The Influence of Environmental Polycyclic Aromatic Hydrocarbons (PAHs) Exposure on DNA Damage among School Children in Urban Traffic Area, Malaysia
Abstract
:1. Introduction
2. Materials and Methods
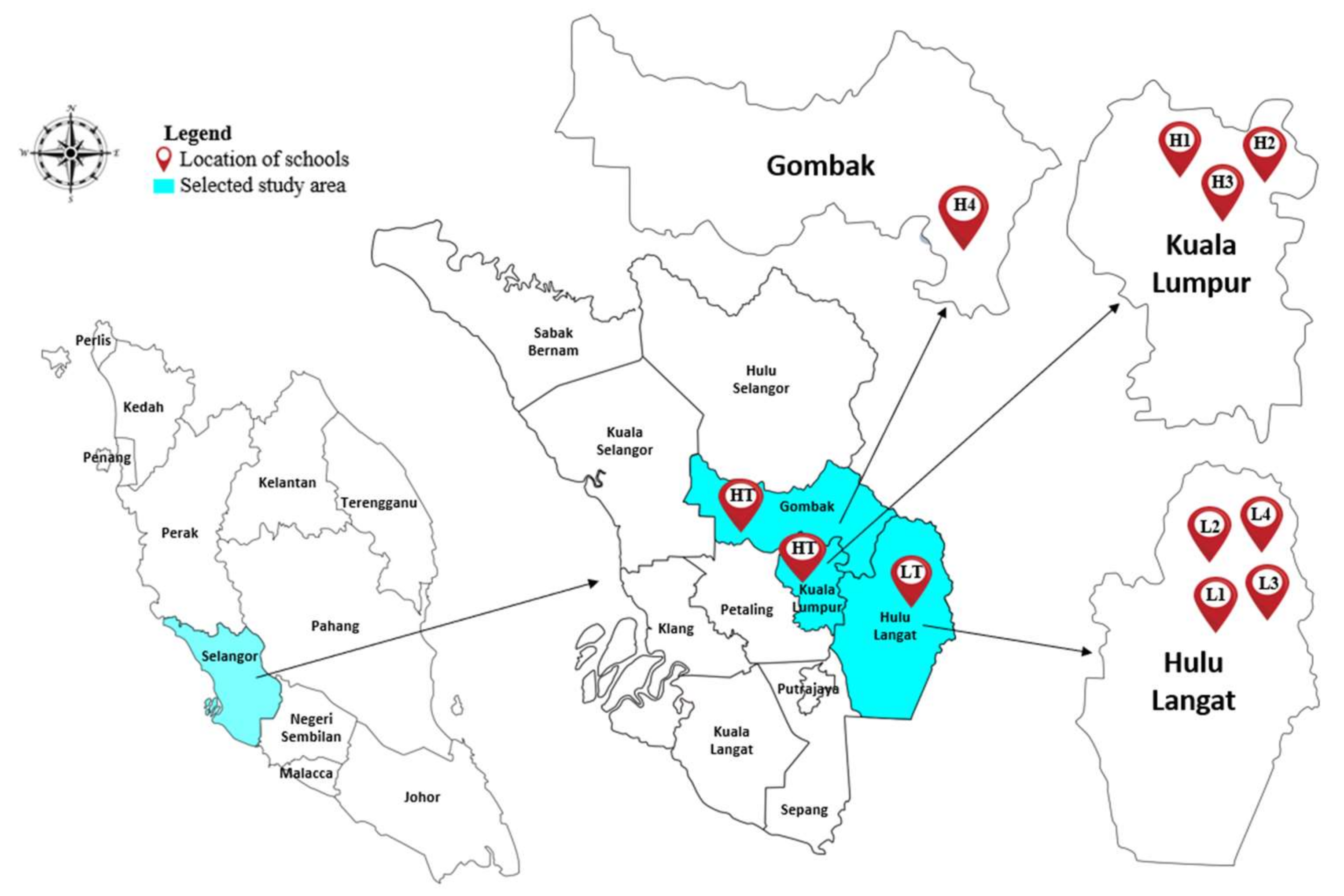
2.1. Study Location
2.2. Study Population
2.3. Questionnaires
2.4. PM2.5 Sampling
2.5. PAHs Extraction
2.6. Health Risk Assessment
| C (ng m−3) | = | TEQ |
| CSF (3.85 mg kg−1 day−1) | = | Inhalation cancer slope factor of BaP |
| BW (kg) | = | Body weight (kg) |
| IR (12 m3 day−1); | = | Inhalation rate |
| ED (6 years) | = | Exposure duration |
| EF (250 day year−1) | = | Exposure frequency |
| AT (70 years × 365 days) | = | Averaging time of carcinogenic PAHs exposure [38] |
| cf | = | Conversion factor |
2.7. Comet Assay
2.8. Statistical Analysis
2.9. Quality Control
3. Results and Discussion
3.1. Distributions of PAHs Species at Schools
3.2. Indoor and Outdoor PAHs
3.3. Distribution of PAHs Based on Number of Rings
3.4. Source Diagnostic Ratio
3.5. Health Risk Assessment
3.6. Individual Factors on DNA Damage
3.7. Factors That Influence DNA Damage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagpure, A.S.; Gurjar, B.; Kumar, V.; Kumar, P. Estimation of exhaust and non-exhaust gaseous, particulate matter and air toxics emissions from on-road vehicles in Delhi. Atmospheric Environ. 2016, 127, 118–124. [Google Scholar] [CrossRef]
- Azhari, A.; Latif, M.T.; Mohamed, A.F. Road traffic as an air pollutant contributor within an industrial park environment. Atmospheric Pollut. Res. 2018, 9, 680–687. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Azhari, A.; Halim, N.; Mohtar, A.; Aiyub, K.; Latif, M.; Ketzel, M. Evaluation and prediction of PM10 and PM2.5 from road source emissions in Kuala Lumpur City Centre. Sustainability 2021, 13, 5402. [Google Scholar] [CrossRef]
- Khan, F.; Latif, M.T.; Juneng, L.; Amil, N.; Nadzir, M.S.M.; Hoque, H.M.S. Physicochemical factors and sources of particulate matter at residential urban environment in Kuala Lumpur. J. Air Waste Manag. Assoc. 2015, 65, 958–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhaimi, N.; Jalaludin, J.; Abu Bakar, S. The influence of Traffic-Related Air Pollution (TRAP) in primary schools and residential proximity to traffic sources on Histone H3 level in selected Malaysian children. Int. J. Environ. Res. Public Heal. 2021, 18, 7995. [Google Scholar] [CrossRef] [PubMed]
- Fadzir, N.A.S.; Jalaludin, J. Indoor particulate matter 2.5 (PM2.5) and lung function among children living near busy road in Cheras, Kuala Lumpur. Health Environ. J. 2013, 4, 1–19. [Google Scholar]
- Hisamuddin, N.H.; Jalaludin, J.; Yusof, A.N.; Tualeka, A.R. Genotoxic effects of exposure to urban traffic related air pollutants on children in Klang Valley, Malaysia. Aerosol Air Qual. Res. 2020, 20, 2614–2623. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alokail, M.S.; Abd-Alrahman, S.H.; Draz, H.M.; Yakout, S.M.; Clerici, M. Polycyclic aromatic hydrocarbon exposure and pediatric asthma in children: A case–control study. Environ. Health 2013, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.F.; Latif, M.T.; Lim, C.H.; Amil, N.; Jaafar, S.A.; Dominick, D.; Mohd Nadzir, M.S.; Sahani, M.; Tahir, N.M. Seasonal effect and source apportionment of polycyclic aromatic hydrocarbons in PM2.5. Atmos. Environ. 2015, 106, 178–190. [Google Scholar] [CrossRef]
- Sulong, N.A.; Latif, M.T.; Sahani, M.; Khan, M.F.; Fadzil, M.F.; Mohd Tahir, N.; Mohamad, N.; Sakai, N.; Fujii, Y.; Othman, M.; et al. Distribution, sources and potential health risks of polycyclic aromatic hydrocarbons (PAHs) in PM2.5 collected during different monsoon seasons and haze episode in Kuala Lumpur. Chemosphere 2019, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Y.; Liu, M.; Ge, R.; Li, S.; Liu, X.; Dong, W.; Qadeer, A. Characterization and source identification of PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in different seasons from Shanghai, China. Sci. Total Environ. 2018, 644, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Bahry, P.S.; Zakaria, M.P.; Bin Abdullah, A.M.; Abdullah, D.K.; Sakari, M.; Chandru, K.; Shahbazi, A. Forensic characterization of Polycyclic Aromatic Hydrocarbons and Hopanes in aerosols from Peninsular Malaysia. Environ. Forensics 2009, 10, 240–252. [Google Scholar] [CrossRef]
- Caricchia, A.M.; Chiavarini, S.; Pezza, M. Polycyclic aromatic hydrocarbons in the urban atmospheric particulate matter in the city of Naples (Italy). Atmospheric Environ. 1999, 33, 3731–3738. [Google Scholar] [CrossRef]
- Okuda, T.; Kumata, H.; Zakaria, M.P.; Naraoka, H.; Ishiwatari, R.; Takada, H. Source identification of Malaysian atmospheric polycyclic aromatic hydrocarbons nearby forest fires using molecular and isotopic compositions. Atmospheric Environ. 2002, 36, 611–618. [Google Scholar] [CrossRef]
- Omar, N.Y.M.; Mon, T.C.; Rahman, N.A.; Bin Abas, M.R. Distributions and health risks of polycyclic aromatic hydrocarbons (PAHs) in atmospheric aerosols of Kuala Lumpur, Malaysia. Sci. Total Environ. 2006, 369, 76–81. [Google Scholar] [CrossRef]
- Omar, N.Y.; Bin Abas, M.; Ketuly, K.A.; Tahir, N.M. Concentrations of PAHs in atmospheric particles (PM-10) and roadside soil particles collected in Kuala Lumpur, Malaysia. Atmospheric Environ. 2002, 36, 247–254. [Google Scholar] [CrossRef]
- Suradi, H.; Khan, F.; Sairi, N.; Rahim, H.; Yusoff, S.; Fujii, Y.; Qin, K.; Bari, A.; Othman, M.; Latif, M. Ambient levels, emission sources and health effect of PM2.5-Bound carbonaceous particles and Polycyclic Aromatic Hydrocarbons in the city of Kuala Lumpur, Malaysia. Atmosphere 2021, 12, 549. [Google Scholar] [CrossRef]
- Jamhari, A.A.; Sahani, M.; Latif, M.T.; Chan, K.M.; Seng, H.; Khan, M.F.; Mohd Tahir, N. Concentration and source identification of polycyclic aromatic hydrocarbons (PAHs) in PM10 of urban, industrial and semi-urban areas in Malaysia. Atmos. Environ. 2014, 86, 16–27. [Google Scholar] [CrossRef]
- Sopian, N.A.; Jalaludin, J. The Application of Biomarker in Determining Genotoxic Potential of Polyaromatic Hydrocarbon Exposure among children. Ann. Trop. Med. Public Heal. 2017, 10, 533. [Google Scholar]
- Hisamuddin, N.H.; Jalaludin, J. Children’s exposure to polycyclic aromatic hydrocarbon (PAHs): A review on urinary 1-hydroxypyrene and associated health effects. Rev. Environ. Health 2021. [Google Scholar] [CrossRef] [PubMed]
- Ruchirawat, M.; Settachan, D.; Navasumrit, P.; Tuntawiroon, J.; Autrup, H. Assessment of potential cancer risk in children exposed to urban air pollution in Bangkok, Thailand. Toxicol. Lett. 2007, 168, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Tuntawiroon, J.; Mahidol, C.; Navasumrit, P.; Autrup, H.; Ruchirawat, M. Increased health risk in Bangkok children exposed to polycyclic aromatic hydrocarbons from traffic-related sources. Carcinogenesis 2006, 28, 816–822. [Google Scholar] [CrossRef]
- Mielżyńska, D.; Siwinska, E.; Kapka, L.; Szyfter, K.; Knudsen, L.E.; Merlo, D.F. The influence of environmental exposure to complex mixtures including PAHs and lead on genotoxic effects in children living in Upper Silesia, Poland. Mutagenesis 2006, 21, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suk, W.A.; Murray, K.; Avakian, M.D. Environmental hazards to children’s health in the modern world. Mutat. Res. Mutat. Res. 2003, 544, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.D.C.; Morais, S. Assessment of exposure to polycyclic aromatic hydrocarbons in preschool children: Levels and impact of preschool indoor air on excretion of main urinary monohydroxyl metabolites. J. Hazard. Mater. 2017, 322, 357–369. [Google Scholar] [CrossRef]
- Jasso-Pineda, Y.; Barriga, F.D.; Yáñez-Estrada, L.; Pérez-Vázquez, F.J.; Pérez-Maldonado, I.N. DNA damage in Mexican children living in high-risk contaminated scenarios. Sci. Total Environ. 2015, 518–519, 38–48. [Google Scholar] [CrossRef]
- Ochoa-Martinez, A.C.; Orta-Garcia, S.T.; Rico-Escobar, E.M.; Carrizales-Yañez, L.; Del Campo, J.D.M.; Pruneda-Alvarez, L.G.; Ruiz-Vera, T.; Gonzalez-Palomo, A.K.; Piña-Lopez, I.G.; Torres-Dosal, A.; et al. Exposure assessment to environmental chemicals in children from Ciudad Juarez, Chihuahua, Mexico. Arch. Environ. Contam. Toxicol. 2016, 70, 657–670. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.C.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef]
- Junaidi, E.S.; Jalaludin, J.; Tualeka, A.R. A Review on the Exposure to Benzene among children in schools, preschools and daycare centres. Asian J. Atmospheric Environ. 2019, 13, 151–160. [Google Scholar] [CrossRef]
- Ministry of Transport. Transport Statistics Malaysia 2019. Available online: https://www.mot.gov.my/en/StatistikTahunanPengangkutan/TransportStatisticsMalaysia2019.pdf (accessed on 30 June 2021).
- Brauer, M.; Reynolds, C.; Hystad, P. Traffic-related air pollution and health in Canada. Can. Med. Assoc. J. 2013, 185, 1557–1558. [Google Scholar] [CrossRef] [Green Version]
- Borrego-Soto, G.; Ortiz-Lopez, R.; Rojas-Martinez, A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.A.; Smibert, E.; Algar, E.M.; Dite, G.; Hopper, J.L. Cancer risks for relatives of children with cancer. J. Cancer Epidemiol. 2014, 2014, 806076. [Google Scholar] [CrossRef]
- Nisbet, I.C.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Othman, M.; Latif, M.T.; Jamhari, A.A.; Abd Hamid, H.H.; Uning, R.; Khan, M.F.; Mohd Nadzir, M.S.; Sahani, M.; Abdul Wahab, M.I.; Chan, K.M. Spatial distribution of fine and coarse particulate matter during a southwest monsoon in Peninsular Malaysia. Chemosphere 2021, 262, 127767. [Google Scholar] [CrossRef]
- Ouyang, R.; Yang, S.; Xu, L. Analysis and risk assessment of PM2.5-Bound PAHs in a comparison of indoor and outdoor environments in a middle school: A Case Study in Beijing, China. Atmosphere 2020, 11, 904. [Google Scholar] [CrossRef]
- US EPA. Exposure Factors Handbook Chapter 6. Available online: https://www.epa.gov/expobox/exposure-factors-handbook-chapter-6 (accessed on 8 September 2021).
- Ismail, M.F.; Fadzil, M.F.; Tahir, N.M.; Latif, M.T.; Mohamad, N. Preliminary assessment of the distribution of PM2.5-bound Polycyclic Aromatic Hydrocarbons in primary school environments in Kuala Lumpur. Univ. Malays. Teren. J. Undergrad. Res. 2019, 1, 51–58. [Google Scholar] [CrossRef]
- Letter, C.; Jäger, G. Simulating the potential of trees to reduce particulate matter pollution in urban areas throughout the year. Environ. Dev. Sustain. 2019, 22, 4311–4321. [Google Scholar] [CrossRef] [Green Version]
- Terzaghi, E.; De Nicola, F.; Cerabolini, B.E.; Posada-Baquero, R.; Ortega-Calvo, J.-J.; Di Guardo, A. Role of photo- and biodegradation of two PAHs on leaves: Modelling the impact on air quality ecosystem services provided by urban trees. Sci. Total Environ. 2020, 739, 139893. [Google Scholar] [CrossRef]
- Sopian, N.; Jalaludin, J.; Abu Bakar, S.; Hamedon, T.; Latif, M. Exposure to particulate PAHs on potential genotoxicity and cancer risk among school children living near the Petrochemical Industry. Int. J. Environ. Res. Public Health 2021, 18, 2575. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Guinot, B.; Li, L.; Ho, S.S.H.; Liu, S.; Li, X.; Cao, J. Concentrations, sources and health effects of parent, oxygenated- and nitrated- polycyclic aromatic hydrocarbons (PAHs) in middle-school air in Xi’an, China. Atmospheric Res. 2017, 192, 1–10. [Google Scholar] [CrossRef]
- Krugly, E.; Martuzevicius, D.; Sidaraviciute, R.; Ciuzas, D.; Prasauskas, T.; Kauneliene, V.; Stasiulaitiene, I.; Kliucininkas, L. Characterization of particulate and vapor phase polycyclic aromatic hydrocarbons in indoor and outdoor air of primary schools. Atmospheric Environ. 2014, 82, 298–306. [Google Scholar] [CrossRef]
- Crist, K.C.; Liu, B.; Kim, M.; Deshpande, S.R.; John, K. Characterization of fine particulate matter in Ohio: Indoor, outdoor, and personal exposures. Environ. Res. 2008, 106, 62–71. [Google Scholar] [CrossRef]
- Srithawirat, T.; Latif, M.T.; Sulaiman, F.R. Indoor PM10 and its heavy metal composition at a roadside residential environment, Phitsanulok, Thailand. Atmósfera 2016, 29, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.C. Outdoor-indoor air pollution in urban environment: Challenges and opportunity. Front. Environ. Sci. 2015, 2, 69. [Google Scholar] [CrossRef]
- Long, C.M.; Sarnat, J.A. Indoor-Outdoor relationships and infiltration behavior of elemental components of outdoor PM2.5 for Boston-Area homes. Aerosol Sci. Technol. 2004, 38, 91–104. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.D.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Khan, F.; Hwa, S.W.; Hou, L.C.; Mustaffa, N.I.H.; Amil, N.; Mohamad, N.; Sahani, M.; Jaafar, S.A.; Nadzir, M.S.M.; Latif, M.T. Influences of inorganic and polycyclic aromatic hydrocarbons on the sources of PM2.5 in the Southeast Asian urban sites. Air Qual. Atmosphere Health 2017, 10, 999–1013. [Google Scholar] [CrossRef]
- Venkataraman, C.; Lyons, J.M.; Friedlander, S.K. Size distributions of Polycyclic Aromatic Hydrocarbons and elemental carbon. 1. Sampling, Measurement Methods, and Source Characterization. Environ. Sci. Technol. 1994, 28, 555–562. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namiesnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Pies, C.; Hoffmann, B.; Petrowsky, J.; Yang, Y.; Ternes, T.A.; Hofmann, T. Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 2008, 72, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, M.; Çabuk, H. Gas–particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci. Total Environ. 2010, 408, 5550–5558. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Bencs, L.; Wauters, E.; de Hoog, J.; Deutsch, F.; Roekens, E.; Bleux, N.; Berghmans, P.; Van Grieken, R. Seasonal and site-specific variation in vapour and aerosol phase PAHs over Flanders (Belgium) and their relation with anthropogenic activities. Atmospheric Environ. 2006, 40, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.-C.; Wu, Y.-S.; Chen, M.-H.; Ho, T.-T.; Huang, S.-H.; Rau, J.-Y. Polycyclic aromatic hydrocarbons study in Taichung, Taiwan, during 2002?2003. Atmospheric Environ. 2004, 38, 3385–3391. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Madureira, J.; De Oliveira Fernandes, E.; Delerue-Matos, C.; Morais, S.; do Carmo Pereira, M. Polycyclic aromatic hydrocarbons in primary school environments: Levels and potential risks. Sci. Total Environ. 2017, 575, 1156–1167. [Google Scholar] [CrossRef] [Green Version]
- Slezakova, K.; Oliveira, M.; Madureira, J.; de Oliveira Fernandes, E.; Delerue-matos, C.; Morais, S.; do Carmo Pereira, M. Polycyclic aromatic hydrocarbons (PAH) in Portuguese educational settings: A comparison between preschools and elementary schools. J. Toxicol. Environ. Health Part A 2017, 80, 630–640. [Google Scholar] [CrossRef]
- Fandi, N.F.M.; Jalaludin, J.; Latif, M.T.; Hamid, H.H.A.; Awang, M.F. BTEX exposure assessment and inhalation health risks to traffic policemen in the Klang Valley region, Malaysia. Aerosol Air Qual. Res. 2020, 20, 1922–1937. [Google Scholar] [CrossRef]
- Miri, M.; Alahabadi, A.; Ehrampoush, M.H.; Ghaffari, H.R.; Sakhvidi, M.J.Z.; Eskandari, M.; Rad, A.; Lotfi, M.H.; Sheikhha, M.H. Environmental determinants of polycyclic aromatic hydrocarbons exposure at home, at kindergartens and during a commute. Environ. Int. 2018, 118, 266–273. [Google Scholar] [CrossRef]
- Tarafdar, A.; Oh, M.-J.; Nguyen-Phuong, Q.; Kwon, J.-H. Profiling and potential cancer risk assessment on children exposed to PAHs in playground dust/soil: A comparative study on poured rubber surfaced and classical soil playgrounds in Seoul. Environ. Geochem. Health 2019, 42, 1691–1704. [Google Scholar] [CrossRef]
- Milić, M.; Ceppi, M.; Bruzzone, M.; Azqueta, A.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.; Møller, P.; Teixeira, J.P.; et al. The hCOMET project: International database comparison of results with the comet assay in human biomonitoring. Baseline frequency of DNA damage and effect of main confounders. Mutat. Res. Mutat. Res. 2021, 787, 108371. [Google Scholar] [CrossRef]
- Piperakis, S.M.; Kontogianni, K.; Karanastasi, G.; Iakovidou-Kritsi, Z.; Piperakis, M.M. The use of comet assay in measuring DNA damage and repair efficiency in child, adult, and old age populations. Cell Biol. Toxicol. 2007, 25, 65–71. [Google Scholar] [CrossRef]
- Locken-Castilla, A.; Pacheco-Pantoja, E.L.; Rodríguez-Brito, F.; May-Kim, S.; López-Rivas, V.; Ceballos-Cruz, A. Smoking index, lifestyle factors, and genomic instability assessed by single-cell gel electrophoresis: A cross-sectional study in subjects from Yucatan, Mexico. Clin. Epigenetics 2019, 11, 150. [Google Scholar] [CrossRef] [Green Version]
- Gajski, G.; Gerić, M.; Oreščanin, V.; Garaj-Vrhovac, V. Cytogenetic status of healthy children assessed with the alkaline comet assay and the cytokinesis-block micronucleus cytome assay. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 750, 55–62. [Google Scholar] [CrossRef]
- How, V.; Hashim, Z.; Ismail, P.; Said, S.M.; Omar, D.; Tamrin, S.B.M. Exploring Cancer Development in Adulthood: Cholinesterase Depression and Genotoxic Effect From Chronic Exposure to Organophosphate Pesticides Among Rural Farm Children. J. Agromedicine 2014, 19, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Setayesh, T.; Nersesyan, A.; Mišík, M.; Ferk, F.; Langie, S.; Andrade, V.M.; Haslberger, A.; Knasmüller, S. Impact of obesity and overweight on DNA stability: Few facts and many hypotheses. Mutat. Res. Rev. Mutat. Res. 2018, 777, 64–91. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.; Kaur, G. Assessment of DNA damage in obese individuals. Res. J. Biol. 2012, 2, 37–44. [Google Scholar]
- Azqueta, A.; Ladeira, C.; Giovannelli, L.; Boutet-Robinet, E.; Bonassi, S.; Neri, M.; Gajski, G.; Duthie, S.; Del Bo’, C.; Riso, P.; et al. Application of the comet assay in human biomonitoring: An hCOMET perspective. Mutat. Res. Mutat. Res. 2019, 783, 108288. [Google Scholar] [CrossRef] [PubMed]
- Zalata, A.; Yahia, S.; Elbakary, A.; Elsheikha, H. Increased DNA damage in children caused by passive smoking as assessed by comet assay and oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2007, 629, 140–147. [Google Scholar] [CrossRef]
- Beyoglu, D.; Ozkozaci, T.; Akici, N.; Omurtag, G.Z.; Akici, A.; Ceran, O.; Sardas, S. Assessment of DNA damage in children exposed to indoor tobacco smoke. Int. J. Hyg. Environ. Health 2010, 213, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-H.; Salehi, S.; Wu, J.; North, M.L.; Kim, J.S.; Chow, C.-W.; Evans, G.J. Indoor measurements of air pollutants in residential houses in urban and suburban areas: Indoor versus ambient concentrations. Sci. Total Environ. 2019, 693, 133446. [Google Scholar] [CrossRef]
- Dusinska, M.; Collins, A. The comet assay in human biomonitoring: Gene-environment interactions. Mutagenesis 2008, 23, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Guerra, M.; Pelallo-Martínez, N.; Barriga, F.D.; Rothenberg, S.J.; Cadena, L.H.; Faugeron, S.; Oropeza-Hernández, L.F.; Guaderrama-Díaz, M.; Quintanilla-Vega, B. Environmental polycyclic aromatic hydrocarbon (PAH) exposure and DNA damage in Mexican children. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 742, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.N.; Jalaludin, J.; Abu Bakar, S.; Hisamuddin, N.H.; Suhaimi, N.F. Association of raffic-Related Air Pollution (TRAP) with DNA damage and respiratory health symptoms among primary school children in Selangor. Asian J. Atmospheric Environ. 2019, 13, 106–116. [Google Scholar] [CrossRef]
- Ruchirawat, M.; Navasumrit, P.; Settachan, D. Exposure to benzene in various susceptible populations: Co-exposures to 1,3-butadiene and PAHs and implications for carcinogenic risk. Chem. Interactions 2010, 184, 67–76. [Google Scholar] [CrossRef]
- Awang, M.F.; Jalaludin, J.; Abu Bakar, S.; Latif, M.T.; Fandi, N.F.M.; Hamid, H.H.A. Assessment of micronucleus frequency and respiratory health symptoms among traffic policemen exposed to BTEX and PM2.5 in Klang Valley, Malaysia. J. Teknol. 2020, 82. [Google Scholar] [CrossRef]





| Parameter | Distribution Mode | HT Schools | LT Schools |
|---|---|---|---|
| TEQ (ng m−3) | Logistic * | 2.32 | 1.02 |
| Inhalation rate (m3 day−1) | Constant | 12 | 12 |
| Exposure frequency (day year−1) | Constant | 250 | 250 |
| Exposure duration (year) | Constant | 6 | 6 |
| Averaging time (days) | Constant | 25, 500 | 25, 500 |
| Body weight (kg) | Log normal * | 33.13, 14.54 | 28.33, 10.86 |
| Cancer slope factor (mg kg−1 day−1) | Constant | 3.85 | 3.85 |
| Variables | HT (N = 113) | LT (N = 115) | All Children (N = 228) | |||
|---|---|---|---|---|---|---|
| GM ± SD | p-Value ‡ | GM ± SD | p-Value ‡ | GM ± SD | p-Value ‡ | |
| Age | ||||||
| 7–9 | 3.15 ± 0.50 | 0.914 | 2.74 ± 0.74 | 0.302 | 2.84 ± 0.71 | 0.040 * |
| 10–11 | 3.13 ± 0.54 | 2.88 ± 0.71 | 3.03 ± 0.62 | |||
| Gender | ||||||
| Boy | 3.09 ± 0.53 | 0.310 | 2.86 ± 0.66 | 0.556 | 2.99 ± 0.60 | 0.773 |
| Girl | 3.19 ± 0.54 | 2.78 ± 0.77 | 2.96 ± 0.71 | |||
| BMI categories | ||||||
| Underweight | 3.20 ± 0.59 | 0.338 | 2.98 ± 0.62 | 0.421 | 3.10 ± 0.60 | 0.138 |
| Normal | 3.19 ± 0.45 | 2.87 ± 0.64 | 3.02 ± 0.58 | |||
| Overweight | 2.84 ± 0.61 | 2.53 ± 1.13 | 2.68 ± 0.88 | |||
| Obese | 3.07 ± 0.63 | 2.69 ± 0.85 | 2.88 ± 0.76 | |||
| House distance from main road | ||||||
| <500 m | 3.13 ± 0.53 | 0.846 | 2.85 ± 0.71 | 0.243 | 2.99 ± 0.64 | 0.465 |
| ≥500 m | 3.16 ± 0.58 | 2.63 ± 0.82 | 2.90 ± 0.75 | |||
| House distance from highway | ||||||
| <500 m | 3.16 ± 0.49 | 0.504 | 2.59 ± 0.44 | 0.393 | 3.11 ± 0.51 | 0.028 * |
| ≥500 m | 3.09 ± 0.59 | 2.83 ± 0.74 | 2.91 ± 0.71 | |||
| Mode of transportation to school | ||||||
| Active mode | 3.04 ± 0.59 | 0.509 | 2.79 ± 0.98 | 0.928 | 2.95 ± 0.73 | 0.899 |
| Motorized mode | 3.15 ± 0.53 | 2.82 ± 0.71 | 2.98 ± 0.65 | |||
| Grilled food | ||||||
| Yes | 3.14 ± 0.54 | 0.990 | 2.94 ± 0.66 | 0.387 | 3.03 ± 0.61 | 0.554 |
| No | 3.13 ± 0.54 | 2.79 ± 0.74 | 2.96 ± 0.67 | |||
| Supplement intake | ||||||
| Yes | 3.12 ± 0.49 | 0.750 | 2.76 ± 0.69 | 0.466 | 2.96 ± 0.61 | 0.763 |
| No | 3.15 ± 0.59 | 2.86 ± 0.75 | 2.99 ± 0.70 | |||
| Fruit consumption | ||||||
| Yes | 3.14 ± 0.54 | 0.617 | 2.85 ± 0.72 | 0.274 | 3.00 ± 0.65 | 0.123 |
| No | 3.06 ± 0.47 | 2.66 ± 0.73 | 2.81 ± 0.66 | |||
| ETS exposure | ||||||
| Yes | 3.16 ± 0.52 | 0.672 | 2.87 ± 0.58 | 0.516 | 3.01 ± 0.57 | 0.495 |
| No | 3.12 ± 0.55 | 2.78 ± 0.80 | 2.95 ± 0.70 | |||
| Variable | B (95% CI) | β | p-Value | Adjusted R2 |
|---|---|---|---|---|
| Constant | 2.734 (2.572, 2.897) | <0.001 * | 0.044 | |
| Indoor tPAHs | 0.063 (0.026, 0.100) | 0.220 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hisamuddin, N.H.; Jalaludin, J.; Abu Bakar, S.; Latif, M.T. The Influence of Environmental Polycyclic Aromatic Hydrocarbons (PAHs) Exposure on DNA Damage among School Children in Urban Traffic Area, Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 2193. https://doi.org/10.3390/ijerph19042193
Hisamuddin NH, Jalaludin J, Abu Bakar S, Latif MT. The Influence of Environmental Polycyclic Aromatic Hydrocarbons (PAHs) Exposure on DNA Damage among School Children in Urban Traffic Area, Malaysia. International Journal of Environmental Research and Public Health. 2022; 19(4):2193. https://doi.org/10.3390/ijerph19042193
Chicago/Turabian StyleHisamuddin, Nur Hazirah, Juliana Jalaludin, Suhaili Abu Bakar, and Mohd Talib Latif. 2022. "The Influence of Environmental Polycyclic Aromatic Hydrocarbons (PAHs) Exposure on DNA Damage among School Children in Urban Traffic Area, Malaysia" International Journal of Environmental Research and Public Health 19, no. 4: 2193. https://doi.org/10.3390/ijerph19042193








