Risk Prescriptions of Strong Opioids in the Treatment of Chronic Non-Cancer Pain by Primary Care Physicians in Catalonia: Opicat Padris Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Population
2.3. Variables Included
- Concomitant risk prescription: defined as the concomitant prescription of SO with any of the active ingredients included in the following pharmacological groups (central nervous system depressant drugs and concomitant drugs with adverse effects proven): antidepressants [15,16], gabapentinoids [13,17,18,19], benzodiazepines and Z-drugs [7,10,11,12]. For extraction they were identified by their Anatomical Therapeutic Chemical Classification (ACT) code.
- The prescription of any of these drugs during the 100 days after the date of prescription of the SO was considered a concomitant prescription (according to chronic pain definition as ≥3 months).
- Doses of pregabalin and gabapentin prescribed in patients with a concomitant prescription of SO (variable included due to dose-related mortality in gabapentinoids concomitancy [18,19]). The dose per unit (tablet, capsule) was obtained and the daily dose was calculated using the dosage interval recommended in the data sheet (gabapentin every 8 h, pregabalin every 12 h).
- Prescription of IRF without prescription of a baseline SO in the previous 100 days (unauthorized prescription [20]).
2.4. Ethical Aspects
2.5. Statistical Analysis
3. Results
3.1. Association between Strong Opioids (SO) and Benzodiazepines
3.2. Association between SO and Antidepressants
3.3. Association between SO and Gabapentinoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales-Espinoza, E.M.; Kostov, B.; Salami, D.C.; Perez, Z.H.; Rosalen, A.P.; Molina, J.O.; Paz, L.G.; Momblona, J.M.S.; Àreu, J.B.; Brito-Zerón, P.; et al. Complexity, Comorbidity, and Health Care Costs Associated with Chronic Widespread Pain in Primary Care. Pain 2016, 157, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.S.; Varillas, A.T.; Garrido, R.C.; García, C.R.G.; García, S.G.; Sánchez, M.G.; Vallejo, F.J.G.; Hernán, J.C.H.; Negrín, F.V.; Pol, E.N. La Atención al Paciente con Dolor Crónico no Oncológico (DCNO) en Atención Primaria (AP). Documento de Consenso; Semg, Semfyc, Semergen: Madrid, Spain, 2017. [Google Scholar]
- Santo, L.; Okeyode, T. National Ambulatory Medical Care Survey: 2018 National Summary Tables; National Center for Health Statistics: Hyattsville, MD, USA, 2018. [Google Scholar]
- AEMPS. Utilización de Medicamentos Opioides en España Durante el Periodo 2010–2018; Ministerio de Sanidad: Madrid, Spain, 2019. [Google Scholar]
- Perelló-Bratescu, A.; Dürsteler, C.; Álvarez-Carrera, M.A.; Granés, L.; Kostov, B.; Sisó-Almirall, A. Trends in the Prescription of Strong Opioids for Chronic Non-Cancer Pain in Primary Care in Catalonia: Opicat-Padris-Project. Pharmaceutics 2022, 14, 237. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network. Scottish Intercollegiate Guidelines Network (SIGN). Management of Chronic Pain. Edinburgh: SIGN (SIGN Publication No. 136). Revised Edition Published August 2019. Available online: https://www.sign.ac.uk/media/1108/sign136_2019.pdf (accessed on 22 December 2021).
- Ministerio de Sanidad. Prácticas Seguras Para el uso de Opioides en Pacientes con Dolor Crónico; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain, 2015. [Google Scholar]
- Petzke, F.; Bock, F.; Hüppe, M.; Nothacker, M.; Norda, H.; Radbruch, L.; Schiltenwolf, M.; Schuler, M.; Tölle, T.; Viniol, A.; et al. Long-Term Opioid Therapy for Chronic Noncancer Pain: Second Update of the German Guidelines. PAIN Rep. 2020, 5, e840. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Morlion, B.; Vowles, K.E.; Bannister, K.; Buchser, E.; Casale, R.; Chenot, J.F.; Chumbley, G.; Drewes, A.M.; Dom, G.; et al. European* Clinical Practice Recommendations on Opioids for Chronic Noncancer Pain—Part 1: Role of Opioids in the Management of Chronic Noncancer Pain. Eur. J. Pain 2021, 25, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Arbonés, E.; Montes, A. Riscos Associats a L’abús D’opioides. Butlletí de Prevenció d’Errors de Medicació de Catalunya; Generalitat de Catalunya, Departament de Salut: Catalonia, Spain, 2016; Volume 4. [Google Scholar]
- Sharma, V.; Weir, D.; Samanani, S.; Simpson, S.H.; Gilani, F.; Jess, E.; Eurich, D.T. Characterisation of Concurrent Use of Prescription Opioids and Benzodiazepine/Z-Drugs in Alberta, Canada: A Population-Based Study. BMJ Open. 2019, 9, e030858. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.R.; Oh, I.-S.; Li, J.; Jeon, H.-L.; Shin, J.-Y. Association between Opioid Analgesic plus Benzodiazepine Use and Death: A Case-Crossover Study. J. Psychosom. Res. 2020, 135, 110153. [Google Scholar] [CrossRef]
- Macleod, J.; Steer, C.; Tilling, K.; Cornish, R.; Marsden, J.; Millar, T.; Strang, J.; Hickman, M. Prescription of Benzodiazepines, z-Drugs, and Gabapentinoids and Mortality Risk in People Receiving Opioid Agonist Treatment: Observational Study Based on the UK Clinical Practice Research Datalink and Office for National Statistics Death Records. PLoS Med. 2019, 16, e1002965. [Google Scholar] [CrossRef]
- Perelló Bratescu, A.; Adriyanov, B.; Dürsteler, C.; Sisó-Almirall, A.; Álvarez Carrera, M.A.; Riera Nadal, N. Opioides Fuertes y Dolor Crónico No Oncológico en Cataluña. Análisis del Patrón de Prescripción por Parte de los Médicos de Familia. Rev. Esp. Anestesiol. Reanim. 2020, 67, 68–75. [Google Scholar] [CrossRef]
- Tapentadol (Palexia): Risk of Seizures and Reports of Serotonin Syndrome When Co-Administered with other Medicines. Available online: https://www.gov.uk/drug-safety-update/tapentadol-palexia-risk-of-seizures-and-reports-of-serotonin-syndrome-when-co-administered-with-other-medicines (accessed on 22 December 2021).
- Agencia Española de Medicamentos y Productos Sanitarios. Boletín Mensual de la AEMPS Sobre Medicamentos de uso Humano; Ministerio de Sanidad: Madrid, Spain, 2018. [Google Scholar]
- Torrance, N.; Veluchamy, A.; Zhou, Y.; Fletcher, E.H.; Moir, E.; Hebert, H.L.; Donnan, P.T.; Watson, J.; Colvin, L.A.; Smith, B.H. Trends in Gabapentinoid Prescribing, Co-Prescribing of Opioids and Benzodiazepines, and Associated Deaths in Scotland. Br. J. Anaesth. 2020, 125, 159–167. [Google Scholar] [CrossRef]
- Gomes, T.; Juurlink, D.N.; Antoniou, T.; Mamdani, M.M.; Paterson, J.M.; van den Brink, W. Gabapentin, Opioids, and the Risk of Opioid-Related Death: A Population-Based Nested Case–Control Study. PLoS Med. 2017, 14, e1002396. [Google Scholar] [CrossRef]
- Gomes, T.; Greaves, S.; van den Brink, W.; Antoniou, T.; Mamdani, M.M.; Paterson, J.M.; Martins, D.; Juurlink, D.N. Pregabalin and the Risk for Opioid-Related Death: A Nested Case–Control Study. Ann. Intern. Med. 2018, 169, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española de Medicamentos y Productos Sanitarios AEMPS. Fentanilo de Liberación Inmediata: Importancia de Respetar las Condiciones de uso Autorizadas; Ministerio de Sanidad: Madrid, Spain, 2018. [Google Scholar]
- González-Bermejo, D.; Rayón-Iglesias, P.; Rodríguez-Pascual, A.; Álvarez-Gutiérrez, A.; Fernández-Dueñas, A.; Montero-Corominas, D.; Huerta-Álvarez, C. Drug Utilization Study on Immediate Release Fentanyl in Spain. Prevalence, Incidence, and Indication. Pharmacoepidemiol. Drug Saf. 2020, 30, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Dépendance Aux Médicaments Opioïdes Aux États-Unis: Une Énorme Épidémie Mortelle Par Surdose. Prescrire Réd. 2017, 37, 622–629.
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm. Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef]
- Cochrane. Traditional Opioids for Chronic Non-Cancer Pain: Untidy, Unsatisfactory, and Probably Unsuitable; Cochrane UK: London, UK, 2022. [Google Scholar]
- Pando, T.; Molina, A.; Carbonell, P.D.J. Informe d’utilització de Medicaments Opioides a Catalunya: Anàlisi del Període 2012–2016; Servei Català de La Salut: Barcelona, Spain, 2017. [Google Scholar]
- Bruguera, E.; Carreras, A.; Manresa, A.; Miravet, S.; Perelló, A.R.M. Documento de Posición del Consejo de Colegios de Médicos de Catalunya (CCMC). In El Dolor y los Fármacos Opioides Mayores: Previniendo Problemas Potenciales; Colegios de Médicos de Catalunya (CCMC): Barcelona, Spain, 2018. [Google Scholar]
- Galan-Martin, M.A.; Montero-Cuadrado, F.; Lluch-Girbes, E.; Coca-López, M.C.; Mayo-Iscar, A.; Cuesta-Vargas, A. Pain Neuroscience Education and Physical Therapeutic Exercise for Patients with Chronic Spinal Pain in Spanish Physiotherapy Primary Care: A Pragmatic Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1201. [Google Scholar] [CrossRef]
- Serrat, M.; Almirall, M.; Musté, M.; Sanabria-Mazo, J.P.; Feliu-Soler, A.; Méndez-Ulrich, J.L.; Luciano, J.V.; Sanz, A. Effectiveness of a Multicomponent Treatment for Fibromyalgia Based on Pain Neuroscience Education, Exercise Therapy, Psychological Support, and Nature Exposure (NAT-FM): A Pragmatic Randomized Controlled Trial. J. Clin. Med. 2020, 9, 3348. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease. Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Vold, J.H.; Skurtveit, S.; Aas, C.; Chalabianloo, F.; Kloster, P.S.; Johansson, K.A.; Fadnes, L.T. Dispensations of Benzodiazepines, z-Hypnotics, and Gabapentinoids to Patients Receiving Opioid Agonist Therapy; a Prospective Cohort Study in Norway from 2013 to 2017. BMC Health Serv. Res. 2020, 20, 352. [Google Scholar] [CrossRef]
- Musich, S.; Wang, S.S.; Slindee, L.B.; Ruiz, J.; Yeh, C.S. Concurrent Use of Opioids with Other Central Nervous System-Active Medications Among Older Adults. Popul. Health Manag. 2020, 23, 286–296. [Google Scholar] [CrossRef]
- Friedman, J.; Kim, D.; Schneberk, T.; Bourgois, P.; Shin, M.; Celious, A.; Schriger, D.L. Assessment of Racial/Ethnic and Income Disparities in the Prescription of Opioids and Other Controlled Medications in California. JAMA Intern. Med. 2019, 179, 469–476. [Google Scholar] [CrossRef]
- Puustinen, J.; Nurminen, J.; Löppönen, M.; Vahlberg, T.; Isoaho, R.; Räihä, I.; Kivelä, S.-L. Use of CNS Medications and Cognitive Decline in the Aged: A Longitudinal Population-Based Study. BMC Geriatr. 2011, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Zin, C.S.; Ismail, F. Co-Prescription of Opioids with Benzodiazepine and Other Co-Medications among Opioid Users: Differential in Opioid Doses. J. Pain Res. 2017, 10, 249–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, E.Y.; Tamblyn, R.; Filion, K.B.; Buckeridge, D.L. Concurrent Prescriptions for Opioids and Benzodiazepines and Risk of Opioid Overdose: Protocol for a Retrospective Cohort Study Using Linked Administrative Data. BMJ Open 2021, 11, e042299. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, J.; Puustinen, J.; Piirtola, M.; Vahlberg, T.; Lyles, A.; Kivela, S.-L. Opioids, Antiepileptic and Anticholinergic Drugs and the Risk of Fractures in Patients 65 Years of Age and Older: A Prospective Population-Based Study. Age Ageing 2013, 42, 318–324. [Google Scholar] [CrossRef]
- Khan, S.R.; Heller, D.A.; Latty, L.L.; Cadieux, R.J.; LaSure, M.; Brown, T.V. Association between Psychotropic Drug Use and Prescription Opioid Use among Older Adults. Geriatr. Nurs. 2020, 41, 776–781. [Google Scholar] [CrossRef]
- SEMFyC; FAECAP; SECPAL. Guía de Consenso Para el Buen uso de Analgésicos Opioides; Socidrogalcohol: Madrid, Spain, 2017. [Google Scholar]
- Yu, D.; Appleyard, T.; Cottrell, E.; Peat, G. Co-Prescription of Gabapentinoids and Opioids among Adults with and without Osteoarthritis in the United Kingdom between 1995 and 2017. Rheumatology 2021, 60, 1942–1950. [Google Scholar] [CrossRef]
- Nielsen, S.; Gisev, N.; Leung, J.; Clare, P.; Bruno, R.; Lintzeris, N.; Larance, B.; Blyth, F.; Hall, W.; Cohen, M.; et al. Clinical Correlates and Outcomes Associated with Pregabalin Use among People Prescribed Opioids for Chronic Non-cancer Pain: A Five-year Prospective Cohort Study. Br. J. Clin. Pharmacol. 2021, 87, 3092–3104. [Google Scholar] [CrossRef]
- Batet, C.; Ferrándiz, M.; Limon, E.; Manresa, A.; Perelló Bratescu, A.; Samper Bernal, D. Consens Català de Dolor Crònic No Oncològic. Camfic, Societat Catalana del Dolor; Camfic, Societat Catalana de Dolor: Barcelona, Spain, 2017. [Google Scholar]
- Briggs, E.V.; Battelli, D.; Gordon, D.; Kopf, A.; Ribeiro, S.; Puig, M.M.; Kress, H.G. Current Pain Education within Undergraduate Medical Studies across Europe: Advancing the Provision of Pain Education and Learning (APPEAL) Study. BMJ Open 2015, 5, e006984. [Google Scholar] [CrossRef]
| No Concomitant Medication (n = 2337) (10.2%) | Concomitant Medication (n = 20,354) (89.7%) | p-Value | |
|---|---|---|---|
| n(%) | n(%) | ||
| Sex | <0.001 | ||
| Female | 1594 (9.1) | 15,915 (90.9) | |
| Male | 743 (14.3) | 4439 (85.7) | |
| Socioeconomic level (in Euros) | <0.001 | ||
| Exempt | 107 (7.8) | 1261 (92.2) | |
| <18,000 | 1910 (10.4) | 16,478 (89.6) | |
| 18,001–100,000 | 316 (10.9) | 2592 (89.1) | |
| >100,000 | 4 (14.8) | 23 (85.2) | |
| Age (in years) | <0.001 | ||
| <50 | 129 (7.1) | 1683 (92.9) | |
| 50–64 | 287 (7.3) | 3661 (92.7) | |
| 65–74 | 340 (9.1) | 3388 (90.9) | |
| 75–84 | 660 (10.4) | 5697 (89.6) | |
| 85–94 | 750 (12.7) | 5143 (87.3) | |
| ≥ 95 | 171 (17.9) | 782 (82.1) | |
| Health region | 0.317 | ||
| Urban | 1490 (10.3) | 12,962 (89.7) | |
| Semi-urban | 568 (10.2) | 4989 (89.8) | |
| Rural | 279 (10.4) | 2400 (89.6) |
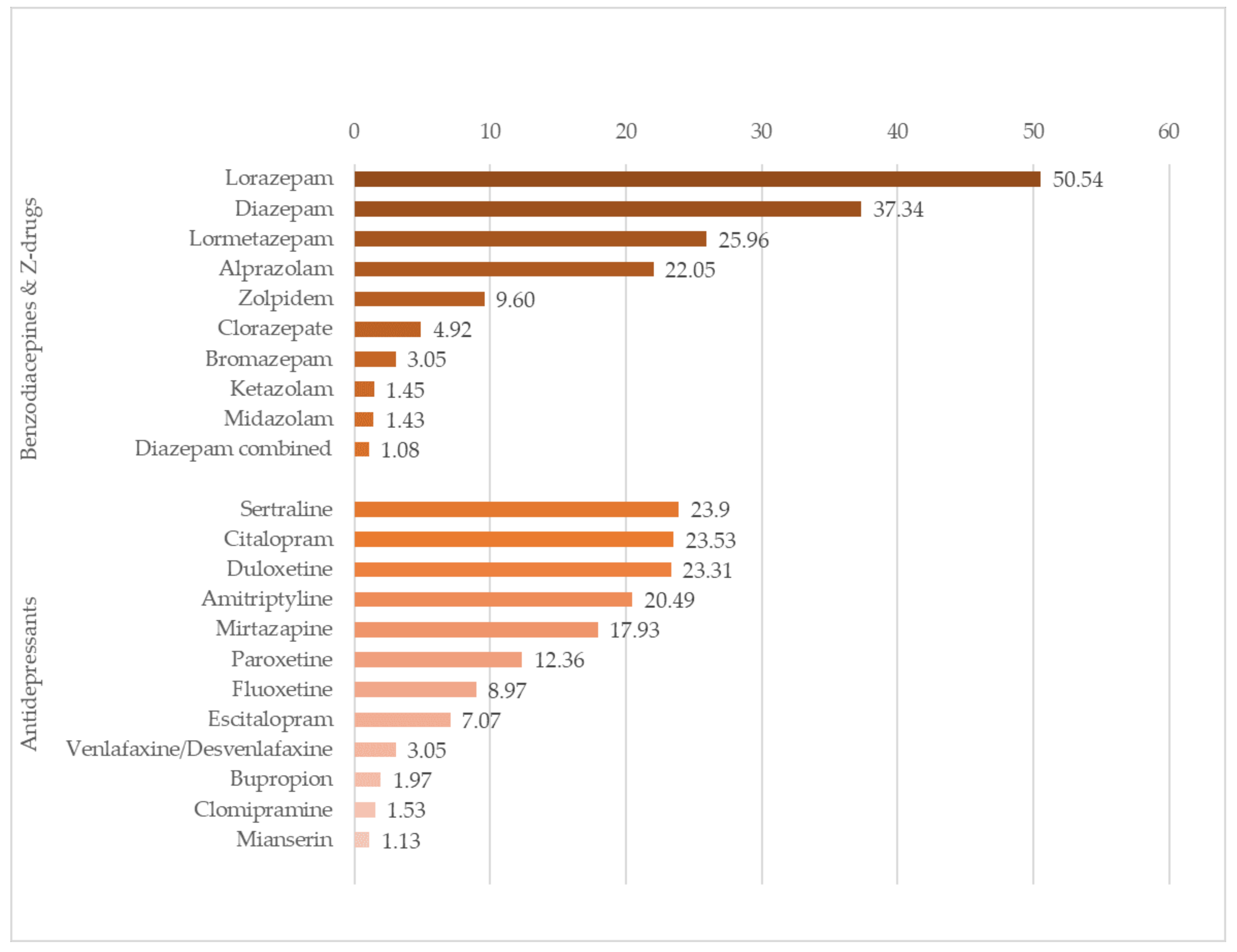
| Benzodiazepines | No. Patients | % |
|---|---|---|
| Lorazepam | 8027 | 50.54 |
| Diazepam | 5931 | 37.34 |
| Lormetazepam | 4124 | 25.96 |
| Alprazolam | 3502 | 22.05 |
| Zolpidem | 1525 | 9.6 |
| Clorazepate | 782 | 4.92 |
| Bromazepam | 485 | 3.05 |
| Ketazolam | 231 | 1.45 |
| Midazolam | 227 | 1.43 |
| Diazepam in combination with pyridoxine and/or sulpiride | 172 | 1.08 |
| Antidepressants | n Patients | % |
|---|---|---|
| Sertraline | 3569 | 23.9 |
| Citalopram | 3514 | 23.53 |
| Duloxetine | 3480 | 23.31 |
| Amitriptyline | 3060 | 20.49 |
| Mirtazapine | 2678 | 17.93 |
| Paroxetine | 1845 | 12.36 |
| Fluoxetine | 1340 | 8.97 |
| Escitalopram | 1056 | 7.07 |
| Venlafaxine/Desvenlafaxine | 456 | 3.05 |
| Bupropion | 294 | 1.97 |
| Clomipramine | 228 | 1.53 |
| Mianserin | 169 | 1.13 |
| Gabapentinoids | No. Patients | % |
|---|---|---|
| Gabapentin | 5573 | 49.46 |
| Pregabalin | 7564 | 67.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelló-Bratescu, A.; Dürsteler, C.; Álvarez-Carrera, M.A.; Granés, L.; Kostov, B.; Sisó-Almirall, A. Risk Prescriptions of Strong Opioids in the Treatment of Chronic Non-Cancer Pain by Primary Care Physicians in Catalonia: Opicat Padris Project. Int. J. Environ. Res. Public Health 2022, 19, 1652. https://doi.org/10.3390/ijerph19031652
Perelló-Bratescu A, Dürsteler C, Álvarez-Carrera MA, Granés L, Kostov B, Sisó-Almirall A. Risk Prescriptions of Strong Opioids in the Treatment of Chronic Non-Cancer Pain by Primary Care Physicians in Catalonia: Opicat Padris Project. International Journal of Environmental Research and Public Health. 2022; 19(3):1652. https://doi.org/10.3390/ijerph19031652
Chicago/Turabian StylePerelló-Bratescu, Aina, Christian Dürsteler, Maria Asunción Álvarez-Carrera, Laura Granés, Belchin Kostov, and Antoni Sisó-Almirall. 2022. "Risk Prescriptions of Strong Opioids in the Treatment of Chronic Non-Cancer Pain by Primary Care Physicians in Catalonia: Opicat Padris Project" International Journal of Environmental Research and Public Health 19, no. 3: 1652. https://doi.org/10.3390/ijerph19031652
APA StylePerelló-Bratescu, A., Dürsteler, C., Álvarez-Carrera, M. A., Granés, L., Kostov, B., & Sisó-Almirall, A. (2022). Risk Prescriptions of Strong Opioids in the Treatment of Chronic Non-Cancer Pain by Primary Care Physicians in Catalonia: Opicat Padris Project. International Journal of Environmental Research and Public Health, 19(3), 1652. https://doi.org/10.3390/ijerph19031652









