Quantitative Microbial Risk Assessment Applied to Legionella Contamination on Long-Distance Public Transport
Abstract
:1. Introduction
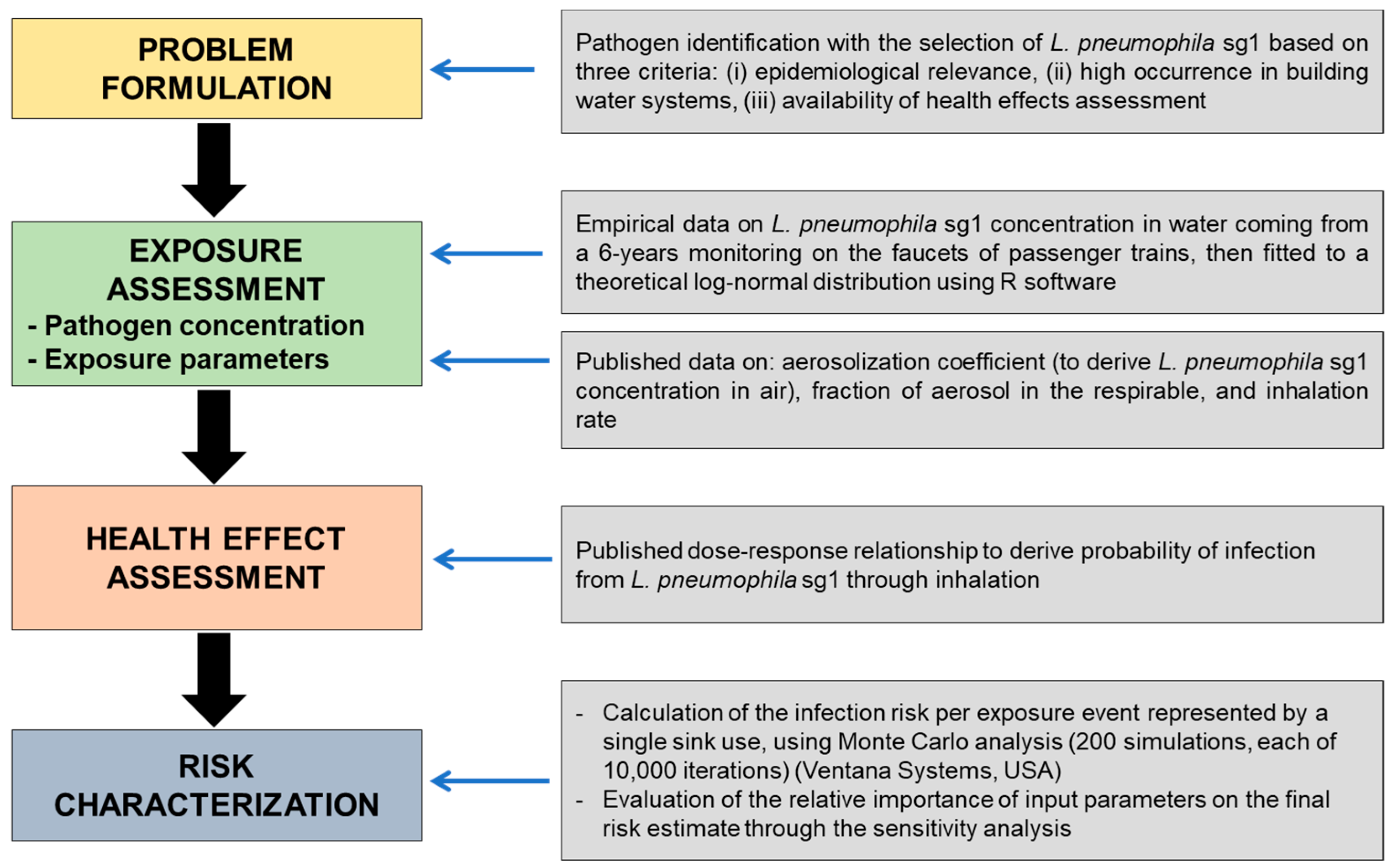
2. Materials and Methods
2.1. Water Sampling and Legionella Analysis
2.2. Statistical Analysis
2.3. QMRA Methodology
2.3.1. Exposure Assessment
2.3.2. Dose–Response Assessment
2.3.3. Risk Characterization and Sensitivity Analysis
3. Results
3.1. Descriptive Evaluation of Monitoring Data
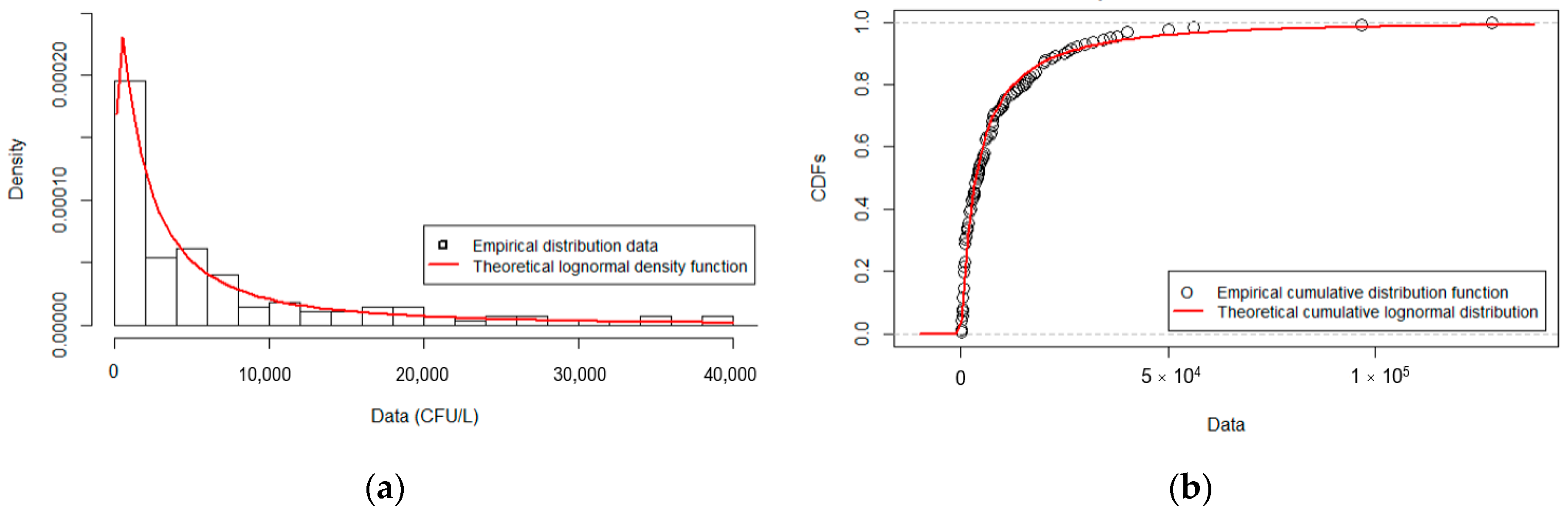
3.2. Fitted Distribution for L. pneumophila Serogroup 1
3.3. QMRA Simulation Results
3.3.1. Infection Risk from Inhalation of L. pneumophila sg1
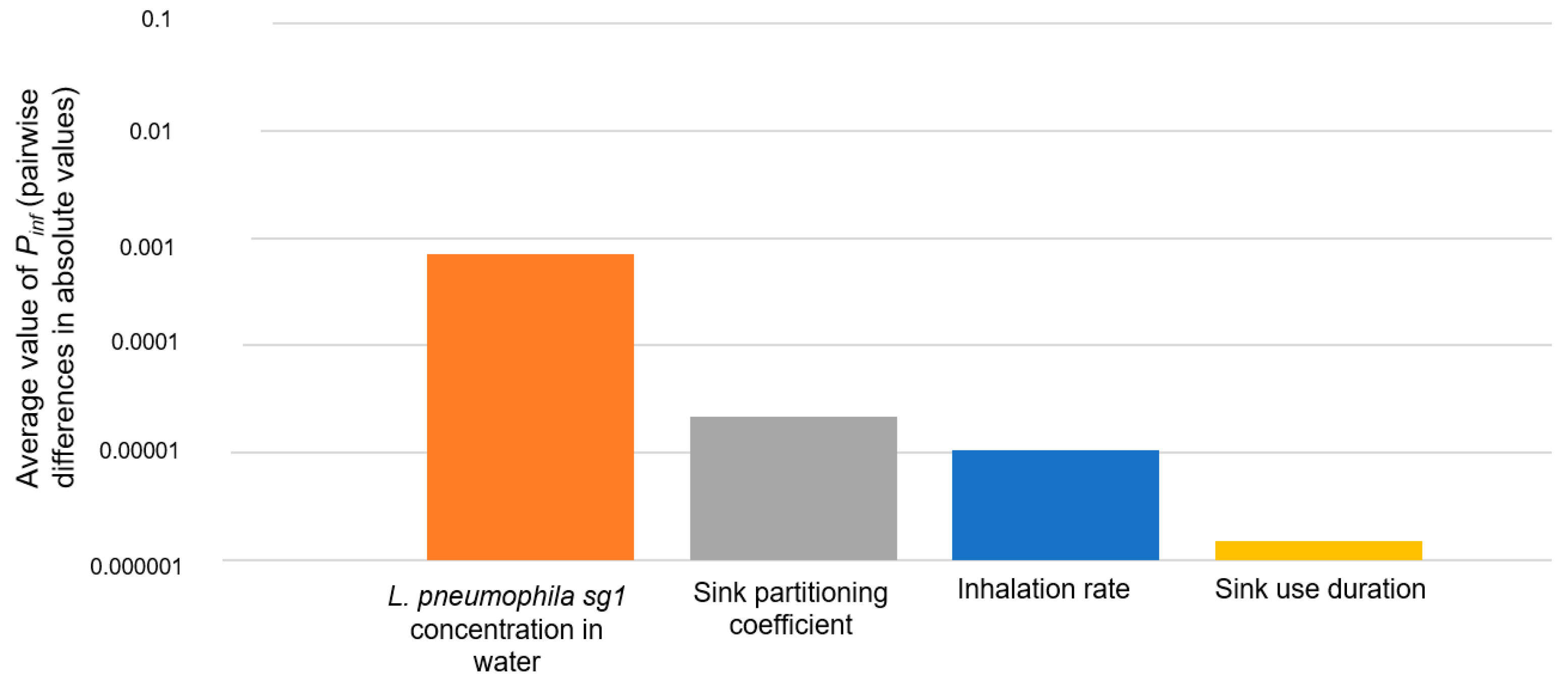
3.3.2. Sensitivity Analysis Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Health Aspects of Plumbing; World Health Organization, World Council of Plumbing, Eds.; World Health Organization (WHO): Geneva, Switzerland, 2006; ISBN 9241563184. Available online: https://www.who.int/publications/i/item/9241563184 (accessed on 1 December 2021).
- Falkinham, J.O.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Falkinham, J.O., 3rd. Living with Legionella and Other Waterborne Pathogens. Microorganisms 2020, 8, 2026. [Google Scholar] [CrossRef]
- Benedict, K.M.; Reses, H.; Vigar, M.; Roth, D.M.; Roberts, V.A.; Mattioli, M.; Cooley, L.A.; Hilborn, E.D.; Wade, T.J.; Fullerton, K.E.; et al. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2013–2014. MMWR Morb. Mortal Wkly. Rep. 2017, 66, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.A.; Prussin, A.J., 2nd; Ahmed, W.; Haas, C.N. Outbreaks of Legionnaires’ Disease and Pontiac Fever 2006–2017. Curr. Environ. Health Rep. 2018, 5, 263–271. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Legionnaires’ Disease. In Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/legionnaires-disease-annual-epidemiological-report-2019 (accessed on 1 December 2021).
- Bauer, M.; Mathieu, L.; Deloge-Abarkan, M.; Remen, T.; Tossa, P.; Hartemann, P.; Zmirou-Navier, D. Legionella bacteria in shower aerosols increase the risk of Pontiac fever among older people in retirement homes. J. Epidemiol. Community Health 2008, 62, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Breiman, R.F.; Fields, B.S.; Sanden, G.N.; Volmer, L.; Meier, A.; Spika, J.S. Association of shower use with Legionnaires’ disease. Possible role of amoebae. JAMA 1990, 263, 2924–2926. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, O.; Fasano, F.; Diella, G.; Lopuzzo, M.; Napoli, C.; Apollonio, F.; Brigida, S.; Calia, C.; Campanale, C.; Marzella, A.; et al. Legionella and legionellosis in touristic-recreational facilities: Influence of climate factors and geostatistical analysis in Southern Italy (2001–2017). Environ. Res. 2019, 178, 108721. [Google Scholar] [CrossRef]
- Katsiaflaka, A.; Pournaras, S.; Kristo, I.; Mouchtouri, V.A.; Kyritsi, M.; Velonakis, E.; Vatopoulos, A.C.; Hadjichristodoulou, C. Epidemiological investigation of Legionella pneumophila serogroup 2 to 14 isolates from water samples by amplified fragment length polymorphism and sequence-based typing and detection of virulence traits. Appl. Environ. Microbiol. 2016, 82, 6102–6108. [Google Scholar] [CrossRef] [Green Version]
- Azara, A.; Piana, A.; Sotgiu, G.; Dettori, M.; Deriu, M.G.; Masia, M.D.; Are, B.M.; Muresu, E. Prevalence study of Legionella spp. contamination in ferries and cruise ships. BMC Public Health 2006, 6, 100. [Google Scholar] [CrossRef] [Green Version]
- Laganà, P.; Gambuzza, M.E.; Delia, S. Legionella risk assessment in cruise ships and ferries. Ann. Agric. Environ. Med. 2017, 24, 276–282. [Google Scholar] [CrossRef]
- De Filippis, P.; Mozzetti, C.; Messina, A.; D’Alò, G.L. Prevalence of Legionella in retirement homes and group homes water distribution systems. Sci. Total Environ. 2018, 643, 715–724. [Google Scholar] [CrossRef]
- Borella, P.; Montagna, M.T.; Romano-Spica, V.; Stampi, S.; Stancanelli, G.; Triassi, M.; Neglia, R.; Marchesi, I.; Fantuzzi, G.; Tatò, D.; et al. Legionella infection risk from domestic hot water. Emerg. Infect. Dis. 2004, 10, 457–464. [Google Scholar] [CrossRef]
- Mathys, W.; Stanke, J.; Harmuth, M.; Junge-Mathys, E. Occurrence of Legionella in hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating. Int. J. Hyg. Environ. Health 2008, 211, 179–185. [Google Scholar] [CrossRef]
- Donohue, M.J.; King, D.; Pfaller, S.; Mistry, J.H. The sporadic nature of Legionella pneumophila, Legionella pneumophila Sg1 and Mycobacterium avium occurrence within residences and office buildings across 36 states in the United States. J. Appl. Microbiol. 2019, 126, 1568–1579. [Google Scholar] [CrossRef] [Green Version]
- Bonetta, S.; Bonetta, S.; Ferretti, E.; Balocco, F.; Carraro, E. Evaluation of Legionella pneumophila contamination in Italian hotel water systems by quantitative real-time PCR and culture methods. J. Appl. Microbiol. 2011, 108, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- WHO. Legionella and the Prevention of Legionellosis; World Health Organization (WHO): Geneva, Switzerland, 2007; ISBN 9241562978. Available online: https://apps.who.int/iris/handle/10665/43233 (accessed on 1 December 2021).
- ESGLI. European Technical Guidelines for the Prevention, Control and Investigation of Infections Caused by Legionella Species; European Society of Clinical Microbiology and Infectious Diseases Study Group for Legionella Infections (ESGLI): Basel, Switzerland, 2017; Available online: https://www.ecdc.europa.eu/en/publications-data/european-technical-guidelines-prevention-control-and-investigation-infections (accessed on 1 December 2021).
- Hamilton, K.A.; Hamilton, M.T.; Johnson, W.; Jjemba, P.; Bukhari, Z.; LeChevallier, M.; Haas, C.N.; Gurian, P.L. Risk-Based Critical Concentrations of Legionella pneumophila for Indoor Residential Water Uses. Environ. Sci. Technol. 2019, 53, 4528–4541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Quantitative Microbial Risk Assessment: Application for Water Safety Management; World Health Organization (WHO): Geneva, Switzerland, 2016. [Google Scholar]
- Quaranta, G.; Vincenti, S.; Ferriero, A.M.; Boninti, F.; Sezzatini, R.; Turnaturi, C.; Gliubizzi, M.D.; Munafò, E.; Ceccarelli, G.; Causarano, C.; et al. Legionella on board trains: Effectiveness of environmental surveillance and decontamination. BMC Public Health 2012, 12, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Italian Ministry of Health. Guidelines for the Prevention and Control of Legionellosis. 2015. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?id=2362 (accessed on 1 December 2021). (In Italian)
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: www.Rproject.org (accessed on 1 December 2021).
- Pouillot, R.; Delignette-Muller, M.L. Evaluating variability and uncertainty in microbial quantitative risk assessment using two R packages. Int. J. Food Microbiol. 2010, 142, 330–340. [Google Scholar] [CrossRef]
- Gan, F.F.; Koehler, K.J. Goodness-of-Fit Tests Based on P-P Probability Plots. Technometrics 1990, 32, 289–303. [Google Scholar] [CrossRef]
- Haas, C.; Rose, J.; Gerba, C. Risk assessment paradigms. In Quantitative Microbial Risk Assessment, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; ISBN 9781118145296. [Google Scholar]
- Whiley, H.; Keegan, A.; Fallowfield, H.; Ross, K. Uncertainties associated with assessing the public health risk from Legionella. Front. Microbiol. 2014, 5, 501. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, K.A.; Haas, C.N. Critical review of mathematical approaches for quantitative microbial risk assessment (QMRA) of Legionella in engineered water systems: Research gaps and a new framework. Environ. Sci. Water Res. Technol. 2016, 2, 599–613. [Google Scholar] [CrossRef]
- Bollin, G.E.; Plouffe, J.F.; Para, M.F.; Hackman, B. Aerosols containing Legionella pneumophila generated by shower heads and hot-water faucets. Appl. Environ. Microbiol. 1995, 50, 1128–1131. [Google Scholar] [CrossRef] [Green Version]
- U.S. Environmental Protection Agency. Exposure Factors Handbook: Chapter 6—Inhalation Rates. 2011. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 (accessed on 1 December 2021).
- Armstrong, T.W.; Haas, C.N. A quantitative microbial risk assessment model for Legionnaires’ disease: Animal model selection and dose-response modeling. Risk Anal. 2007, 27, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Federigi, I.; Bonadonna, L.; Bonanno Ferraro, G.; Briancesco, R.; Cioni, L.; Coccia, A.M.; Della Libera, S.; Ferretti, E.; Gramaccioni, L.; Iaconelli, M.; et al. Quantitative Microbial Risk Assessment as support for bathing water profiling. Mar. Pollut. Bull. 2020, 157, 111318. [Google Scholar] [CrossRef]
- Ratcliff, R.M.; Lanser, J.A.; Manning, P.A.; Heuzenroeder, M.W. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J. Clin. Microbiol. 1998, 36, 1560–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Academies of Sciences, Engineering, and Medicine. Management of Legionella in Water Systems; The National Academies Press: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Yu, V.L.; Plouffe, J.F.; Pastoris, M.C.; Stout, J.E.; Schousboe, M.; Widmer, A.; Summersgill, J.; File, T.; Heath, C.M.; Paterson, D.L.; et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: An international collaborative survey. J. Infect. Dis. 2002, 186, 127–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, S.; Scaturro, M.; Rota, M.C.; Caporali, M.G.; Ricci, M.L. Molecular typing of Legionella pneumophila serogroup 1 clinical strains isolated in Italy. Int. J. Med. Microbiol. 2014, 304, 597–602. [Google Scholar] [CrossRef]
- Beauté, J. Legionnaires’ disease in Europe, 2011 to 2015. European Legionnaires’ Disease Surveillance Network. Eurosurveillance 2017, 22, 30566. [Google Scholar] [CrossRef] [Green Version]
- Schoen, M.E.; Ashbolt, N.J. An in-premise model for Legionella exposure during showering events. Water Res. 2011, 45, 5826–5836. [Google Scholar] [CrossRef]
- Azuma, K.; Uchiyama, I.; Okumura, J. Assessing the risk of Legionnaires’ disease: The inhalation exposure model and the estimated risk in residential bathrooms. Regul. Toxicol. Pharmacol. 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Schijven, J.F.; Schalk, J.A.; de Roda Husman, A.M. Quantitative risk estimation for a Legionella pneumophila infection due to whirlpool use. Risk Anal. 2013, 33, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Blanky, M.; Sharaby, Y.; Rodríguez-Martínez, S.; Halpern, M.; Friedler, E. Greywater reuse—Assessment of the health risk induced by Legionella pneumophila. Water Res. 2017, 125, 410–417. [Google Scholar] [CrossRef]
- Hines, S.A.; Chappie, D.J.; Lordo, R.A.; Miller, B.D.; Janke, R.J.; Lindquist, H.A.; Fox, K.R.; Ernst, H.S.; Taft, S.C. Assessment of relative potential for Legionella species or surrogates inhalation exposure from common water uses. Water Res. 2014, 56, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Polat, Y.; Ergin, C.; Kaleli, I.; Pinar, A. Riskli bir meslek olarak profesyonel uzun yol süruocolerinde Legionella pneumophila seropozitifldginin araştirilmasi [Investigation of Legionella pneumophila seropositivity in the professional long distance drivers as a risky occupation]. Mikrobiyol. Bul. 2007, 41, 211–217. (In Turkish) [Google Scholar] [PubMed]



| Input Variables | Description | Unit | Characterization | Source and Comments |
|---|---|---|---|---|
| Cwater | L. pneumophila sg1 concentration in water | CFU/L | lognormal distribution 1 (µ = 8.166848, σ = 1.521021) | This article, based on a 6-year monitoring period |
| PC | Sink partitioning coefficient | L/m3 | lognormal distribution 1 (μ = −13.3, σ = 3.49) truncated on the interval [0, 2.35 × 10−3] | Hamilton et al., 2019 [20]. Data analysis of 19 paired water and air samples from hot-water faucets |
| F1–8 | Percentage of aerosols in respirable range (between 1 and 8 μm reported) for partitioning coefficient | % | point estimate (50) | Bollin et al., 1985 [30]. Monitoring of air samples from hot-water faucets, and approximately half of recovered Legionella were between 1 and 8 μm aerosol |
| IR | Inhalation rate | m3/min | uniform distribution (min = 0.013, max = 0.017) | USEPA 2011 [31]. Inhalation rate for individuals engaging in light activities |
| ET | Sink use duration | min | uniform distribution (min = 0.5, max = 1.5) | An assumption on the duration of an individual would stay in the toilet for hand washing. |
| L. pneumophila Total | L. pneumophila sg1 | L. pneumophila sg 2–14 | Mixed L. pneumophila sg 1 and sg 2–14 | |
|---|---|---|---|---|
| Positive samples (n°, %) | 217/398 (54.5%) | 139/217 (64.1%) | 58/217 (26.7%) | 20/217 (9.2%) |
| Count (CFU/L) | ||||
| Geometric mean | 4.93 × 103 ± 4.97 | 3.67 × 103 ± 4.96 | 5.72 × 103 ± 5.74 | 7.00 × 103 ± 2.45 |
| Median | 5.00 × 103 | 4.10 × 103 | 6.68 × 103 | 6.63 × 103 |
| IQR (1°–3° quartiles) | 1.50 × 103–1.30 × 104 | 1.00 × 103–1.14 × 104 | 2.13 × 103–1.93 × 104 | 3.79 × 103–1.22 × 104 |
| Load distribution categories (n°, %) | ||||
| ≤100 CFU/L | 4/217 (1.8%) | 1/139 (0.7%) | 3/58 (5.2%) | 0/20 (0%) |
| 101–1000 CFU/L | 44/217 (20.3%) | 39/139 (28.1%) | 5/58 (8.6%) | 0/20 (0%) |
| 1001–10,000 CFU/L | 100/217 (46.1%) | 61/139 (43.9%) | 27/58 (46.6%) | 12/20 (60%) |
| ≥10,001 CFU/L | 69/217 (31.8%) | 38/139 (27.3%) | 23/58 (39.7%) | 8/20 (40%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Federigi, I.; De Giglio, O.; Diella, G.; Triggiano, F.; Apollonio, F.; D’Ambrosio, M.; Cioni, L.; Verani, M.; Montagna, M.T.; Carducci, A. Quantitative Microbial Risk Assessment Applied to Legionella Contamination on Long-Distance Public Transport. Int. J. Environ. Res. Public Health 2022, 19, 1960. https://doi.org/10.3390/ijerph19041960
Federigi I, De Giglio O, Diella G, Triggiano F, Apollonio F, D’Ambrosio M, Cioni L, Verani M, Montagna MT, Carducci A. Quantitative Microbial Risk Assessment Applied to Legionella Contamination on Long-Distance Public Transport. International Journal of Environmental Research and Public Health. 2022; 19(4):1960. https://doi.org/10.3390/ijerph19041960
Chicago/Turabian StyleFederigi, Ileana, Osvalda De Giglio, Giusy Diella, Francesco Triggiano, Francesca Apollonio, Marilena D’Ambrosio, Lorenzo Cioni, Marco Verani, Maria Teresa Montagna, and Annalaura Carducci. 2022. "Quantitative Microbial Risk Assessment Applied to Legionella Contamination on Long-Distance Public Transport" International Journal of Environmental Research and Public Health 19, no. 4: 1960. https://doi.org/10.3390/ijerph19041960








