Effectiveness and Cost-Effectiveness of Non-Pharmacological Interventions among Chinese Adults with Prediabetes: A Protocol for Network Meta-Analysis and CHIME-Modeled Cost-Effectiveness Analysis
Abstract
:1. Introduction
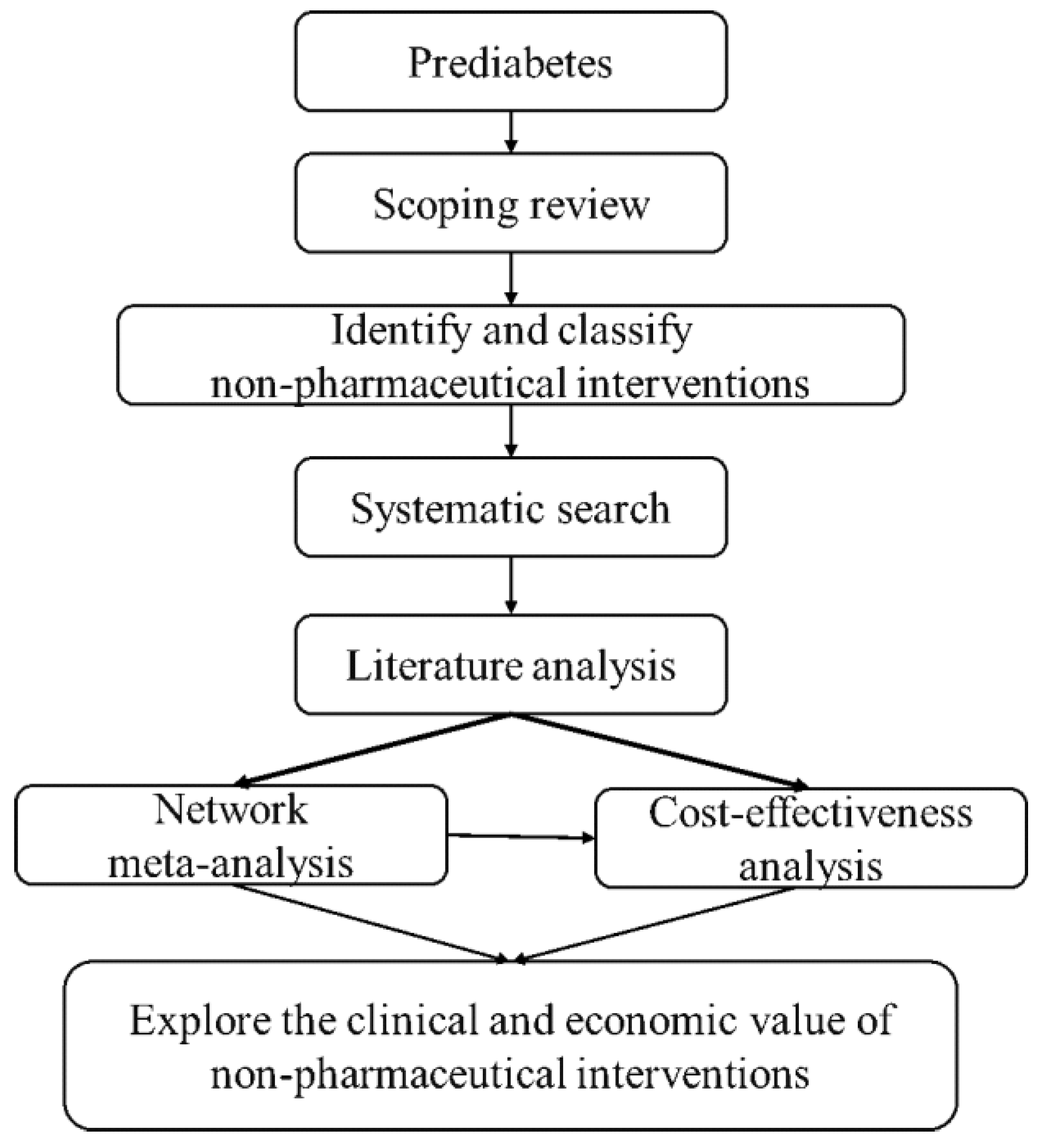
2. Methods and Analysis
2.1. Inclusion and Exclusion Criteria
2.2. Data Sources and Analysis
2.3. Data Synthesis and Statistical Methods
2.4. Cost-Effectiveness Analysis
2.4.1. Model Design
2.4.2. Model Input
2.4.3. Economic Decision
2.4.4. Dealing with Uncertainty
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Kalra, K.; Chandrabose, S.T.; Ramasamy, T.S.; Kasim, N. Advances in the Generation of Functional β-cells from Induced Pluripotent Stem Cells as a Cure for Diabetes Mellitus. Curr. Drug Targets 2018, 19, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, S.; Sawatani, T.; Van Mulders, A.; De Leu, N.; Heremans, Y.; Heimberg, H.; Cnop, M.; Staels, W. Towards a Functional Cure for Diabetes Using Stem Cell-Derived Beta Cells: Are We There Yet? Cells 2021, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Fouli, G.E.; Gnudi, L. The Future: Experimental Therapies for Renal Disease in Diabetes. Nephron 2019, 143, 3–7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Khan, R.; Chua, Z.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [Green Version]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Yusuf, S.; Bosch, J.; Pogue, J.; Sheridan, P.; Dinccag, N.; Hanefeld, M.; Hoogwerf, B.; Laakso, M.; Mohan, V.; et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [CrossRef] [Green Version]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- National Cardiovascular Intelligence Network (NCVIN)/NHS Diabetes Prevention Programme (NHS DPP). Nondiabetic Hyperglycaemia; Public Health England: London, UK, 2015; Available online: https://www.england.nhs.uk/diabetes/diabetes-prevention/ (accessed on 21 December 2021).
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Sussman, J.B.; Kent, D.M.; Nelson, J.P.; Hayward, R.A. Improving diabetes prevention with benefit based tailored treatment: Risk based reanalysis of Diabetes Prevention Program. BMJ 2015, 350, h454. [Google Scholar] [CrossRef] [Green Version]
- Raina, E.C.; Kenealy, T. Lifestyle interventions reduced the long-term risk of diabetes in adults with impaired glucose tolerance. Evid. Based Med. 2008, 13, 173. [Google Scholar] [CrossRef]
- Rao, S.S.; Disraeli, P.; McGregor, T. Impaired glucose tolerance and impaired fasting glucose. Am. Fam. Physician 2004, 69, 1961–1968. [Google Scholar]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Færch, K.; Blond, M.B.; Bruhn, L.; Amadid, H.; Vistisen, D.; Clemmensen, K.; Vainø, C.; Pedersen, C.; Tvermosegaard, M.; Dejgaard, T.F.; et al. The effects of dapagliflozin, metformin or exercise on glycaemic variability in overweight or obese individuals with prediabetes (the PRE-D Trial): A multi-arm, randomised, controlled trial. Diabetologia 2021, 64, 42–55. [Google Scholar] [CrossRef]
- Li, R.; Zhang, P.; Barker, L.E.; Chowdhury, F.M.; Zhang, X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: A systematic review. Diabetes Care 2010, 33, 1872–1894. [Google Scholar] [CrossRef] [Green Version]
- Glechner, A.; Keuchel, L.; Affengruber, L.; Titscher, V.; Sommer, I.; Matyas, N.; Wagner, G.; Kien, C.; Klerings, I.; Gartlehner, G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2018, 12, 393–408. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, W.; Cai, Y.; Sun, D.; Zhao, J. Clinical-and cost-effectiveness of telemedicine in type 2 diabetes mellitus: A systematic review and meta-analysis. Medicine 2014, 93, e312. [Google Scholar] [CrossRef]
- Huang, L.; Fang, Y.; Tang, L. Comparisons of different exercise interventions on glycemic control and insulin resistance in prediabetes: A network meta-analysis. BMC Endocr. Disord. 2021, 21, 181. [Google Scholar] [CrossRef]
- Sheng, Z.; Cao, J.; Pang, Y.; Xu, H.; Chen, J.; Yuan, J.; Wang, R.; Zhang, C.; Wang, L.; Dong, J. Effects of lifestyle modification and anti-diabetic medicine on prediabetes progress: A systematic review and meta-analysis. Front. Endocrinol. 2019, 10, 455. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhang, G.; Hu, D. Progress of diabetes prevention and control and countermeasures. Chin. J. Public Health Manag. 2018, 34, 779–782. [Google Scholar] [CrossRef]
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef]
- Hutton, B.; Catala-Lopez, F.; Moher, D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated health economic evaluation reporting standards (CHEERS) statement. Int. J. Technol. Assess 2013, 29, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Society, C.D. Chinese Guideline for the Prevention and Treatment of Type 2 Diabetes Mellitus (2020 edition). Int. J. Endocrinol. Metab./Int. J. Endocrinol. Metab. 2021, 41, 482–548. [Google Scholar]
- Spencer-Bonilla, G.; Ponce, O.J.; Rodriguez-Gutierrez, R.; Alvarez-Villalobos, N.; Erwin, P.J.; Larrea-Mantilla, L.; Rogers, A.; Montori, V.M. A systematic review and meta-analysis of trials of social network interventions in type 2 diabetes. BMJ Open 2017, 7, e16506. [Google Scholar] [CrossRef] [Green Version]
- Curtin, F.; Elbourne, D.; Altman, D.G. Meta-analysis combining parallel and cross-over clinical trials. III: The issue of carry-over. Stat. Med. 2002, 21, 2161–2173. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Moggio, L.; Marinaro, C.; Pino, I.; Barletta, M.; Petraroli, A.; Pepe, D.; Lavano, F.; Ammendolia, A. Comparative effectiveness of breathing exercises in patients with chronic obstructive pulmonary disease. Complement. Clin. 2020, 41, 101260. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 2012, 3, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, D142. [Google Scholar] [CrossRef] [Green Version]
- Quan, J.; Ng, C.S.; Kwok, H.; Zhang, A.; Yuen, Y.H.; Choi, C.H.; Siu, S.C.; Tang, S.Y.; Wat, N.M.; Woo, J.; et al. Development and validation of the CHIME simulation model to assess lifetime health outcomes of prediabetes and type 2 diabetes in Chinese populations: A modeling study. PLoS Med 2021, 18, e1003692. [Google Scholar] [CrossRef]
- Research Group of “Guidelines for Evaluation of Chinese Pharmacoeconomics”. China Pharmacoeconomic Evaluation Guidelines; China Market Press: Beijing, China, 2019; p. 16. [Google Scholar]
- Palmer, A.J.; Roze, S.; Valentine, W.J.; Minshall, M.E.; Foos, V.; Lurati, F.M.; Lammert, M.; Spinas, G.A. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr. Med. Res. Opin. 2004, 20 (Suppl. 1), S5–S26. [Google Scholar] [CrossRef]
- McEwan, P.; Evans, M.; Bergenheim, K. A population model evaluating the costs and benefits associated with different oral treatment strategies in people with type 2 diabetes. Diabetes Obes. Metab. 2010, 12, 623–630. [Google Scholar] [CrossRef]
- Richter, B.; Hemmingsen, B.; Metzendorf, M.I.; Takwoingi, Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev. 2018, 10, D12661. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S17. [Google Scholar]

| Type of Intervention | Intervention Measures | Occupation | |
|---|---|---|---|
| Non-drug therapies | Nutritional therapy | 1. diet OR dietary OR supplementation 2. weight loss OR weight reduction 3. smoking cessation 4. alcohol restriction | Nutritionist |
| Physical activity | 1. exercise OR training OR sport OR practice OR activity 2. cardio OR anaerobic exercise OR resistance therapy OR physical therapy OR kinesiotherapy 3. bicycle OR walking OR swimming OR yoga OR qigong OR tai chi OR dance | Fitness coach | |
| Psychological interventions | 1. psychological intervention 2. mental health OR emotion OR mood OR neuropsychological 3. meditation OR music OR speech therapy OR interview | Psychologist | |
| Social network interventions | 1. peer support 2. society support OR community support OR family support OR friend support | Peer facilitator | |
| Self-management and education | 1. self-management OR self-management group 2. knowledge, attitude/belief, practice 3. health consultation OR health education | Self-management planner | |
| Media-related interventions | 1. telephone OR mobile OR smartphone 2. application OR software OR internet OR online OR technique OR digital 3. message OR e-mail OR wechat 4. telemedicine OR telehealth OR mhealth OR ehealth OR digital health 5. artificial Intelligence | Network nurse | |
| Chinese medicine | 1. traditional Chinese medicine 2. acupuncture OR acupressure OR massage OR guasha | Physiotherapist | |
| Multidisciplinary interventions | 1. patient care team OR general practitioner | General practitioner team | |
| Drug therapies | Metformin | ||
| Sulfonylureas | |||
| Thiazolidinedione | |||
| Alpha-glucosidase inhibitors | |||
| Dipeptidyl peptidase IV (DPP-4) | |||
| Sodium-glucose cotransporter 2 inhibitor (SGLT2i) | |||
| GLP-1 * receptor agonists | |||
| No. | Search Items |
|---|---|
| #1 | ‘impaired glucose tolerance’/exp |
| #2 | prediabetic: ti, ab, kw OR prediabetes: ti, ab, kw |
| #3 | (progress: ti OR conversion: ti OR develop: ti OR delay: ti OR latent: ti OR potential: ti OR prevent: ti OR prevention: ti) AND (diabetes: ti OR diabetic: ti OR t2dm:ti OR t2d:ti OR niddm: ti) |
| #4 | ‘glucose intolerance’/exp |
| #5 | glucose: ti, ab, kw AND (intolerance: ti, ab, kw OR intolerances: ti, ab, kw OR dysregulation: ti, ab, kw) |
| #6 | impaired: ti, ab, kw AND glucose: ti, ab, kw AND (tolerance: ti, ab, kw OR tolerances: ti, ab, kw OR sensitivity: ti, ab, kw OR metabolism: ti, ab, kw OR regulation: ti, ab, kw) |
| #7 | IGT: ti, ab, kw OR IFG: ti, ab, kw |
| #8 | ‘impaired fasting’: ti, ab, kw AND (glucose: ti, ab, kw OR glycaemia: ti, ab, kw) |
| #9 | intermediate: ti, ab, kw AND (hyperglycemia: ti, ab, kw OR ‘glycemic control’: ti, ab, kw) |
| #10 | borderline: ti, ab, kw AND (diabetes: ti, ab, kw OR diabetic: ti, ab, kw OR hba1c: ti, ab, kw OR hyperglycemia: ti, ab, kw OR ‘hemoglobin a1c: ti, ab, kw OR a1c: ti, ab, kw) |
| #11 | impaired AND (fpg: ti, ab, kw OR ‘fasting plasma glucose’: ti, ab, kw OR ‘fasting blood glucose’: ti, ab, kw) |
| #12 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 |
| #13 | pregnancy: ti, ab, kw |
| #14 | T1DM: ti, ab, kw OR (‘type 1: ti, ab, kw AND ‘diabetes’: ti, ab, kw) OR T1D: ti, ab, kw |
| #15 | #12 NOT #13 NOT #14 |
| #16 | protocol: ti OR guidelines: ti OR consensus: ti OR case: ti |
| #17 | #15 NOT #16 |
| #18 | ‘animal’/exp NOT ‘human’/exp |
| #19 | #17 NOT #18 |
| #20 | ‘influencing factors’: ti, kw OR mechanism: ti, kw OR ‘risk factors’: ti, kw |
| #21 | #19 NOT #20 |
| #22 | ‘crossover procedure’: de OR ‘double-blind procedure’: de OR ‘randomized controlled trial’: de OR ‘single-blind procedure’: de OR (random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEAR/1 blind* OR singl* NEAR/1 blind* OR assign* OR allocat* OR volunteer*): de, ab, ti |
| #23 | ‘cohort analysis’/exp OR ‘longitudinal study’/exp OR ‘prospective study’/exp OR ‘follow up’/exp OR cohort*: ab, ti |
| #24 | #22 OR #23 |
| #25 | ‘ecological study’: ti OR ‘case study’: ti OR ‘case report’: ti OR ‘cross section’: ti OR ‘editorial’: ti OR ‘letter’: ti OR news: ti OR ‘newspaper article’: ti |
| #26 | #24 NOT #25 |
| #27 | #21 AND #26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Tu, Y.; Zhao, M.; Tang, W. Effectiveness and Cost-Effectiveness of Non-Pharmacological Interventions among Chinese Adults with Prediabetes: A Protocol for Network Meta-Analysis and CHIME-Modeled Cost-Effectiveness Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1622. https://doi.org/10.3390/ijerph19031622
Yin Y, Tu Y, Zhao M, Tang W. Effectiveness and Cost-Effectiveness of Non-Pharmacological Interventions among Chinese Adults with Prediabetes: A Protocol for Network Meta-Analysis and CHIME-Modeled Cost-Effectiveness Analysis. International Journal of Environmental Research and Public Health. 2022; 19(3):1622. https://doi.org/10.3390/ijerph19031622
Chicago/Turabian StyleYin, Yue, Yusi Tu, Mingye Zhao, and Wenxi Tang. 2022. "Effectiveness and Cost-Effectiveness of Non-Pharmacological Interventions among Chinese Adults with Prediabetes: A Protocol for Network Meta-Analysis and CHIME-Modeled Cost-Effectiveness Analysis" International Journal of Environmental Research and Public Health 19, no. 3: 1622. https://doi.org/10.3390/ijerph19031622






