When Bodybuilding Goes Wrong—Bilateral Renal Artery Thrombosis in a Long-Term Misuser of Anabolic Steroids Treated with AngioJet Rheolytic Thrombectomy
Abstract
:1. Introduction
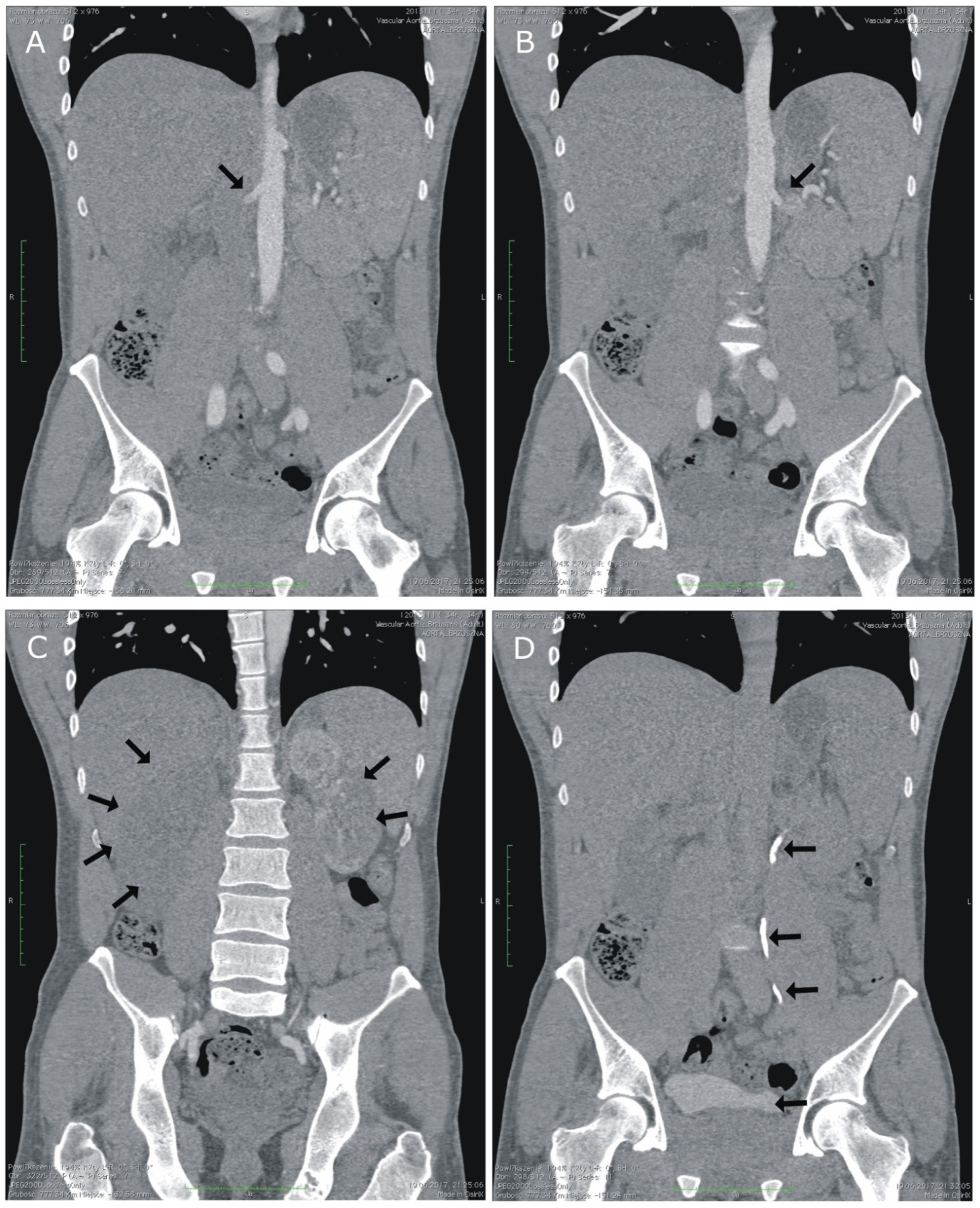
2. Case Presentation
3. Discussion
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Lee, J.Y.; Na, Y.J.; Lim, S.Y.; Kim, M.G.; Jo, S.K.; Cho, W. Risk factors and outcomes of acute renal infarction. Kidney Res. Clin. Pract. 2016, 35, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.J.; Liu, L.J.; Chen, M.; Zhou, F.D. Asynchronous Bilateral Renal Infarction and Thrombophilia With Associated Gene Mutations in a 43-Year-Old Man: A Case Report. Medicine 2016, 95, e3258. [Google Scholar] [CrossRef] [PubMed]
- Eren, N.; Gungor, O.; Kocyigit, I.; Guzel, F.B.; Erken, E.; Altunoren, O.; Tatar, E.; Eroglu, E.; Senel, E.; Kaya, B.; et al. Acute renal infarction in Turkey: A review of 121 cases. Int. Urol. Nephrol. 2018, 50, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Mihout, F.; Joseph, L.; Brocheriou, I.; Leblond, V.; Varnous, S.; Ronco, P.; Plaisier, E. Bilateral kidney infarction due to primary Al amyloidosis: A first case report. Medicine 2015, 94, e777. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Song, S.Y.; Lee, C.H.; Ko, B.H.; Lee, S.; Kang, B.K.; Kim, M.M. Spontaneous Renal Artery Dissection as a Cause of Acute Renal Infarction: Clinical and MDCT Findings. J. Korean Med. Sci. 2017, 32, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Añazco, P.H.; Balta, F.M.; Córdova-Cueva, L. Bilateral renal infarction in a patient with severe COVID-19 infection. J. Bras. Nefrol. 2021, 43, 127–131. [Google Scholar] [CrossRef]
- Ayach, T.; Kazory, A. Bilateral renal infarction: An uncommon presentation of fibromuscular dysplasia. Clin. Kidney J. 2013, 6, 646–649. [Google Scholar] [CrossRef] [Green Version]
- Madhrira, M.M.; Mohan, S.; Markowitz, G.S.; Pogue, V.A.; Cheng, J.T. Acute bilateral renal infarction secondary to cocaine-induced vasospasm. Kidney Int. 2009, 76, 576–580. [Google Scholar] [CrossRef] [Green Version]
- Leifman, H.; Rehnman, C.; Sjöblom, E.; Holgersson, S. Anabolic androgenic steroids-use and correlates among gym users-an assessment study using questionnaires and observations at gyms in the Stockholm region. Int. J. Environ. Res. Public Health 2011, 8, 2656–2674. [Google Scholar] [CrossRef]
- Kanayama, G.; Kaufman, M.J.; Pope, H.G. Public health impact of androgens. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 218–223. [Google Scholar] [CrossRef]
- Uzunova, A.D.; Ramey, E.R.; Ramwell, P.W. Arachidonate-Induced thrombosis in mice: Effects of gender or testosterone and estradiol administration. Prostaglandins 1977, 13, 995–1002. [Google Scholar] [CrossRef]
- Chang, S.; Münster, A.M.B.; Gram, J.; Sidelmann, J.J. Anabolic Androgenic Steroid Abuse: The Effects on Thrombosis Risk, Coagulation, and Fibrinolysis. Semin. Thromb. Hemost. 2018, 44, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Kouidi, E.J.; Kaltsatou, A.; Anifanti, M.A.; Deligiannis, A.P. Early Left Ventricular Diastolic Dysfunction, Reduced Baroreflex Sensitivity, and Cardiac Autonomic Imbalance in Anabolic–Androgenic Steroid Users. Int. J. Environ. Res. Public Health 2021, 18, 6974. [Google Scholar] [CrossRef] [PubMed]
- Christou, G.A.; Christou, K.A.; Nikas, D.N.; Goudevenos, J.A. Acute myocardial infarction in a young bodybuilder taking anabolic androgenic steroids: A case report and critical review of the literature. Eur. J. Prev. Cardiol. 2016, 23, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, R.D.; Besocke, A.G.; Romano, L.M.; Ioli, P.L.; Gonorazky, S.E. Ischemic stroke related to anabolic abuse. Clin. Neuropharmacol. 2008, 31, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, M.; Karlsson, J.; Ortenwall, P. Peripheral arterial thrombosis in two young men using anabolic steroids. Eur. J. Vasc. Endovasc. Surg. 1997, 13, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Torrisi, M.; Pennisi, G.; Russo, I.; Amico, F.; Esposito, M.; Liberto, A.; Cocimano, G.; Salerno, M.; Rosi, G.L.; Di Nunno, N.; et al. Sudden cardiac death in anabolic-androgenic steroid users: A literature review. Medicina 2020, 56, 587. [Google Scholar] [CrossRef]
- Leong, F.T.; Freeman, L.J. Acute renal infarction. J. R. Soc. Med. 2005, 98, 121–122. [Google Scholar] [CrossRef]
- Carman, T.L.; Olin, J.W.; Julianna, C. Noninvasive imaging of the renal arteries. Urol. Clin. N. Am. 2001, 28, 815–826. [Google Scholar] [CrossRef]
- Oh, Y.K.; Yang, C.W.; Kim, Y.L.; Kang, S.W.; Park, C.W.; Kim, Y.S.; Lee, E.Y.; Han, B.G.; Lee, S.H.; Kim, S.H.; et al. Clinical Characteristics and Outcomes of Renal Infarction. Am. J. Kidney Dis. 2016, 67, 243–250. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Xhepa, G.; Piffaretti, G.; Bacuzzi, A.; Tozzi, M.; Carbone, M.; Barile, A.; Squillaci, E.; Fonio, P.; Brunese, L.; et al. Clinical experience with Angiojet: A comprehensive review. Int. Angiol. 2015, 34, 1–14. [Google Scholar] [PubMed]
- Latacz, P.; Simka, M.; Brzegowy, P.; Serednicki, W.; Konduracka, E.; Mrowiecki, W.; Słowik, A.; Łasocha, B.; Mrowiecki, T.; Popiela, T. Treatment of high- and intermediate-risk pulmonary embolism using the AngioJet percutaneous mechanical thrombectomy system in patients with contraindications for thrombolytic treatment—A pilot study. Wideochir. Inne Tech. Maloinwazyjne 2018, 13, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, D.; Menes, T.; Rimon, U.; Salomon, O.; Halak, M. Acute renal artery occlusion: Presentation, treatment, and outcome. J. Vasc. Surg. 2016, 64, 1026–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siablis, D.; Liatsikos, E.N.; Goumenos, D.; Karnabatidis, D.; Voudoukis, T.; Barbalias, G.; Vlahogiannis, J. Percutaneous rheolytic thrombectomy for treatment of acute renal-artery thrombosis. J. Endourol. 2005, 19, 68–71. [Google Scholar] [CrossRef]
- Greenberg, J.M.; Steiner, M.A.; Marshall, J.J. Acute renal artery thrombosis treated by percutaneous rheolytic thrombectomy. Catheter. Cardiovasc. Interv. 2002, 56, 66–68. [Google Scholar] [CrossRef]
- Favi, E.; Iesari, S.; Cina, A.; Citterio, F. Spontaneous renal allograft rupture complicated by urinary leakage: Case report and review of the literature. BMC Urol. 2015, 15, 114. [Google Scholar] [CrossRef] [Green Version]
- Shahrokh, H.; Rasouli, H.; Zargar, M.A.; Karimi, K.; Zargar, K. Spontaneous kidney allograft rupture. Transplant. Proc. 2005, 37, 3079–3080. [Google Scholar] [CrossRef]
| Day of Stay | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Creatinine [mg/dL] | 1.82 | 2.66 | 3.82 | 4.34 | 4.98 | 4.43 | 3.89 |
| eGFR [mL/min/1.73 m2] | 47 | 30 | 19 | 17 | 14 | 16 | 19 |
| Urine output [mL] | n.d. | 2100 | 450 | 780 | 4500 | 8400 | 2900 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemiński, A.; Kubis, M.; Kaczmarek, K.; Gołąb, A.; Kazimierczak, A.; Kotfis, K.; Słojewski, M. When Bodybuilding Goes Wrong—Bilateral Renal Artery Thrombosis in a Long-Term Misuser of Anabolic Steroids Treated with AngioJet Rheolytic Thrombectomy. Int. J. Environ. Res. Public Health 2022, 19, 2122. https://doi.org/10.3390/ijerph19042122
Lemiński A, Kubis M, Kaczmarek K, Gołąb A, Kazimierczak A, Kotfis K, Słojewski M. When Bodybuilding Goes Wrong—Bilateral Renal Artery Thrombosis in a Long-Term Misuser of Anabolic Steroids Treated with AngioJet Rheolytic Thrombectomy. International Journal of Environmental Research and Public Health. 2022; 19(4):2122. https://doi.org/10.3390/ijerph19042122
Chicago/Turabian StyleLemiński, Artur, Markiian Kubis, Krystian Kaczmarek, Adam Gołąb, Arkadiusz Kazimierczak, Katarzyna Kotfis, and Marcin Słojewski. 2022. "When Bodybuilding Goes Wrong—Bilateral Renal Artery Thrombosis in a Long-Term Misuser of Anabolic Steroids Treated with AngioJet Rheolytic Thrombectomy" International Journal of Environmental Research and Public Health 19, no. 4: 2122. https://doi.org/10.3390/ijerph19042122
APA StyleLemiński, A., Kubis, M., Kaczmarek, K., Gołąb, A., Kazimierczak, A., Kotfis, K., & Słojewski, M. (2022). When Bodybuilding Goes Wrong—Bilateral Renal Artery Thrombosis in a Long-Term Misuser of Anabolic Steroids Treated with AngioJet Rheolytic Thrombectomy. International Journal of Environmental Research and Public Health, 19(4), 2122. https://doi.org/10.3390/ijerph19042122








