Survival of Frail Elderly with Delirium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Statistical Methods
3. Results
4. Discussion
Limitations
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Setters, B.; Solberg, L.M. Delirium. Prim Care 2017, 44, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Westendorp, R.G.J.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.E.; Mart, M.F.; Cunningham, C.; Shehabi, Y.; Girard, T.D.; MacLullich, A.M.J.; Slooter, A.J.C.; Ely, E.W. Delirium. Nat. Rev. Dis. Prim. 2020, 6, 90. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Inouye, S.K.; Oh, E.S. Delirium in the Elderly. Clin. Geriatr. Med. 2020, 36, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Czok, M.; Pluta, M.P.; Putowski, Z.; Krzych, Ł.J. Postoperative Neurocognitive Disorders in Cardiac Surgery: Investigating the Role of Intraoperative Hypotension. A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 786. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Park, S.Y.; Lee, J.H.; Lee, S.H.; Park, J.H.; Kim, S.K.; Suh, S.W. Incidence & Risk Factors of Postoperative Delirium After Spinal Surgery in Older Patients. Sci. Rep. 2020, 10, 9232. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.; Pereira, M.A.; Hiebert, B.; Song, X.; Rockwood, K.; Tangri, N.; Arora, R.C. The impact of frailty on postoperative delirium in cardiac surgery patients. J. Thorac. Cardiovasc. Surg. 2015, 149, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipowski, Z.J. Delirium (acute confusional states). JAMA 1987, 258, 1789–1792. [Google Scholar] [CrossRef]
- Magny, E.; Le Petitcorps, H.; Pociumban, M.; Bouksani-Kacher, Z.; Pautas, É.; Belmin, J.; Bastuji-Garin, S.; Lafuente-Lafuente, C. Predisposing and precipitating factors for delirium in community-dwelling older adults admitted to hospital with this condition: A prospective case series. PLoS ONE 2018, 13, e0193034. [Google Scholar] [CrossRef]
- Cano-Escalera, G.; Graña, M.; Irazusta, J.; Labayen, I.; Besga, A. Risk factors for prediction of delirium at hospital admittance. Expert Syst. 2021, e12698. [Google Scholar] [CrossRef]
- Ocagli, H.; Bottigliengo, D.; Lorenzoni, G.; Azzolina, D.; Acar, A.S.; Sorgato, S.; Stivanello, L.; Degan, M.; Gregori, D. A Machine Learning Approach for Investigating Delirium as a Multifactorial Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 7105. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.G.; Bailey, R.; Bonnycastle, M.; McCusker, J.; Fung, S.; Ciampi, A.; Belzile, E.; Bai, C. Partial and No Recovery from Delirium in Older Hospitalized Adults: Frequency and Baseline Risk Factors. J. Am. Geriatr. Soc. 2015, 63, 2340–2348. [Google Scholar] [CrossRef]
- Morandi, A.; Davis, D.; Fick, D.M.; Turco, R.; Boustani, M.; Lucchi, E.; Guerini, F.; Morghen, S.; Torpilliesi, T.; Gentile, S.; et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J. Am. Med. Dir. Assoc. 2014, 15, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Davis, D.; Growdon, M.E.; Albuquerque, A.; Inouye, S.K. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015, 14, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.Y.; Wickel, J.; Brunkhorst, F.M.; Geis, C. Sepsis-Associated Encephalopathy: From Delirium to Dementia? J. Clin. Med. 2020, 9, 703. [Google Scholar] [CrossRef] [Green Version]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.; Tangen, C.; Walston, J.; Newman, A.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L.; Fried, L.P. Frailty in the clinical scenario. Lancet 2015, 385, e7–e9. [Google Scholar] [CrossRef]
- Besga, A.; Ayerdi, B.; Alcalde, G.; Manzano, A.; Lopetegui, P.; Graña, M.; González-Pinto, A. Risk Factors for Emergency Department Short Time Readmission in Stratified Population. BioMed Res. Int. 2015, 2015, 685067. [Google Scholar] [CrossRef] [Green Version]
- Verloo, H.; Goulet, C.; Morin, D.; von Gunten, A. Association between frailty and delirium in older adult patients discharged from hospital. Clin. Interv. Aging 2016, 11, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Joosten, E.; Demuynck, M.; Detroyer, E.; Milisen, K. Prevalence of frailty and its ability to predict in hospital delirium, falls, and 6-month mortality in hospitalized older patients. BMC Geriatr. 2014, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.S.; Liang, C.K.; Chou, M.Y.; Lin, Y.T.; Hsu, C.J.; Chou, P.H.; Chu, C.L. Short-Form Mini Nutritional Assessment as a useful method of predicting the development of postoperative delirium in elderly patients undergoing orthopedic surgery. Gen. Hosp. Psychiatry 2016, 38, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bellelli, G.; Moresco, R.; Panina-Bordignon, P.; Arosio, B.; Gelfi, C.; Morandi, A.; Cesari, M. Is Delirium the Cognitive Harbinger of Frailty in Older Adults? A Review about the Existing Evidence. Front. Med. 2017, 4, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucht, G.; Gustafson, Y.; Sandberg, O. Epidemiology of delirium. Dement. Geriatr. Cogn. Disord. 1999, 10, 315–318. [Google Scholar] [CrossRef]
- Gibb, K.; Seeley, A.; Quinn, T.; Siddiqi, N.; Shenkin, S.; Rockwood, K.; Davis, D. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: A systematic review and meta-analysis study. Age Ageing 2020, 49, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Flaherty, J.H.; Morley, J.E. Delirium in the Nursing Home. J. Am. Med. Dir. Assoc. 2013, 14, 632–634. [Google Scholar] [CrossRef]
- Ohl, I.C.B.; Chavaglia, S.R.R.; Ohl, R.I.B.; Lopes, M.C.B.T.; Campanharo, C.R.V.; Okuno, M.F.P.; Batista, R.E.A. Evaluation of delirium in aged patients assisted at emergency hospital service. Rev. Bras. Enferm. 2019, 72, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Feldman, J.; Yaretzky, A.; Kaizimov, N.; Alterman, P.; Vigder, C. Delirium in an acute geriatric unit: Clinical aspects. Arch. Gerontol. Geriatr. 1999, 28, 37–44. [Google Scholar] [CrossRef]
- Fong, T.G.; Tulebaev, S.R.; Inouye, S.K. Delirium in elderly adults: Diagnosis, prevention and treatment. Nat. Rev. Neurol. 2009, 5, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.V.Q.; Pelletier, L.; Payot, I.; Latour, J. The Delirium Drug Scale is associated with delirium incidence in the emergency department. Int. Psychogeriatr. 2018, 30, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Chen, M.; Yang, Q.; Cao, X.; Ge, L.; Di, B. Delirium screening tools in the emergency department: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e24779. [Google Scholar] [CrossRef] [PubMed]
- Aung Thein, M.Z.; Pereira, J.V.; Nitchingham, A.; Caplan, G.A. A call to action for delirium research: Meta-analysis and regression of delirium associated mortality. BMC Geriatr. 2020, 20, 325. [Google Scholar] [CrossRef]
- Klein Klouwenberg, P.M.C.; Zaal, I.J.; Spitoni, C.; Ong, D.S.Y.; van der Kooi, A.W.; Bonten, M.J.M.; Slooter, A.J.C.; Cremer, O.L. The attributable mortality of delirium in critically ill patients: Prospective cohort study. BMJ 2014, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolters, A.E.; van Dijk, D.; Pasma, W.; Cremer, O.L.; Looije, M.F.; de Lange, D.W.; Veldhuijzen, D.S.; Slooter, A.J. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: A prospective cohort study. Crit. Care 2014, 18, R125. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, D.; Brennan, K.; Al Sayfe, M.; Shunker, S.A.; Bogdanoski, T.; Hedges, S.; Hou, Y.C.; Lynch, J.; Hunt, L.; Alexandrou, E.; et al. Frailty, delirium and hospital mortality of older adults admitted to intensive care: The Delirium (Deli) in ICU study. Crit. Care 2020, 24, 609. [Google Scholar] [CrossRef]
- Duprey, M.S.; van den Boogaard, M.; van der Hoeven, J.G.; Pickkers, P.; Briesacher, B.A.; Saczynski, J.S.; Griffith, J.L.; Devlin, J.W. Association between incident delirium and 28- and 90-day mortality in critically ill adults: A secondary analysis. Crit. Care 2020, 24, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ely, E.W.; Shintani, A.; Truman, B.; Speroff, T.; Gordon, S.M.; Harrell, F.E., Jr.; Inouye, S.K.; Bernard, G.R.; Dittus, R.S. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. JAMA 2004, 291, 1753–1762. [Google Scholar] [CrossRef] [Green Version]
- Gower, L.E.J.; Gatewood, M.O.; Kang, C.S. Emergency department management of delirium in the elderly. West J. Emerg. Med. 2012, 13, 194–201. [Google Scholar] [CrossRef]
- Diwell, R.A.; Davis, D.H.; Vickerstaff, V.; Sampson, E.L. Key components of the delirium syndrome and mortality: Greater impact of acute change and disorganised thinking in a prospective cohort study. BMC Geriatr. 2018, 18, 24. [Google Scholar] [CrossRef]
- Avelino-Silva, T.J.; Campora, F.; Curiati, J.A.E.; Jacob-Filho, W. Association between delirium superimposed on dementia and mortality in hospitalized older adults: A prospective cohort study. PLoS Med. 2017, 14, e1002264. [Google Scholar] [CrossRef] [Green Version]
- Blodgett, J.M.; Theou, O.; Howlett, S.E.; Rockwood, K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. GeroScience 2017, 39, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inouye, S.K.; van Dyck, C.H.; Alessi, C.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying Confusion: The Confusion Assessment Method. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef]
- Graña, M.; Besga, A. Fragility and deliriium data from UHA [Data set]. Zenodo 2021. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.; Barthel, D. Functional Evaluation: The Barthel Index. Md State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. JNHA J. Nutr. Health Aging 2009, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Erkinjuntti, T.; Sulkava, R.; Wikström, J.; Autio, L. Short Portable Mental Status Questionnaire as a Screening Test for Dementia and Delirium Among the Elderly. J. Am. Geriatr. Soc. 1987, 35, 412–416. [Google Scholar] [CrossRef]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Barakat, A.; Mittal, A.; Ricketts, D.; Rogers, B.A. Understanding survival analysis: Actuarial life tables and the Kaplan–Meier plot. Br. J. Hosp. Med. 2019, 80, 642–646. [Google Scholar] [CrossRef]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.J.; Nussenbaum, B.; Wang, E.W. A practical guide to understanding Kaplan–Meier curves. Otolaryngol. Head Neck Surg. 2010, 143, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Everitt, B.; Hothorn, T. A Handbook of Statistical Analyses Using R; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Harith, S.; Mohamed, R.; Fazimah, N. Hospitalized Geriatric Malnutrition: A Perspective of Prevalence, Identification and Implications to Patient and Healthcare Cost. Health Environ. J. 2013, 4, 55–67. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kline, K.A.; Bowdish, D.M.E. Infection in an aging population. Curr. Opin. Microbiol. 2016, 29, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Owen, L.H.; Jackson, T.A.; Rockwood, K.; Sampson, E.L.; Davis, D. Delirium, Frailty, and Mortality: Interactions in a Prospective Study of Hospitalized Older People. J. Gerontol. Ser. A 2017, 73, 415–418. [Google Scholar] [CrossRef]
- Yaghi, S.; Elkind, M.S. Lipids and Cerebrovascular Disease. Stroke 2015, 46, 3322–3328. [Google Scholar] [CrossRef] [Green Version]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef] [Green Version]
- Maddocks, M.; Kon, S.S.C.; Jones, S.E.; Canavan, J.L.; Nolan, C.M.; Higginson, I.J.; Gao, W.; Polkey, M.I.; Man, W.D.C. Bioelectrical impedance phase angle relates to function, disease severity and prognosis in stable chronic obstructive pulmonary disease. Clin. Nutr. 2015, 34, 1245–1250. [Google Scholar] [CrossRef]
- Oikawa, S.Y.; Holloway, T.M.; Phillips, S.M. The Impact of Step Reduction on Muscle Health in Aging: Protein and Exercise as Countermeasures. Front. Nutr. 2019, 6, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieracci, F.M.; Eachempati, S.R.; Shou, J.; Hydo, L.J.; Barie, P.S. Use of long-term anticoagulation is associated with traumatic intracranial hemorrhage and subsequent mortality in elderly patients hospitalized after falls: Analysis of the New York State Administrative Database. J. Trauma 2007, 63, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Díez-Manglano, J.; Bernabeu-Wittel, M.; Murcia-Zaragoza, J.; Escolano-Fernández, B.; Jarava-Rol, G.; Hernández-Quiles, C.; Oliver, M.; Sanz-Baena, S. Oral anticoagulation in patients with atrial fibrillation and medical non-neoplastic disease in a terminal stage. Intern Emerg. Med. 2017, 12, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Kamaruzzaman, S.B.; Tan, M.P. The consumption of two or more fall risk-increasing drugs rather than polypharmacy is associated with falls. Geriatr. Gerontol. Int. 2017, 17, 463–470. [Google Scholar] [CrossRef]
- de Vries, M.; Seppala, L.J.; Daams, J.G.; van de Glind, E.M.M.; Masud, T.; van der Velde, N. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: I. Cardiovascular Drugs. J. Am. Med. Dir. Assoc. 2018, 19, 371.e1–371.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seppala, L.J.; van de Glind, E.M.M.; Daams, J.G.; Ploegmakers, K.J.; de Vries, M.; Wermelink, A.M.A.T.; van der Velde, N. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-analysis: III. Others. J. Am. Med. Dir. Assoc. 2018, 19, 372.e1–372.e8. [Google Scholar] [CrossRef] [Green Version]
- Axmon, A.; Sandberg, M.; Ahlström, G.; Midlöv, P. Fall-risk-increasing drugs and falls requiring health care among older people with intellectual disability in comparison with the general population: A register study. PLoS ONE 2018, 13, e0199218. [Google Scholar] [CrossRef]
- Hajjar, E.R.; Cafiero, A.C.; Hanlon, J.T. Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 2007, 5, 345–351. [Google Scholar] [CrossRef]
- Hart, L.A.; Phelan, E.A.; Yi, J.Y.; Marcum, Z.A.; Gray, S.L. Use of Fall Risk-Increasing Drugs Around a Fall-Related Injury in Older Adults: A Systematic Review. J. Am. Geriatr. Soc. 2020, 68, 1334–1343. [Google Scholar] [CrossRef]
- Rossi, M.I.; Young, A.; Maher, R.; Rodriguez, K.L.; Appelt, C.J.; Perera, S.; Hajjar, E.R.; Hanlon, J.T. Polypharmacy and health beliefs in older outpatients. Am. J. Geriatr. Pharmacother. 2007, 5, 317–323. [Google Scholar] [CrossRef]
- Al-Musawe, L.; Martins, A.P.; Raposo, J.F.; Torre, C. The association between polypharmacy and adverse health consequences in elderly type 2 diabetes mellitus patients; a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2019, 155, 107804. [Google Scholar] [CrossRef]
- Formica, M.; Politano, P.; Marazzi, F.; Tamagnone, M.; Serra, I.; Marengo, M.; Falconi, D.; Gherzi, M.; Tattoli, F.; Bottaro, C.; et al. Acute Kidney Injury and Chronic Kidney Disease in the Elderly and Polypharmacy. Blood Purif 2018, 46, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Steptoe, A.; Wei, L.; Zaninotto, P. The impact of high-risk medications on mortality risk among older adults with polypharmacy: Evidence from the English Longitudinal Study of Ageing. BMC Med. 2021, 19, 321. [Google Scholar] [CrossRef] [PubMed]
- Wastesson, J.W.; Morin, L.; Tan, E.C.K.; Johnell, K. An update on the clinical consequences of polypharmacy in older adults: A narrative review. Expert Opin. Drug Saf. 2018, 17, 1185–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehling, M. Morbus diureticus in the elderly: Epidemic overuse of a widely applied group of drugs. J. Am. Med. Dir. Assoc. 2013, 14, 437–442. [Google Scholar] [CrossRef]
- Ribas, C.D. Mortality and β-Agonists, or the Risk of Statistical Inference. Arch. Bronconeumol. 2007, 43, 355–357. [Google Scholar] [CrossRef]
- Schneider, L.S.; Dagerman, K.S.; Insel, P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA 2005, 294, 1934–1943. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.S.; Park, J.S.; Cho, Y.J.; Yoon, H.I.; Lee, S.M.; Lee, J.H.; Lee, C.T.; Lee, Y.J. Efficacy of Low-Dose Prophylactic Quetiapine on Delirium Prevention in Critically Ill Patients: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Med. 2020, 9, 69. [Google Scholar] [CrossRef] [Green Version]



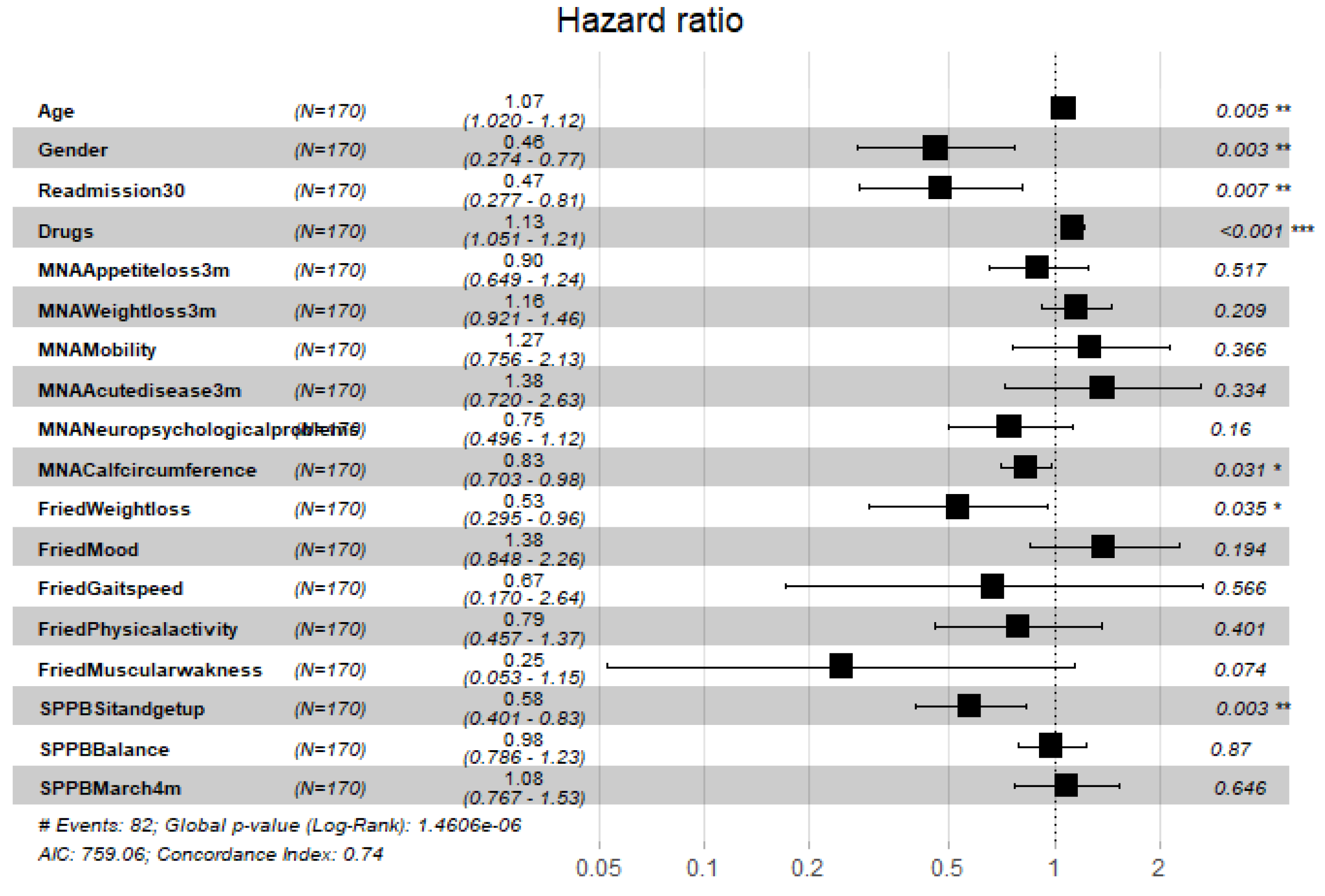


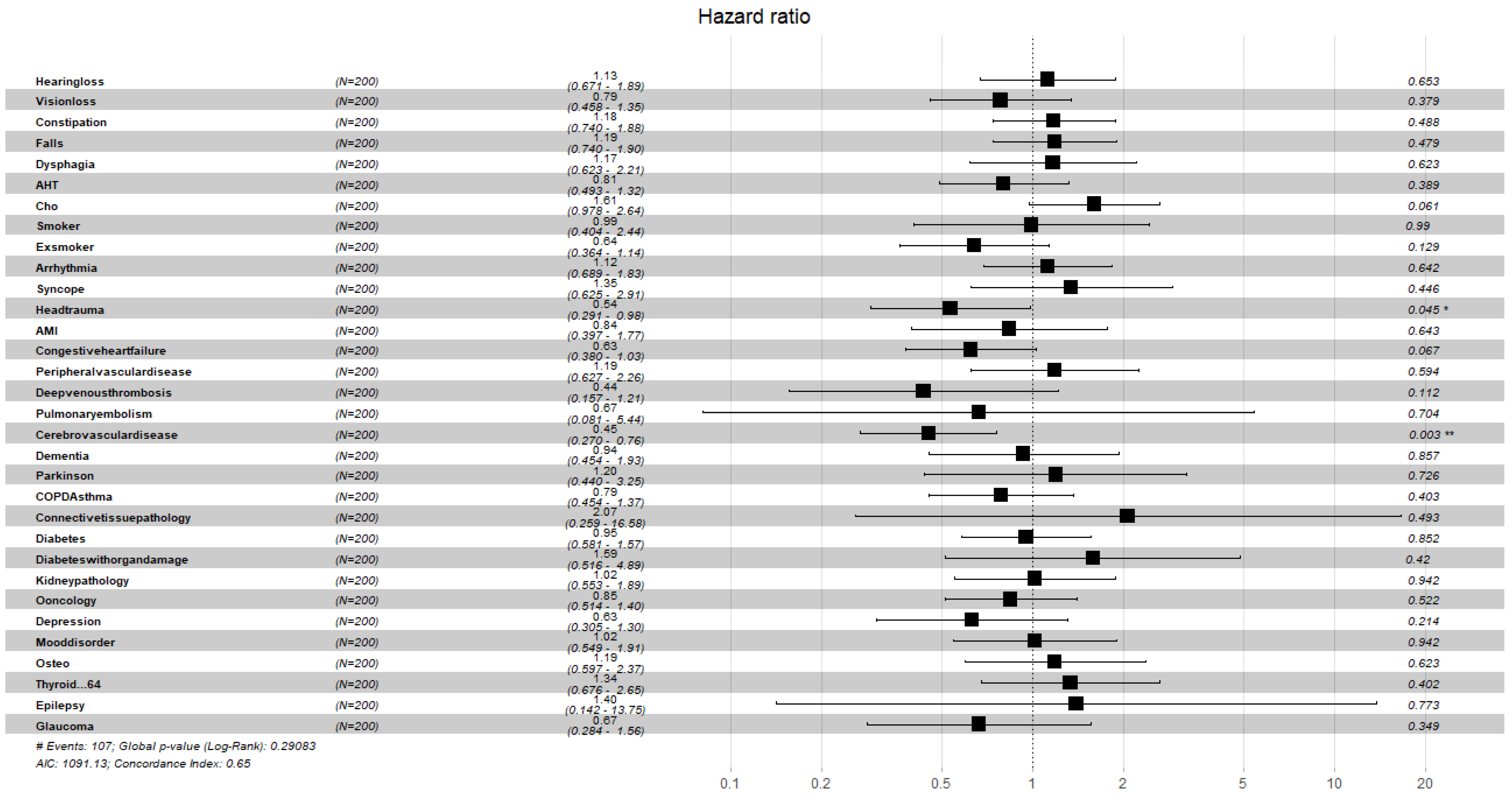
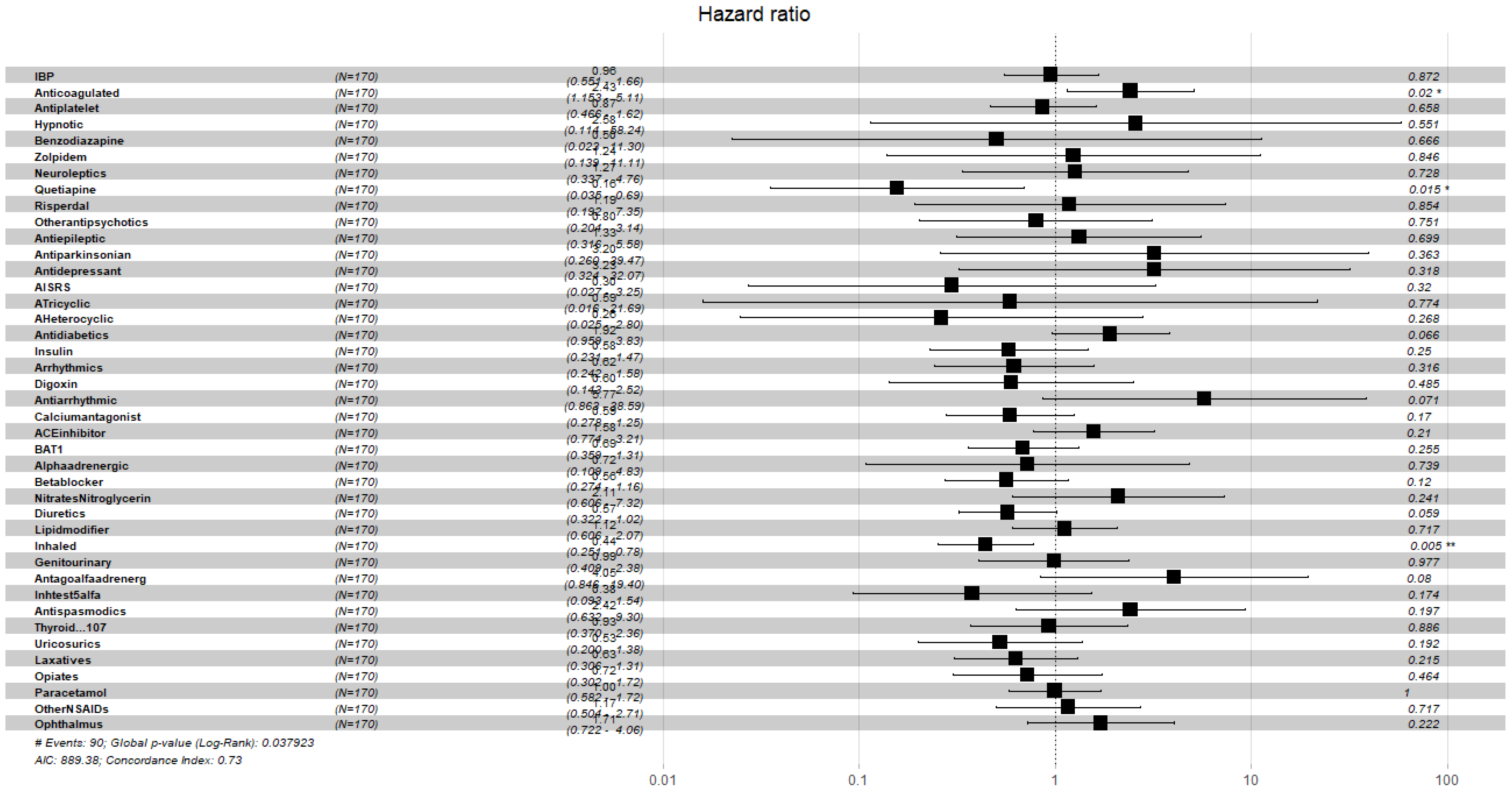

| Variable | Categories | N Total | % | ND (N = 541) | P (N = 170) | I (N = 200) | ||
|---|---|---|---|---|---|---|---|---|
| % | % | % | ||||||
| Gender | Male | 382 | 51.55 | 53.97 | 43.53 | 0.017 | 45.00 | 0.030 |
| Female | 359 | 48.45 | 46.03 | 56.47 | 55.00 | |||
| Weight (kg) | Mean (SD) | 67.43 (13.60) | 68.02 (13.00) | 67.43 (13.61) | <0.001 | 67.43 (13.60) | <0.001 | |
| Age (years) | Mean (SD) | 84.37 (6.76) | 83.43 (6.67) | 84.37 (6.76) | <0.001 | 84.37 (6.76) | <0.001 | |
| MS | Married | 265 | 35.60 | 36.41 | 30.58 | 0.109 | 33.00 | 0.543 |
| Single | 65 | 8.90 | 9.06 | 8.24 | 0.778 | 8.00 | 0.652 | |
| Divorced | 13 | 1.80 | 1.48 | 2.35 | 0.499 | 2.50 | 0.348 | |
| Widowed | 265 | 35.70 | 31.98 | 47.65 | <0.001 | 47.00 | <0.001 | |
| NA | 133 | 18.00 | 21.07 | 11.18 | 0.009 | 9.50 | <0.001 | |
| HD | Yes | 501 | 67.61 | 62.47 | 79.41 | 0.002 | 81.50 | <0.001 |
| No | 241 | 32.39 | 37.53 | 20.59 | 18.50 | |||
| NWS | Yes | 419 | 56.54 | 52.12 | 66.47 | 0.443 | 68.00 | 0.189 |
| No | 322 | 43.46 | 47.88 | 33.53 | 32.00 | |||
| Living at | Own home | 530 | 71.52 | 70.42 | 69.41 | <0.001 | 70.50 | <0.001 |
| Alone | 195 | 26.31 | 25.69 | 25.88 | 0.454 | 24.00 | 0.120 | |
| Other’s home | 62 | 8.36 | 6.28 | 11.18 | 0.248 | 13.00 | 0.018 | |
| Retirement house | 64 | 8.63 | 6.09 | 16.47 | <0.001 | 14.50 | 0.003 | |
| Polypharmacy | Oligopharma <5 | 178 | 24.02 | 25.32 | 22.35 | 0.562 | 20.50 | 0.173 |
| Moderate (5–9) | 358 | 48.31 | 49.72 | 44.71 | 0.267 | 45.00 | 0.254 | |
| Severe (>9) | 205 | 27.67 | 24.96 | 32.94 | 0.072 | 34.50 | 0.010 | |
| Prevalent delirium | 170 | 22.94 | ||||||
| Incident delirium | 200 | 26.99 | ||||||
| Reason for Admission | N | % |
|---|---|---|
| Heart failure | 216 | 29.14 |
| Infection | 323 | 43.58 |
| Anemia | 79 | 10.66 |
| CAL | 101 | 13.63 |
| DM | 48 | 6.47 |
| Delirium | 170 | 22.94 |
| Fall | 119 | 16.05 |
| Neurological | 67 | 9.04 |
| Oncology | 35 | 4.72 |
| OC | 63 | 8.50 |
| Kidney failure | 96 | 12.95 |
| Test | Score | N | % |
|---|---|---|---|
| SPPB | Minimum (10–12) | 95 | 12.91 |
| Light (7–9) | 169 | 22.97 | |
| Moderate (4–6) | 232 | 31.52 | |
| Severe (0–3) | 240 | 32.60 | |
| FFI | Robust (0) | 17 | 2.32 |
| Pre-frail (1–3) | 213 | 29.14 | |
| Frail (>3) | 501 | 68.54 | |
| BIS | Independent | 237 | 35.06 |
| Mild (>60) | 362 | 53.55 | |
| Moderate (40–55) | 57 | 8.43 | |
| Severe (20–35) | 13 | 1.92 | |
| Total Dependence (<20) | 7 | 1.04 | |
| MNA-SF | Normal (12–14) | 261 | 35.56 |
| At Risk (8–11) | 348 | 47.41 | |
| Poor nutrition (0–7) | 125 | 17.03 | |
| PBSTD | Normal (0–2) | 465 | 63.27 |
| Light (3–4) | 128 | 17.41 | |
| Moderate (5–7) | 108 | 14.70 | |
| Severe (8–10) | 34 | 4.62 |
| Cohort | 1 month | 6 month | 1 year | 2 years |
|---|---|---|---|---|
| prevalent | *** | *** | *** | *** |
| incident | *** | *** | *** | *** |
| 1 month | 6 months | 1 year | 2 years | |
|---|---|---|---|---|
| Variable | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Age | 1.05 (1.00,1.09) | 1.04 (1.00,1.09) | 1.05 (1.00,1.10) | 1.05 (1.00,1.10) |
| Gender | 0.63 (0.38,1.02) | 0.52 (0.31,0.86) | 0.48 (0.28,0.80) | 0.48 (0.28,0.80) |
| R30 | 0.65 (0.38,1.11) | 0.53 (0.31,0.90) | 0.53 (0.30,0.89) | 0.52 (0.30,0.89) |
| ND | 1.08 (1.01,1.15) | 1.10 (1.03,1.18) | 1.13 (1.05,1.21) | 1.13 (1.05,1.21) |
| MNA-SF-CC | 0.90 (0.76,1.06) | 0.85 (0.72,1.00) | 0.83 (0.70,0.98) | 0.83 (0.70,0.98) |
| FFI-WL | 0.55 (0.30,0.99) | 0.59 (0.32,1.05) | 0.50 (0.27,0.90) | 0.53 (0.29,0.94) |
| SPPB-SUG | 0.72 (0.504,01.02) | 0.68 (0.47,0.98) | 0.65 (0.45,0.94) | 0.64 (0.44,0.92) |
| Falls | 1.65 (0.98,2.74) | 1.53 (0.91,2.56) | 1.46 (0.87,2.46) | 1.40 (0.83,2.35) |
| Cholesterol | 1.55 (0.91,2.61) | 1.61 (0.95,2.72) | 1.63 (0.96,2.76) | 1.52 (0.89,2.57) |
| Head Trauma | 0.51 (0.262,1.00) | 0.54 (0.28,1.07) | 0.53 (0.26,1.06) | 0.54 (0.27,1.06) |
| CD | 0.49 (0.27,0.87) | 0.47 (0.26,0.84) | 0.48 (0.26,0.87) | 0.52 (0.28,0.96) |
| Anticoagulated | 1.74 (0.85,3.54) | 2.11 (1.01,4.38) | 2.37 (1.12,4.98) | 2.43 (1.15,5.11) |
| Quetiapine | 0.23 (0.05,0.93) | 0.16 (0.03,0.67) | 0.15 (0.03,0.66) | 0.16 (0.03,0.69) |
| Diuretics | 0.61 (0.34,1.08) | 0.58 (0.32,1.03) | 0.60 (0.33,1.06) | 0.57 (0.32,1.02) |
| IB | 0.51 (0.29,0.87) | 0.43 (0.24,0.75) | 0.43 (0.24,0.75) | 0.44 (0.25,0.78) |
| 1 month | 6 months | 1 year | 2 years | |
|---|---|---|---|---|
| Variable | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Age | 1.05 (1.00,1.1) | 1.05 (1.01,1.10) | 1.06 (1.01,1.11) | 1.07 (1.02,1.11) |
| Gender | 0.65 (0.42,1.0) | 0.48 (0.30,0.75) | 0.43 (0.26,0.69) | 0.43 (0.26,0.69) |
| R30 | 0.62 (0.39,1.0) | 0.51 (0.32,0.83) | 0.51 (0.31,0.81) | 0.50 (0.30,0.80) |
| ND | 1.07 (1.00,1.14) | 1.08 (1.02,1.15) | 1.10 (1.03,1.17) | 1.10 (1.03,1.18) |
| FFI | 1.72 (0.92,3.2) | 1.82 (0.98,3.41) | 1.83 (0.98,3.41) | 1.81 (0.97,3.36) |
| FFI-WL | 0.50 (0.30,0.83) | 0.51 (0.31,0.84) | 0.42 (0.25,0.70) | 0.45 (0.26,0.74) |
| SPPB-SGU | 0.74 (0.55,1.01) | 0.71 (0.52,0.97) | 0.68 (0.50,0.93) | 0.67 (0.49,0.92) |
| Cholesterol | 1.62 (0.99,2.64) | 1.68 (1.03,2.76) | 1.70 (1.03,2.80) | 1.61 (0.97,2.64) |
| Head Trauma | 0.54 (0.29,0.99) | 0.56 (0.30,1.03) | 0.56 (0.30,1.04) | 0.54 (0.29,0.98) |
| CD | 0.47 (0.29,0.77) | 0.44 (0.26,0.72) | 0.44 (0.26,0.73) | 0.45 (0.27,0.76) |
| Diuretics | 0.58 (0.35,0.97) | 0.59 (0.34,0.98) | 0.59 (0.35,0.99) | 0.57 (0.34,0.96) |
| -Adrenergic Antagonist | 2.68 (0.74,9.65) | 3.69 (0.98,13.78) | 3.92 (1.07,14.37) | 4.17 (1.09,15.87) |
| Testosterone inhibitors | 0.36 (0.11,1.18) | 0.31 (0.09,1.04) | 0.28 (0.08,0.94) | 0.28 (0.08,0.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Escalera, G.; Graña, M.; Irazusta, J.; Labayen, I.; Besga, A. Survival of Frail Elderly with Delirium. Int. J. Environ. Res. Public Health 2022, 19, 2247. https://doi.org/10.3390/ijerph19042247
Cano-Escalera G, Graña M, Irazusta J, Labayen I, Besga A. Survival of Frail Elderly with Delirium. International Journal of Environmental Research and Public Health. 2022; 19(4):2247. https://doi.org/10.3390/ijerph19042247
Chicago/Turabian StyleCano-Escalera, Guillermo, Manuel Graña, Jon Irazusta, Idoia Labayen, and Ariadna Besga. 2022. "Survival of Frail Elderly with Delirium" International Journal of Environmental Research and Public Health 19, no. 4: 2247. https://doi.org/10.3390/ijerph19042247
APA StyleCano-Escalera, G., Graña, M., Irazusta, J., Labayen, I., & Besga, A. (2022). Survival of Frail Elderly with Delirium. International Journal of Environmental Research and Public Health, 19(4), 2247. https://doi.org/10.3390/ijerph19042247









