Ultra-High Frequency Distortion Product Otoacoustic Emissions for Detection of Hearing Loss and Tinnitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Statistical Analysis
3. Results
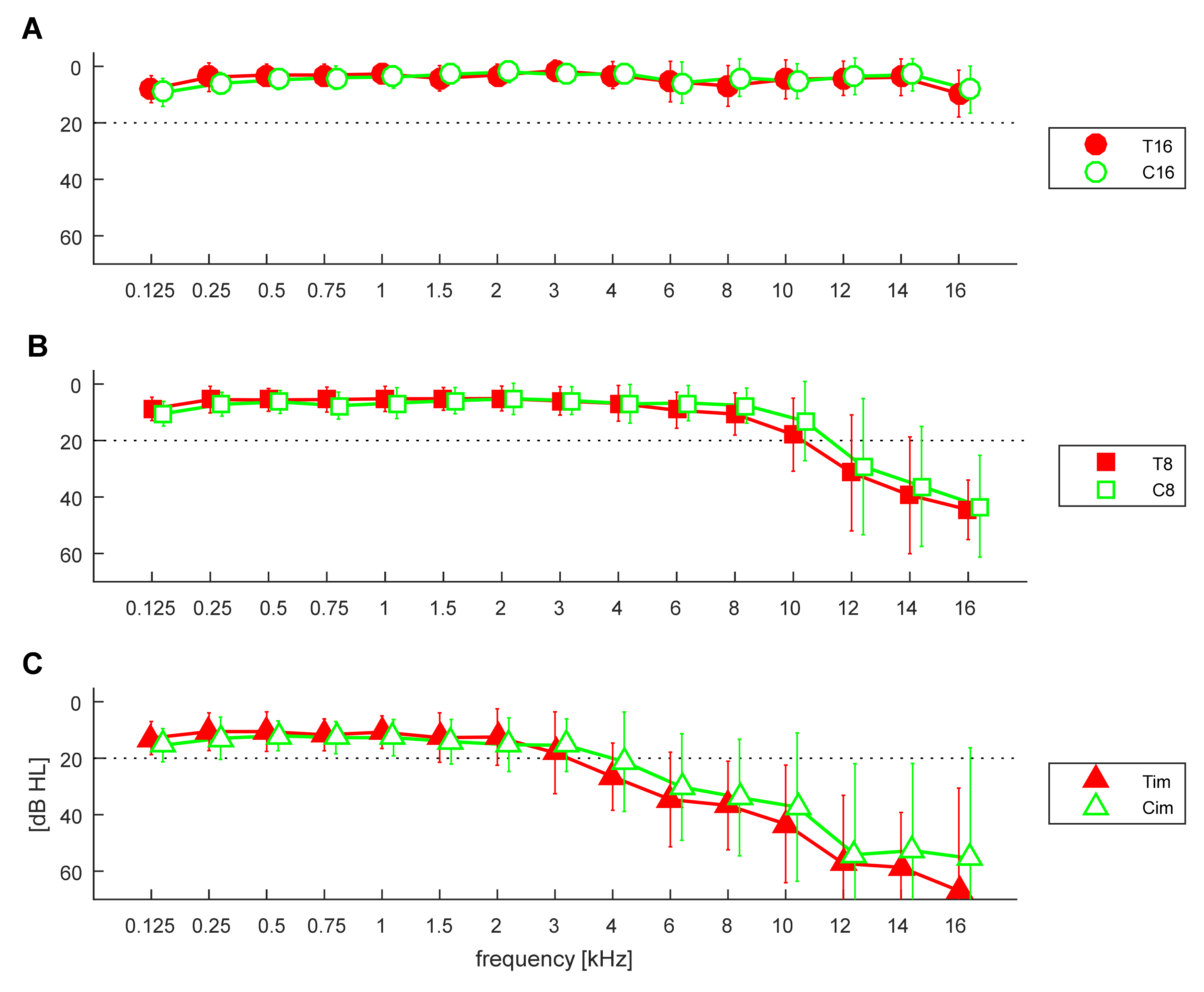
3.1. Pure Tone Thresholds
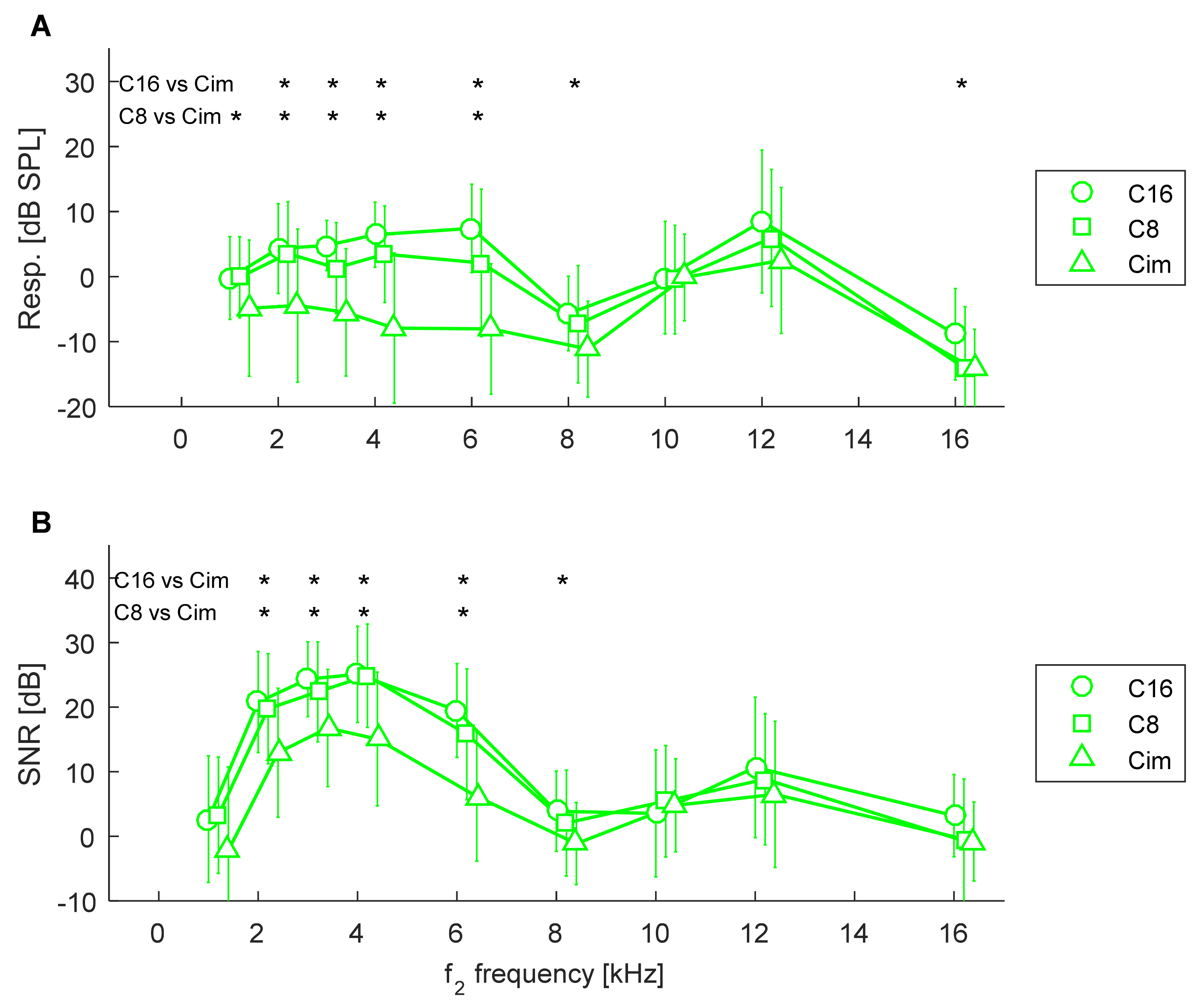
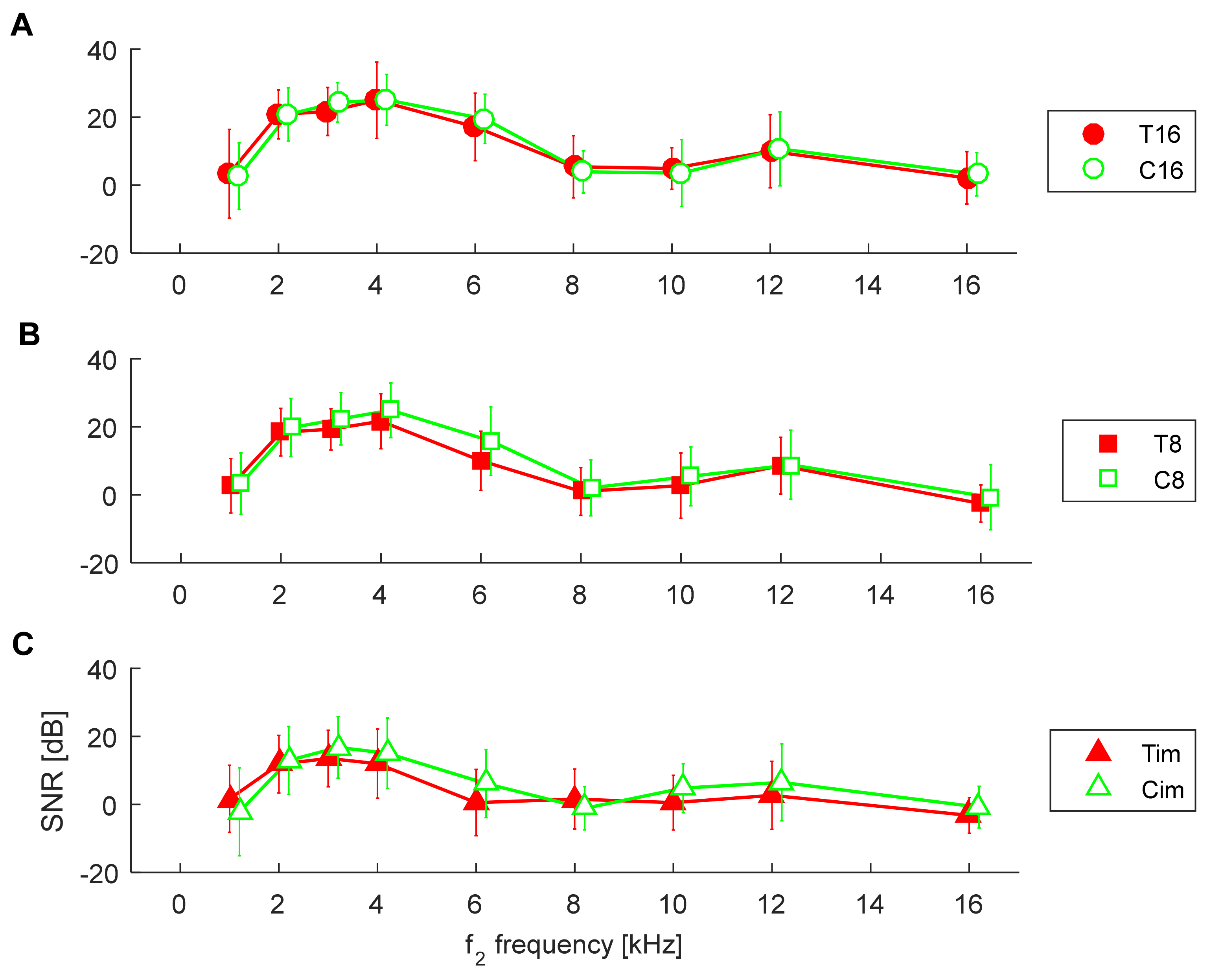
3.2. Comparison of Groups in Relation to Hearing Loss
3.3. Comparison between Controls and Tinnitus Subjects
3.4. Discrimination of Hearing Loss
3.5. Discrimination of Tinnitus
3.6. Examples of Criteria and Their Performance
4. Discussion
4.1. Relationship of DPOAEs to Tinnitus
4.2. Relationship between DPOAEs and HL
4.3. Limitations
4.4. Thoughts on the Usefulness of OAEs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haider, H.F.; Hoare, D.J.; Ribeiro, S.F.; Ribeiro, D.; Caria, H.; Trigueiros, N.; Borrego, L.M.; Szczepek, A.J.; Papoila, A.L.; Elarbed, A.; et al. Evidence for biological markers of tinnitus: A systematic review. Prog. Brain Res. 2021, 262, 345–398. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Vijendren, A.; Phillips, J. Objective Measures of Tinnitus: A Systematic Review. Otol. Neurotol. 2019, 40, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Probst, R.; Lonsbury-Martin, B.L.; Martin, G.K. A review of otoacoustic emissions. J. Acoust. Soc. Am. 1991, 89, 2027–2067. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Roberts, L.E.; Caspary, D.M.; Theodoroff, S.M.; Salvi, R.J. Underlying mechanisms of tinnitus: Review and clinical implications. J. Am. Acad. Audiol. 2014, 25, 5–22. [Google Scholar] [CrossRef]
- Olze, H.; Szczepek, A.J.; Haupt, H.; Zirke, N.; Graebel, S.; Mazurek, B. The impact of cochlear implantation on tinnitus, stress and quality of life in postlingually deafened patients. Audiol. Neurootol. 2012, 17, 2–11. [Google Scholar] [CrossRef]
- Marshall, L.; Lapsley Miller, J.A.; Heller, L.M. Distortion-Product Otoacoustic Emissions as a Screening Tool for Noise-Induced Hearing Loss. Noise Health 2001, 3, 43–60. [Google Scholar]
- De PCampos, U.; Sanches, S.G.; Hatzopoulos, S.; Carvallo, R.M.; Kochanek, K.; Skarżyński, H. Alteration of distortion product otoacoustic emission input/output functions in subjects with a previous history of middle ear dysfunction. Med. Sci. Monit. 2012, 18, MT27–MT31. [Google Scholar] [CrossRef] [Green Version]
- Ozimek, E.; Wicher, A.; Szyfter, W.; Szymiec, E. Distortion product otoacoustic emission (DPOAE) in tinnitus patients. J. Acoust. Soc. Am. 2006, 119, 527–538. [Google Scholar] [CrossRef]
- Ami, M.; Abdullah, A.; Awang, M.A.; Liyab, B.; Saim, L. Relation of distortion product otoacoustic emission with tinnitus. Laryngoscope 2008, 118, 712–717. [Google Scholar] [CrossRef]
- Fabijańska, A.; Smurzyński, J.; Hatzopoulos, S.; Kochanek, K.; Bartnik, G.; Raj-Koziak, D.; Mazzoli, M.; Skarżyński, P.H.; Jędrzejczak, W.W.; Szkiełkowska, A.; et al. The relationship between distortion product otoacoustic emissions and extended high-frequency audiometry in tinnitus patients. Part 1: Normally hearing patients with unilateral tinnitus. Med. Sci. Monit. 2012, 18, CR765–CR770. [Google Scholar] [CrossRef] [Green Version]
- Park, J.P.; Lim, H.W.; Shim, B.S.; Kim, T.S.; Chung, J.W.; Yoon, T.H.; Park, H.J. Interaural differences of distortion product otoacoustic emission amplitudes in patients with unilateral tinnitus. Otolaryngol. Head Neck Surg. 2013, 148, 456–459. [Google Scholar] [CrossRef]
- Modh, D.; Katarkar, A.; Alam, N.; Jain, A.; Shah, P. Relation of distortion product otoacoustic emission and tinnitus in normal hearing patients: A pilot study. Noise Health 2014, 16, 69–72. [Google Scholar] [CrossRef]
- Gouveris, H.; Maurer, J.; Mann, W. DPOAE-grams in patients with acute tonal tinnitus. Otolaryngol. Head Neck Surg. 2005, 132, 550–553. [Google Scholar] [CrossRef]
- Sztuka, A.; Pospiech, L.; Gawron, W.; Dudek, K. DPOAE in estimation of the function of the cochlea in tinnitus patients with normal hearing. Auris Nasus Larynx 2010, 37, 55–60. [Google Scholar] [CrossRef]
- Husain, F.T. Effect of tinnitus on distortion product otoacoustic emissions varies with hearing loss. Am. J. Audiol. 2013, 22, 125–134. [Google Scholar] [CrossRef]
- Onishi, E.T.; Fukuda, Y.; Suzuki, F.A. Distortion product otoacoustic emissions in tinnitus patients. Int. Tinnitus J. 2004, 10, 13–16. [Google Scholar]
- Serra, L.; Novanta, G.; Sampaio, A.L.; Oliveira, C.A.; Granjeiro, R.; Braga, S.C. The study of otoacoustic emissions and the suppression of otoacoustic emissions in subjects with tinnitus and normal hearing: An insight to tinnitus etiology. Int. Arch. Otorhinolaryngol. 2015, 19, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreisbach, L.E.; Siegel, J.H. Distortion-product otoacoustic emissions measured at high frequencies in humans. J. Acoust. Soc. Am. 2001, 110 Pt 1, 2456–2469. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.G.; Moraes, F.; Casseb, J.; Cota, J.; Freire, K.; Roberts, L.E. Tinnitus is associated with reduced sound level tolerance in adolescents with normal audiograms and otoacoustic emissions. Sci. Rep. 2016, 6, 27109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunckley, K.T.; Dreisbach, L.E. Gender effects on high frequency distortion product otoacoustic emissions in humans. Ear Hear. 2004, 25, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Kei, J.; Brazel, B.; Crebbin, K.; Richards, A.; Willeston, N. High frequency distortion product otoacoustic emissions in children with and without middle ear dysfunction. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 125–133. [Google Scholar] [CrossRef]
- Trzaskowski, B.; Jedrzejczak, W.W.; Pilka, E.; Cieslicka, M.; Skarzynski, H. Otoacoustic emissions before and after listening to music on a personal player. Med. Sci. Monit. 2014, 20, 1426–1431. [Google Scholar] [CrossRef] [Green Version]
- Dreisbach, L.; Zettner, E.; Chang Liu, M.; Fernhoff, C.M.; MacPhee, I.; Boothroyd, A. High-Frequency Distortion-Product Otoacoustic Emission Repeatability in a Patient Population. Ear Hear. 2018, 39, 85–100. [Google Scholar] [CrossRef]
- Arnold, D.J.; Lonsbury-Martin, B.L.; Martin, G.K. High-frequency hearing influences lower-frequency distortion-product otoacoustic emissions. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Zagólski, O.; Stręk, P. Comparison of characteristics observed in tinnitus patients with unilateral vs bilateral symptoms, with both normal hearing threshold and distortion-product otoacoustic emissions. Acta Otolaryngol. 2017, 137, 174–178. [Google Scholar] [CrossRef]
- Blankenship, C.M.; Hunter, L.L.; Keefe, D.H.; Feeney, M.P.; Brown, D.K.; McCune, A.; Fitzpatrick, D.F.; Lin, L. Optimizing Clinical Interpretation of Distortion Product Otoacoustic Emissions in Infants. Ear Hear. 2018, 39, 1075–1090. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—Practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Pienkowski, M. On the Etiology of Listening Difficulties in Noise Despite Clinically Normal Audiograms. Ear Hear. 2017, 38, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Guest, H.; Munro, K.J.; Prendergast, G.; Howe, S.; Plack, C.J. Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hear. Res. 2007, 344, 265–274. [Google Scholar] [CrossRef]
- Dreisbach, L.E.; Long, K.M.; Lees, S.E. Repeatability of high-frequency distortion-product otoacoustic emissions in normal-hearing adults. Ear Hear. 2006, 27, 466–479. [Google Scholar] [CrossRef]
- Pilka, E.; Jedrzejczak, W.W.; Trzaskowski, B.; Skarzyński, H. Variability of distortion product otoacoustic emissions at 10, 12 and 16 kHz: A preliminary study. J. Hear. Sci. 2014, 4, 59–64. [Google Scholar]
- Pilka, E.; Jedrzejczak, W.W.; Olszewski, L.; Skarzynski, H. High-frequency distortion product otoacoustic emissions measured by two systems: An example of a subject with normal hearing. J. Hear. Sci. 2016, 6, 57–61. [Google Scholar] [CrossRef]
- Torre, P., 3rd; Cruickshanks, K.J.; Nondahl, D.M.; Wiley, T.L. Distortion product otoacoustic emission response characteristics in older adults. Ear Hear. 2003, 24, 20–29. [Google Scholar] [CrossRef]
- Charaziak, K.K.; Shera, C.A. Compensating for ear-canal acoustics when measuring otoacoustic emissions. J. Acoust. Soc. Am. 2017, 141, 515. [Google Scholar] [CrossRef] [Green Version]
- Riga, M.; Katotomichelakis, M.; Danielides, V. The potential role of the medial olivocochlear bundle in the generation of tinnitus: Controversies and weaknesses in the existing clinical studies. Otol. Neurotol. 2015, 36, 201–208. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Gos, E.; Pilka, E.; Skarzynski, P.H.; Skarzynski, H.; Hatzopoulos, S. Pitfalls in the Detection of Hearing Loss via Otoacoustic Emissions. Appl. Sci. 2021, 11, 2184. [Google Scholar] [CrossRef]
- Zimmerman, B.J.; Abraham, I.; Schmidt, S.A.; Baryshnikov, Y.; Husain, F.T. Dissociating tinnitus patients from healthy controls using resting-state cyclicity analysis and clustering. Netw. Neurosci. 2018, 3, 67–89. [Google Scholar] [CrossRef]
- Han, L.; Na, Z.; Chunli, L.; Yuchen, C.; Pengfei, Z.; Hao, W.; Xu, C.; Peng, Z.; Zheng, W.; Zhenghan, Y.; et al. Baseline Functional Connectivity Features of Neural Network Nodes Can Predict Improvement After Sound Therapy Through Adjusted Narrow Band Noise in Tinnitus Patients. Front. Neurosci. 2019, 13, 614. [Google Scholar] [CrossRef]

| AUC | Symmetry Cutoff Value | |||||
|---|---|---|---|---|---|---|
| F [kHz] | C16 vs. Cim | C8 vs. Cim | C16 vs. C8 | C16 vs. Cim | C8 vs. Cim | C16 vs. C8 |
| 1 | 0.63 | 0.63 | 0.52 | −0.9 | −4.1 | 1.3 |
| 2 | 0.72 | 0.73 | 0.51 | 2 | 0.6 | 8.3 |
| 3 | 0.86 | 0.74 | 0.63 | 2.1 | 2.2 | 4.4 |
| 4 | 0.87 | 0.79 | 0.62 | 0.5 | −0.3 | 5.8 |
| 6 | 0.87 | 0.75 | 0.60 | 1.6 | −3.2 | 4.1 |
| 8 | 0.71 | 0.64 | 0.55 | −8 | −9.2 | −5 |
| 10 | 0.50 | 0.48 | 0.51 | −0.1 | −0.1 | −0.1 |
| 12 | 0.63 | 0.60 | 0.53 | 5.7 | 0.5 | 5.7 |
| 16 | 0.70 | 0.48 | 0.70 | −12.6 | −12.7 | −12.6 |
| AUC | Symmetry Cutoff Value | |||||
|---|---|---|---|---|---|---|
| F [kHz] | C16 vs. Cim | C8 vs. Cim | C16 vs. C8 | C16 vs. Cim | C8 vs. Cim | C16 vs. C8 |
| 1 | 0.66 | 0.66 | 0.48 | −2.2 | −2.1 | 1.9 |
| 2 | 0.74 | 0.72 | 0.52 | 17.9 | 17.4 | 25.6 |
| 3 | 0.79 | 0.70 | 0.55 | 23.3 | 22.9 | 26.2 |
| 4 | 0.78 | 0.77 | 0.51 | 20.5 | 21.2 | 26.1 |
| 6 | 0.86 | 0.75 | 0.60 | 12.5 | 11.9 | 14.4 |
| 8 | 0.73 | 0.62 | 0.59 | 3.2 | 1 | 3.2 |
| 10 | 0.44 | 0.53 | 0.42 | 8.3 | 7.5 | 8.3 |
| 12 | 0.58 | 0.56 | 0.51 | 1.8 | 6.5 | 10.4 |
| 16 | 0.64 | 0.47 | 0.68 | −1.4 | −0.6 | 2.3 |
| AUC | Symmetry Cutoff Value | |||||
|---|---|---|---|---|---|---|
| F [kHz] | C16 vs. T16 | C8 vs. T8 | Cim vs. Tim | C16 vs. T16 | C8 vs. T8 | Cim vs. Tim |
| 1 | 0.44 | 0.42 | 0.46 | −1.6 | 4.1 | −5.4 |
| 2 | 0.39 | 0.48 | 0.43 | 9.5 | 5.1 | −1.7 |
| 3 | 0.54 | 0.53 | 0.54 | 3 | 2.6 | −0.8 |
| 4 | 0.56 | 0.54 | 0.53 | 6.7 | 4.4 | −6.4 |
| 6 | 0.57 | 0.59 | 0.57 | 6.4 | 6.5 | −14.6 |
| 8 | 0.38 | 0.58 | 0.42 | −8 | −9.2 | −7.6 |
| 10 | 0.42 | 0.56 | 0.69 | 3.6 | −2.6 | −1.3 |
| 12 | 0.60 | 0.51 | 0.52 | 5.7 | 10.3 | 5.5 |
| 16 | 0.56 | 0.55 | 0.60 | −8.8 | −15.7 | −15.6 |
| No. of Frequencies | Sensitivity (%) | Specificity (%) | Efficiency (%) |
|---|---|---|---|
| 1 of 6 | 25 | 100 | 59 |
| 2 of 6 | 67 | 95 | 80 |
| 3 of 6 | 79 | 95 | 86 |
| 4 of 6 | 83 | 85 | 84 |
| 5 of 6 | 88 | 50 | 70 |
| 6 of 6 | 100 | 15 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedrzejczak, W.W.; Pilka, E.; Ganc, M.; Kochanek, K.; Skarzynski, H. Ultra-High Frequency Distortion Product Otoacoustic Emissions for Detection of Hearing Loss and Tinnitus. Int. J. Environ. Res. Public Health 2022, 19, 2123. https://doi.org/10.3390/ijerph19042123
Jedrzejczak WW, Pilka E, Ganc M, Kochanek K, Skarzynski H. Ultra-High Frequency Distortion Product Otoacoustic Emissions for Detection of Hearing Loss and Tinnitus. International Journal of Environmental Research and Public Health. 2022; 19(4):2123. https://doi.org/10.3390/ijerph19042123
Chicago/Turabian StyleJedrzejczak, W. Wiktor, Edyta Pilka, Malgorzata Ganc, Krzysztof Kochanek, and Henryk Skarzynski. 2022. "Ultra-High Frequency Distortion Product Otoacoustic Emissions for Detection of Hearing Loss and Tinnitus" International Journal of Environmental Research and Public Health 19, no. 4: 2123. https://doi.org/10.3390/ijerph19042123







