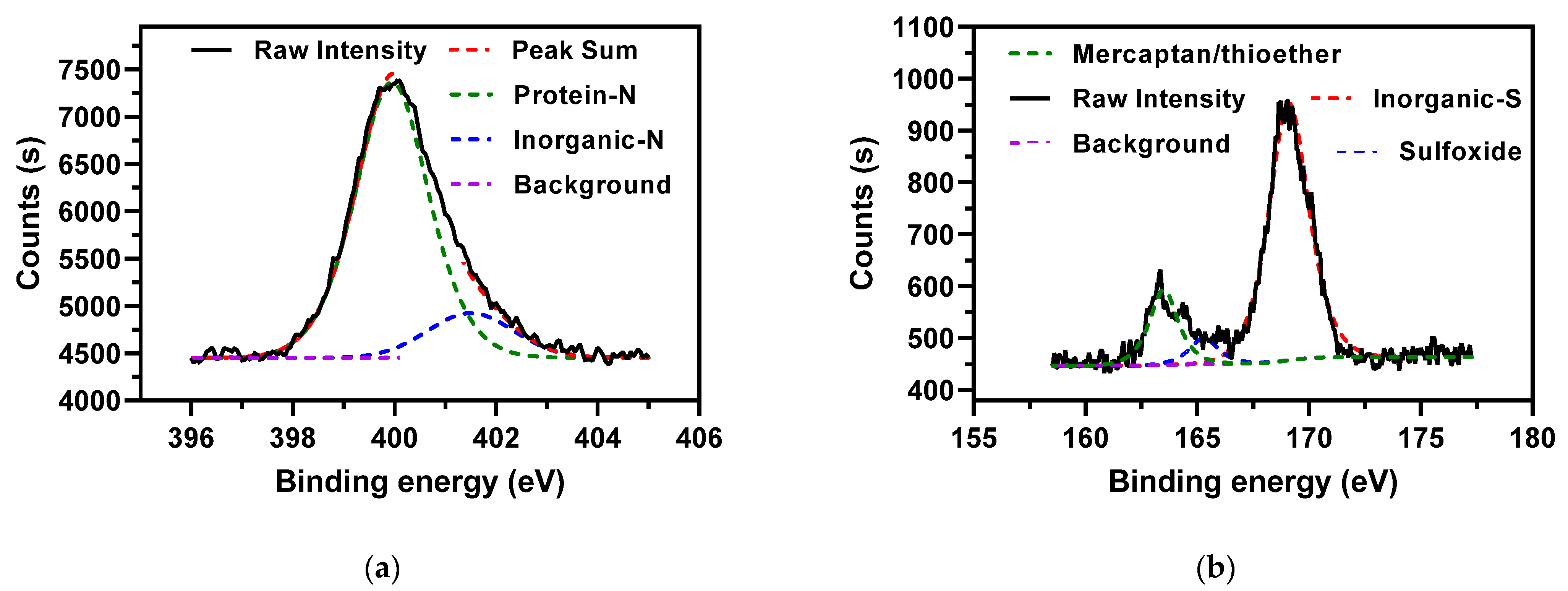
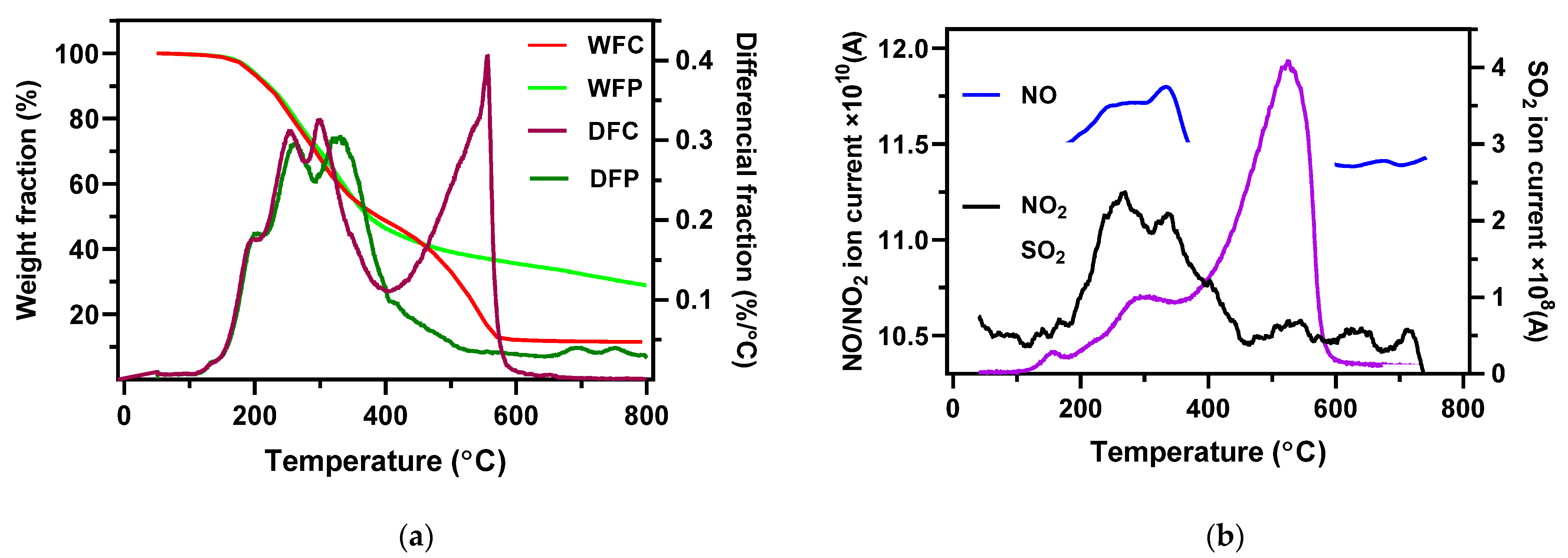
3.1. XPS and TGA-MS Analysis of AMR
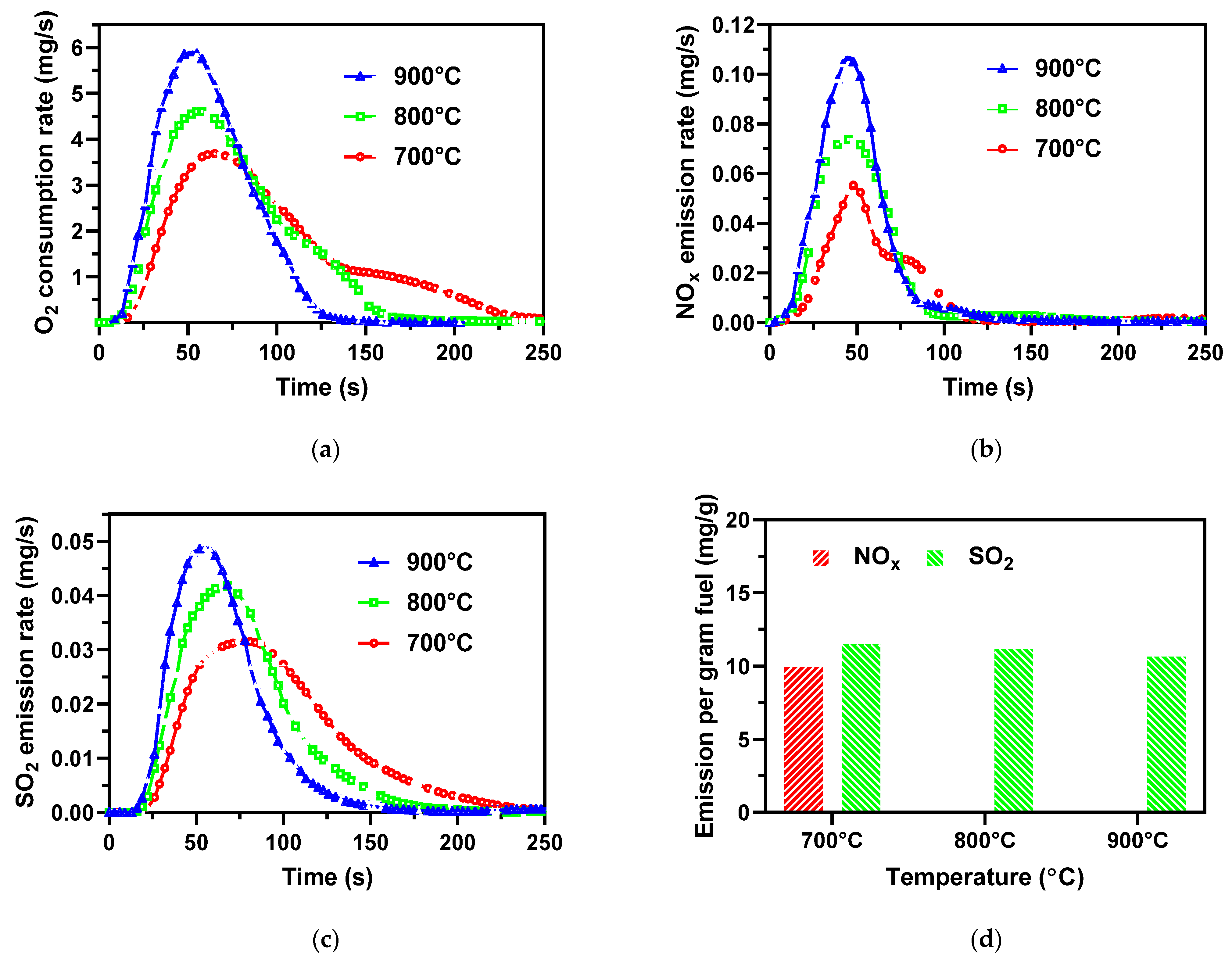
3.2. Effect of Combustion Temperature
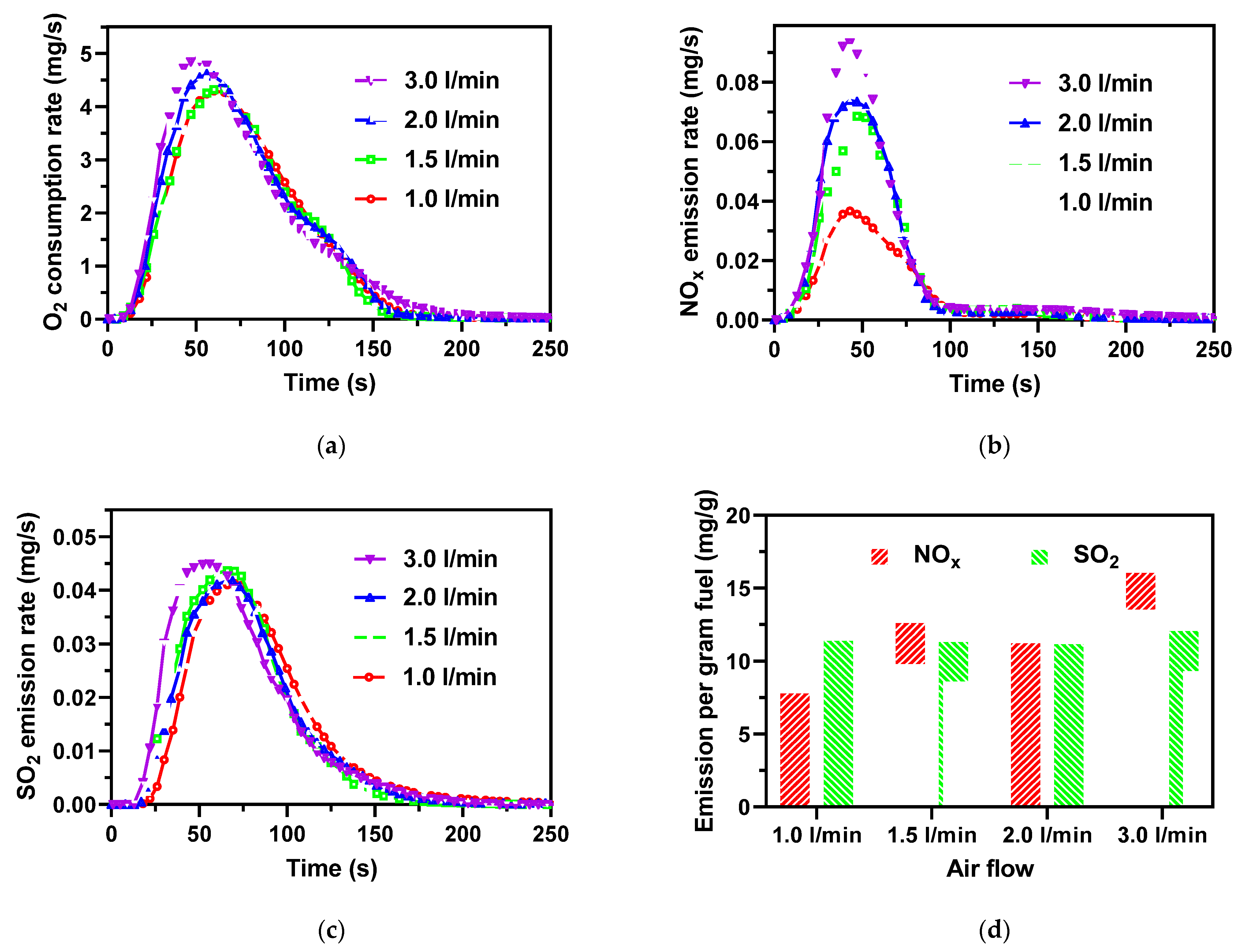
3.3. Effect of Excess Air
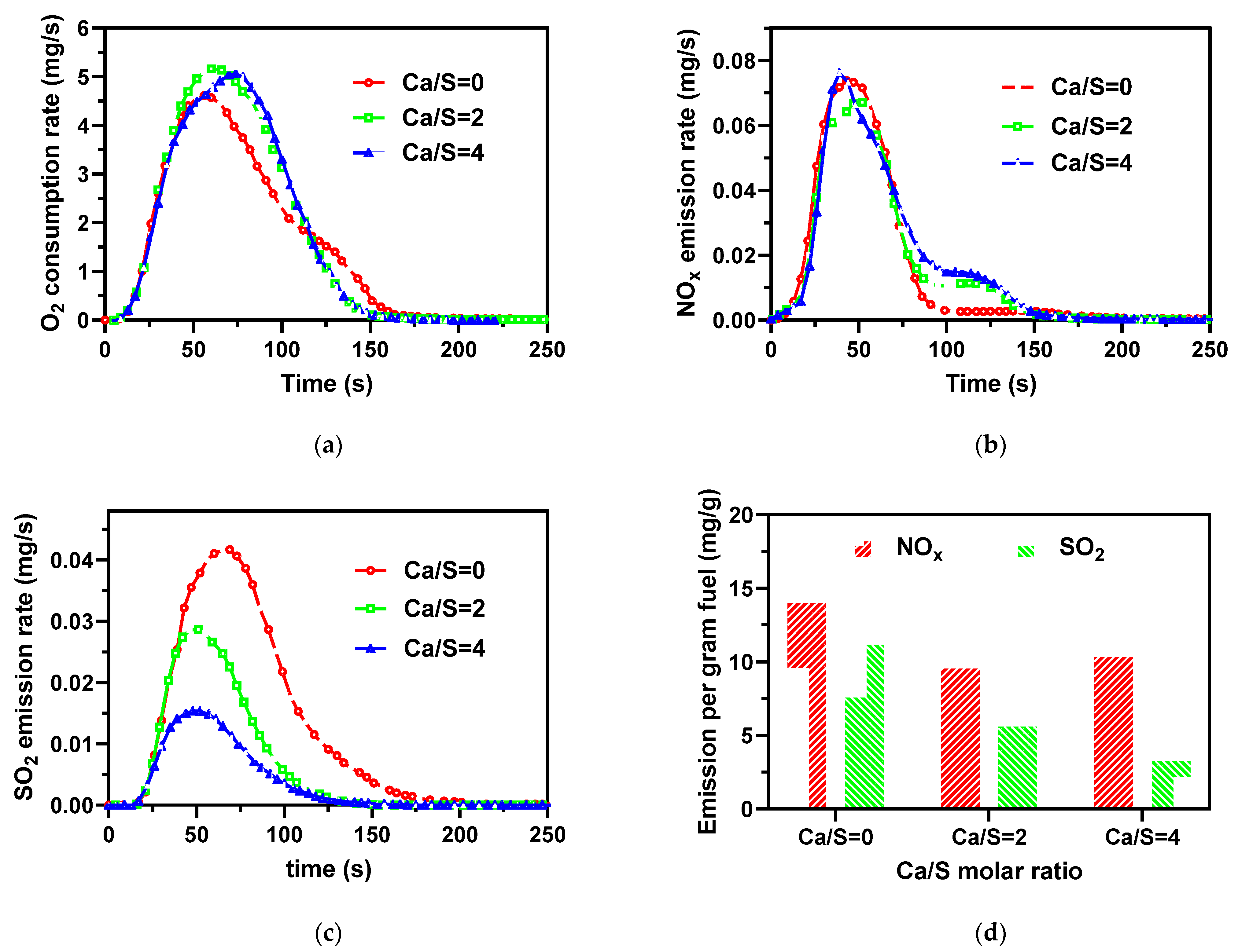
3.4. Desulfurization and Catalytic Effect of CaO
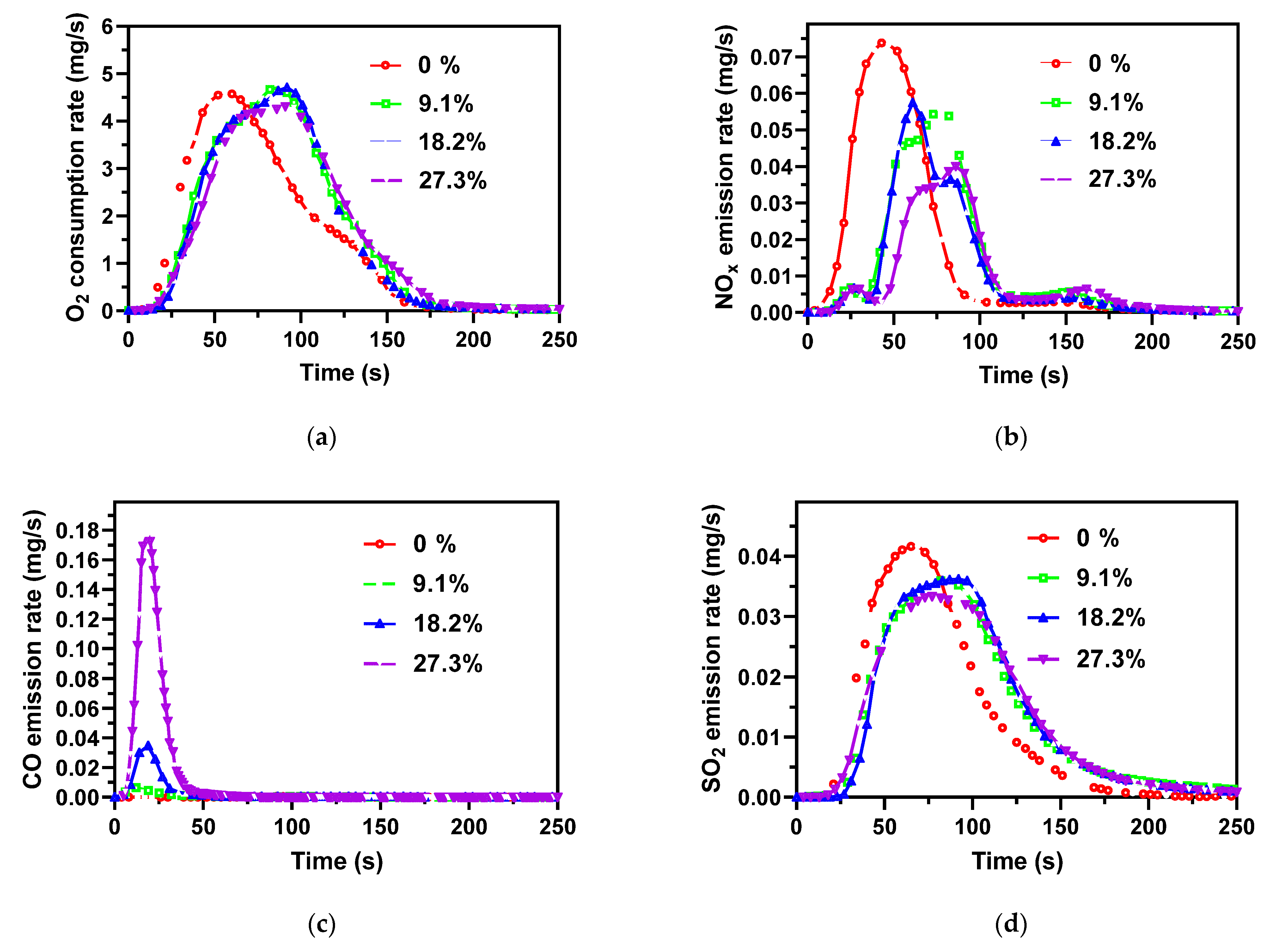
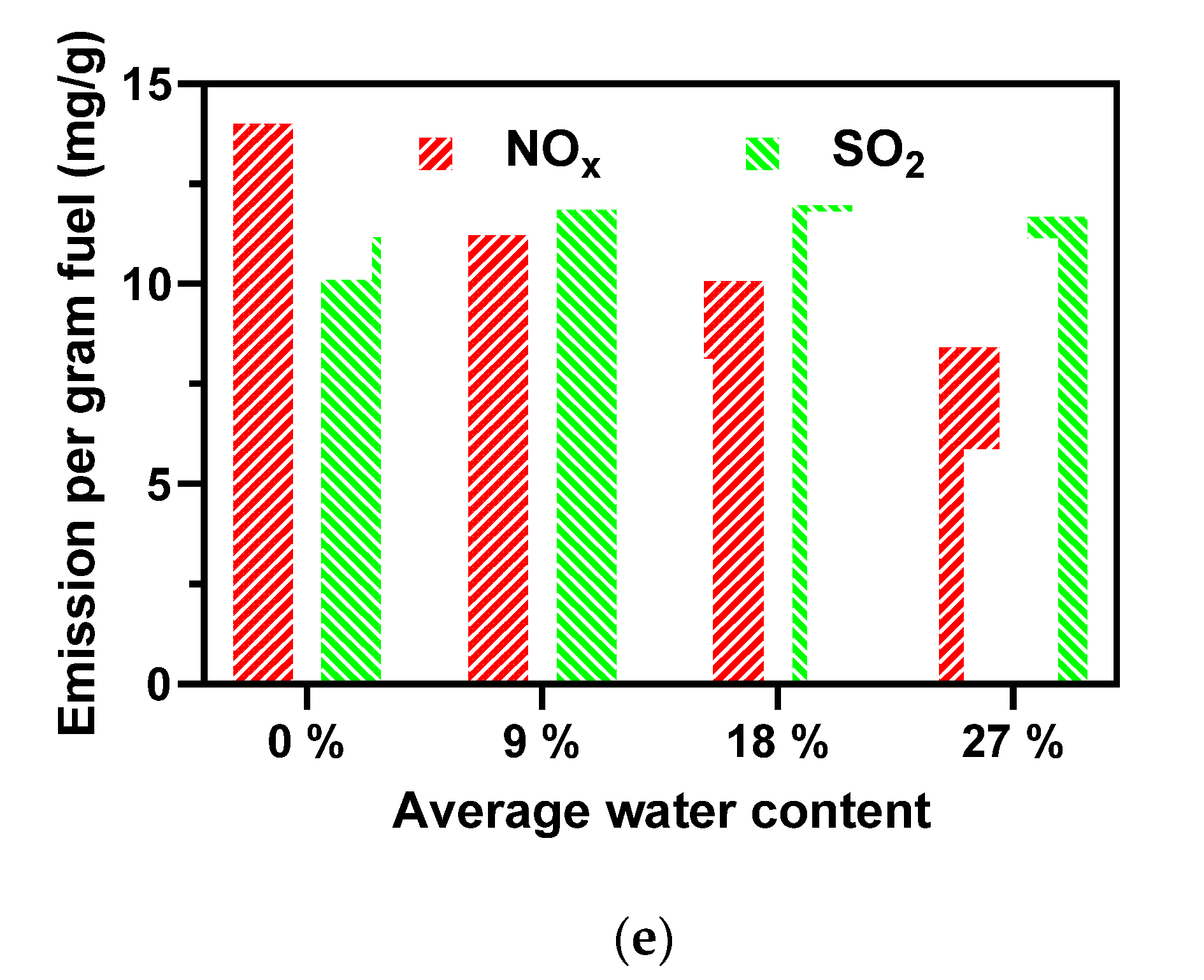
3.5. Effect of Water Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, C.; Liu, H.; Wang, B. Performance of microwave treatment for disintegration of cephalosporin mycelial dreg (CMD) and degradation of residual cephalosporin antibiotics. J. Hazard. Mater. 2017, 331, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, L.; Li, S.; Song, W.; Jensen, P.A.; Lin, W. Pyrolysis of antibiotic mycelial dreg and characterization of obtained gas, liquid and biochar. J. Hazard. Mater. 2021, 402, 123826. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, G.; Zhang, Z.; Ma, D.; Wang, L.; Xu, G. Hydrothermal pretreatment for biogas production from anaerobic digestion of antibiotic mycelial residue. Chem. Eng. J. 2015, 279, 530–537. [Google Scholar] [CrossRef]
- Jiang, X.; Feng, Y.; Lv, G.; Du, Y.; Qin, D.; Li, X.; Chi, Y.; Yan, J.; Liu, X. Bioferment residue: TG-FTIR study and cocombustion in a MSW incineration plant. Environ. Sci. Technol. 2012, 46, 13539–13544. [Google Scholar] [CrossRef]
- Hvistendahl, M. China takes aim at rampant antibiotic resistance. Science 2012, 336, 795. [Google Scholar] [CrossRef]
- Naquin, A.; Shrestha, A.; Sherpa, M.; Nathaniel, R.; Boopathy, R. Presence of antibiotic resistance genes in a sewage treatment plant in Thibodaux, Louisiana, USA. Bioresour. Technol. 2015, 188, 79–83. [Google Scholar] [CrossRef]
- Zhong, W.; Li, Z.; Yang, J.; Liu, C.; Tian, B.; Wang, Y.; Chen, P. Effect of thermal-alkaline pretreatment on the anaerobic digestion of streptomycin bacterial residues for methane production. Bioresour. Technol. 2014, 151, 436–440. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Ma, D.; Zhang, Z.; Xu, G. Anaerobic digestion of antibiotic residue in combination with hydrothermal pretreatment for biogas. Bioresour. Technol. 2015, 192, 257–265. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Pyrolysis behaviors of antibiotic fermentation residue and wastewater sludge from penicillin production: Kinetics, gaseous products distribution, and nitrogen transformation. J. Anal. Appl. Pyrolysis 2021, 158, 105208. [Google Scholar] [CrossRef]
- Li, C.; Xie, S.; You, F.; Zhu, X.; Li, J.; Xu, X.; Yu, G.; Wang, Y.; Angelidaki, I. Heavy metal stabilization and improved biochar generation via pyrolysis of hydrothermally treated sewage sludge with antibiotic mycelial residue. Waste Manag. 2021, 119, 152–161. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, L.; Li, Z.; Zhou, X.; Zhang, W.; Cheng, S.; Zhang, L. Thermal decomposition of antibiotic mycelial fermentation residues in Ar, air, and CO2–N2 atmospheres by TG-FTIR method. J. Therm. Anal. Calorim. 2019, 137, 2053–2060. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, W.; Wu, H.; Jensen, P.A.; Song, W.; Du, L.; Li, S. Steam gasification of char derived from penicillin mycelial dreg and lignocellulosic biomass: Influence of P, K and Ca on char reactivity. Energy 2021, 228, 120605. [Google Scholar] [CrossRef]
- Chen, G.; Liu, H.; LI, J.; Yan, B.; Dong, L. Treatment of antibiotic mycelial fermentation residue: The critical review. Environ. Chem. 2021, 40, 459–473. (In Chinese) [Google Scholar] [CrossRef]
- Lapara, T.M.; Burch, T.R.; McNamara, P.J.; Tan, D.T.; Yan, M.; Eichmiller, J.J. Tertiary-treated municipal wastewater is a significant point source of antibiotic resistance genes into Duluth-Superior Harbor. Environ. Sci. Technol. 2011, 45, 9543–9549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEachran, A.D.; Blackwell, B.R.; Hanson, J.D.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, bacteria, and antibiotic resistance genes: Aerial transport from cattle feed yards via particulate matter. Environ. Health Perspect. 2015, 123, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Liu, H.; Ge, Y.; Gao, S. Gaseous emission and ash characteristics from combustion of high ash content antibiotic mycelial residue in fluidized bed and the impact of additional water vapor. Fuel 2017, 202, 66–77. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, G.; Areeprasert, C.; Li, C.; Shen, Y.; Yoshikawa, K.; Xu, G. Characterization of NO emission in combustion of hydrothermally treated antibiotic mycelial residue. Chem. Eng. J. 2016, 284, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Zhang, G.; Areeprasert, C.; Lina, G.; Yoshikawa, K. NO emission characteristics of hydrothermally pretreated antibiotic mycelial dreg combustionin a drop tube reactor. Energy Procedia 2014, 61, 743–746. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Zhang, G.; Cui, L.; Gao, S. Characteristics of NOx and SO2 emission from combustion of antibiotic mycelial residue with high water content in fluidized bed reactor. CIESC J. 2017, 68, 3250–3257. (In Chinese) [Google Scholar] [CrossRef]
- Wang, C.; Zhou, L.; Fan, G.; Yuan, M.; Zhao, L.; Tang, G.; Liu, C.; Che, D. Experimental study on ash morphology, fusibility, and mineral transformation during co-combustion of antibiotic filter residue and biomass. Energy 2021, 217, 119345. [Google Scholar] [CrossRef]
- Gao, X.; Wang, C.; Liu, C.; Fan, G.; Mao, Q.; Che, D. Numerical simulation study on co-combustion characteristics of biogas and antibiotic filter residue in a grate furnace. In Proceedings of the International Conference on Applied Energy 2020, Bangkok, Thailand, 1–10 December 2020; pp. 1–4. [Google Scholar]
- Özsin, G.; Pütün, A.E. Co-pyrolytic behaviors of biomass and polystyrene: Kinetics, thermodynamics and evolved gas analysis. Korean J. Chem. Eng. 2018, 35, 428–437. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Peng, H.; Leng, S.; Li, H.; Qu, W.; Hu, Y.; Li, H.; Jiang, S.; Zhou, W.; et al. Effect of biomass type and pyrolysis temperature on nitrogen in biochar, and the comparison with hydrochar. Fuel 2021, 291, 120128. [Google Scholar] [CrossRef]
- Liu, L.; Tan, Z.; Ye, Z. Transformation and transport mechanism of nitrogenous compounds in a biochar “preparation-returning to the field” process studied by employing an isotope tracer method. ACS Sustain. Chem. Eng. 2018, 6, 1780–1791. [Google Scholar] [CrossRef]
- Nan, H.; Xiao, Z.; Zhao, L.; Yang, F.; Xu, H.; Xu, X.; Qiu, H. Nitrogen transformation during pyrolysis of various N-containing biowastes with participation of mineral calcium. ACS Sustain. Chem. Eng. 2020, 8, 12197–12207. [Google Scholar] [CrossRef]
- Chang, H.; Wang, C.; Zeng, F.; Li, J.; Li, W.; Xie, K. XPS comparative analysis of coal macerals with different reducibility. J. Fuel Chem. Technol. 2006, 34, 389–394. (In Chinese) [Google Scholar]
- Knudsen, J.N.; Jensen, P.A.; Lin, W.; Frandsen, F.J.; Dam-Johansen, K. Sulfur transformations during thermal conversion of herbaceous biomass. Energy Fuels 2004, 18, 810–819. [Google Scholar] [CrossRef]
- Yuan, R.; Yu, S.; Shen, Y. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 2019, 87, 86–96. [Google Scholar] [CrossRef]
- Roque-Diaz, P.; University, C.; Villas, L.; Cuba, C.V.; Lavrenko, V.A.; Khristich, V.A. Studies on thermal decomposition and combustion mechanism of bagasse under non-isothermal conditions. Thermochim. Acta 1985, 93, 349–352. [Google Scholar] [CrossRef]
- Strydom, C.A. The thermal decomposition of (NH4)4V2O11 in a nitrogen atmosphere and the kinetics of the first decomposition step. J. Therm. Anal. 1993, 40, 1069–1075. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y.; Liu, D.; Zhang, H.; Liu, W.; Cui, G. SO2/NOx emissions and ash formation from algae biomass combustion: Process characteristics and mechanisms. Energy 2016, 113, 821–830. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Bultrini, G.; Chiozzini, G. Correlation between the surface acid-base nature of solid metal oxides and temperature of CaSO4 decomposition. J. Therm. Anal. Calorim. 2001, 66, 27–35. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Clarke, S.; Preto, F. Biomass Burn Characteristics. Available online: https://www.researchgate.net/profile/Fernando-Preto/publication/265498636_Biomass_Burn_Characteristics/links/5592bb9808aed7453d463ce8/Biomass-Burn-Characteristics.pdf (accessed on 29 December 2021).
- Qi, J.; Han, K.; Wang, Q.; Gao, J. Carbonization of biomass: Effect of additives on alkali metals residue, SO2 and NO emission of chars during combustion. Energy 2017, 130, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Liu, X.; Zhao, B.; Liu, W.; Xu, M. The effect of Ca on distribution of nitrogen species during coal pyrolysis in N2, CO2 and Ar atmospheres. Asia-Pac. J. Chem. Eng. 2013, 8, 567–577. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.U.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
| Proximate Analysis (wt.%, as Received) | |
| Moisture | 2.8 |
| Volatile matter | 85.0 |
| Ash | 10.2 |
| Fixed carbon | 2.0 |
| Ultimate analysis (wt.%, dry ash-free) | |
| C | 57.7 |
| H | 9.1 |
| N | 7.9 |
| O | 22.3 |
| S | 2.9 |
| High Heating Value (MJ/kg) | 23.58 |
| Species | SO3 | CaO | P2O5 | K2O | SiO2 | MgO | Na2O | Al2O3 | Fe2O3 | SrO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 40.83 | 35.02 | 14.62 | 3.74 | 2.27 | 1.12 | 0.90 | 0.65 | 0.40 | 0.002 | 99.74 |
| Elements | Element Species | Content (%) |
|---|---|---|
| N | Protein nitrogen | 84.6 |
| Inorganic nitrogen | 15.4 | |
| S | Mercaptan/thioether | 16.8 |
| Sulfoxide | 5.8 | |
| Inorganic sulfur | 77.4 |
| Gas Curve | O2 Consumption | NOx Emission | SO2 Emission |
|---|---|---|---|
| tmax (s) (700 °C, 2.0 L/min, Ca/S = 0, dry) | 65 | 49 | 81 |
| tmax (s) (800 °C, 2.0 L/min, Ca/S = 0, dry) | 56 | 44 | 68 |
| tmax (s) (900 °C, 2.0 L/min, Ca/S = 0, dry) | 52 | 46 | 58 |
| tmax (s) (800 °C, 1.0 L/min, Ca/S = 0, dry) | 61 | 43 | 71 |
| tmax (s) (800 °C, 1.5 L/min, Ca/S = 0, dry) | 61 | 48 | 68 |
| tmax (s) (800 °C, 3.0 L/min, Ca/S = 0, dry) | 51 | 42 | 52 |
| tmax (s) (800 °C, 2.0 L/min, Ca/S = 2, dry) | 61 | 50 | 46 |
| tmax (s) (800 °C, 2.0 L/min, Ca/S = 4, dry) | 73 | 40 | 48 |
| Combustion Condition | Fuel-N to NOx, XN | Fuel-N to SO2, XS | Burn out Ratio, XN |
|---|---|---|---|
| 700 °C, 2.0 L/min, Ca/S = 0, dry | 6.9 | 23.9 | 98.8 |
| 800 °C, 2.0 L/min, Ca/S = 0, dry | 9.7 | 23.3 | 100 |
| 900 °C, 2.0 L/min, Ca/S = 0, dry | 11.9 | 22.2 | 100 |
| 800 °C, 1.0 L/min, Ca/S = 0, dry | 5.4 | 23.8 | 100 |
| 800 °C, 1.5 L/min, Ca/S = 0, dry | 8.8 | 23.6 | 100 |
| 800 °C, 3.0 L/min, Ca/S = 0, dry | 11.2 | 25.1 | 99.9 |
| 800 °C, 2.0 L/min, Ca/S = 2, dry | 9.7 | 11.7 | 100 |
| 800 °C, 2.0 L/min, Ca/S = 4, dry | 10.4 | 6.8 | 100 |
| 800 °C, 2.0 L/min, Ca/S = 0, 9.1% | 7.8 | 24.1 | 99.8 |
| 800 °C, 2.0 L/min, Ca/S = 0, 18.2% | 7.0 | 24.9 | 99.5 |
| 800 °C, 2.0 L/min, Ca/S = 0, 27.3% | 5.9 | 24.3 | 99.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.; Zhang, G.; Zhang, J.; Zhang, W.; Cui, L. Emission Characteristics of NOx and SO2 during the Combustion of Antibiotic Mycelial Residue. Int. J. Environ. Res. Public Health 2022, 19, 1581. https://doi.org/10.3390/ijerph19031581
Ge Y, Zhang G, Zhang J, Zhang W, Cui L. Emission Characteristics of NOx and SO2 during the Combustion of Antibiotic Mycelial Residue. International Journal of Environmental Research and Public Health. 2022; 19(3):1581. https://doi.org/10.3390/ijerph19031581
Chicago/Turabian StyleGe, Yaxin, Guangyi Zhang, Jianling Zhang, Wennan Zhang, and Lijie Cui. 2022. "Emission Characteristics of NOx and SO2 during the Combustion of Antibiotic Mycelial Residue" International Journal of Environmental Research and Public Health 19, no. 3: 1581. https://doi.org/10.3390/ijerph19031581






