Abstract
This pilot randomised control trial (RCT) aimed to evaluate the feasibility and preliminary effectiveness of conducting a full-powered trial for a newly developed pelvic floor muscle training (PFMT) app among pregnant women with urinary incontinence (UI) in Malaysia. This was a prospective, single-centre, single-blind, parallel, randomised controlled, pilot feasibility study—the Kegel Exercise Pregnancy Training app (KEPT app) trial. In total, 26 pregnant women with urinary incontinence from an urban healthcare clinic were recruited and randomly assigned to either intervention or waitlist control group. The intervention group received the KEPT app, while the control group received usual antenatal care (waitlist control). Of the 26 pregnant women, 16 (61.5%) completed the two-month follow-up. The recruitment rate was 54.2%, and the retention rate was 62.5% in the intervention group and 60% in the control group. There was a significant difference between intervention and control groups’ baseline measurement in the severity of UI (p = 0.031). The app improved their knowledge (p = 0.011) and self-efficacy (p = 0.038) after the first month and attitude (p = 0.034) after two months of intervention, compared with the control group. This study supports the feasibility of our future cluster RCT. The KEPT app demonstrates a promising effect in improving PFMT attitude and self-efficacy and potentially enhancing exercise adherence among pregnant women with UI. Trial registration: This study was prospectively registered on ClinicalTrials.gov on 19 February 2021 (NCT04762433).
1. Introduction
Pelvic floor muscle training (PFMT), or Kegel exercise, is the gold standard and is recommended for pregnant women to strengthen pelvic floor muscles [1,2]. The correct performance of PFMT may help first-time pregnant women to shorten their first and second stages of labour [3]. The same exercise can prevent pelvic floor dysfunction, for example, urinary incontinence, which commonly occurs in late pregnancy and the early post-partum period [4]. Moreover, a meta-analysis study has demonstrated positive results with training exercise among pregnant women at any parity in improving quality of life [5].
Urinary incontinence (UI) is defined as involuntary urinary leakage involving about two-fifth of our local population in a single-centred, cross-sectional study [6,7]. Worldwide UI prevalence demonstrated variations ranging from 9% to 75% [8]. Having UI does not add risk to maternal mortality but affects their quality of life and causes psychological morbidities [9,10]. Additionally, they may suffer difficulties in social–emotional relationships, performing exercises, restriction travelling, and sleeping disturbances [11].
Previous studies highlighted that pregnant women face challenges adhering to PFMT, as they consider having UI is ‘normal’. This misconception led to a barrier in seeking help from healthcare providers. Furthermore, there were limited credible sources for PFMT information, [12] preventing them from knowing the benefit of the exercise during pregnancy. Recent guidelines proposed that three sets of exercises are needed to improve the pelvic floor muscle strength [12]. Three sets of daily exercises during their busy schedules make them experience difficulties remembering to perform the exercise [13]. Subsequently, health personnel struggles to discuss and offer pelvic exercise advice due to inadequate knowledge [14], which is not routinely practised [15]. These factors will further reduce the availability of the services and affect the accessibility of PFMT to pregnant women [16].
mHealth apps have shown their effectiveness in self-management pregnancy and improving healthcare delivery [17]. Evidence suggests that the apps can provide audio guidance for PFMT [18] and reminders to improve motivation and adherence [18]. Furthermore, delivering pregnancy-related education and self-management using the mHealth app reported promising outcomes [17] and may be used for self-empowering among pregnant women.
Therefore, this pilot RCT aimed to assess the preliminary effectiveness of a newly developed, validated mHealth app—Kegel Exercise Pregnancy Trial (KEPT app) [19]. Their knowledge, attitude, practice, self-efficacy, and adherence to PFMT with their severity urinary incontinence symptoms and quality of life were assessed in this pilot RCT.
2. Materials and Methods
2.1. Design Overview
An eight-week, two-arm, parallel-group, pilot RCT was undertaken at an urban government health clinic in Ampang, Selangor. Participants were randomly allocated to the intervention group who received the Kegel Exercise Pregnancy Training app (KEPT app) or waitlist control (receiving KEPT app after completing the study). The assessments were conducted at baseline, one month, and after two months of the study. The study was prospectively registered with ClinicalTrials.gov on 19 February 2021 (NCT04762433). The study protocol was designed and reported according to the Consolidated Standards of Reporting Trials (CONSORT) extension for randomised pilot and feasibility trials [20,21] and has been published recently [22].
2.2. Participants
Women aged 18 and above with urinary incontinence were recruited from June 2021 to September 2021. Detailed inclusion and exclusion criteria are described in Table 1. Participants were recruited using e-poster strategies delivered via WhatsApp by the researcher’s team. The pilot RCT obtained ethics approval from the Ethics Committee for Research Involving Human Subjects, Universiti Putra Malaysia (JKEUPM-2019-368) Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia (NMRR-19-412-45606) in August 2019.

Table 1.
Participant inclusion and exclusion criteria.
The trial was undertaken in compliance with the Declaration of Helsinki [23]. Interested participants were provided with an online participant information statement from the researcher’s team. They filled in the google forms survey for our team to assess their study eligibility. An online consent form was provided to eligible participants for their digital signature prior to the study commencement. The study protocol is designed and has been published elsewhere [22].
2.3. Intervention
Participants allocated to the intervention group were provided 8-weeks behavioural change intervention (pelvic floor muscle training) via a newly developed mHealth app (KEPT app). The KEPT app was an interactive Android version that focused on the PFMT program (educational video, training timer, UI symptoms calendar chart, daily reminder notification, progress chart, and frequently asked questions). KEPT app recommendations were promoted through an evidence-based program that has been validated [18] and undergone expert usability testing [26]. The following program components were featured:
- Educational video: Participants watched a PFMT educational video demonstrated by a certified physiotherapist for six minutes. The video has been approved for education in the rehabilitation department tertiary hospital.
- Training timer: Participants performed the exercise according to the tailored timer performance ability (beginner: 2 s contraction, intermediate: 6 s contraction, and expert: 10 s contraction) and 6 s rest between each repetition, every day for three times daily. There were slow-velocity, close-to-maximum contractions of exercise. The first (beginner) performed the quick muscle contractions for two seconds and rested for six seconds while breathing normally. The quick contractions were required to perform 3 times daily, for 10 repetitions each cycle. After gaining confidence and skills, they proceeded to the longer durations, where the same muscles they contracted with longer durations of 6 to 10 seconds, for 10 repetitions, 3 times daily.
- Symptoms calendar charting: Participants recorded their UI symptoms for their self-monitoring.
- Progress chart: Participants could self-monitor their progress of UI symptoms and PFMT adherence.
- Frequently asked questions: Participants could read further the details of anatomy and PFMT techniques.
- Notification reminder: Participants received a daily notification to remind their pelvic exercise.
2.4. Control Group
Participants allocated to the control group were provided with the KEPT app after completing the eight-week follow-up appointment. They continued their usual antenatal follow-up as scheduled.
2.5. Outcome Measures (Preliminary Effectiveness)
The feasibility of this study was assessed by determining the proportions of respondents who meet the eligibility criteria, recruitment rate, and retention rate [27]. All outcomes were measured at baseline, one month, and two months post-intervention. Participant completion of each outcome measure was traced to measure the feasibility of the data collection procedures [28]. The primary outcome was to assess the PFMT adherence among the study participants. Secondary outcomes, including urinary incontinence, quality of life, PFMT knowledge, attitude, practice, and self-efficacy, are described in Table 2.

Table 2.
Preliminary effectiveness outcomes.
2.6. Sample Size
It is not required to have a powered sample size for the pilot study [32]. Previous analysis suggested a minimum of 12–30 participants per group as an appropriate sample in feasibility studies [33] and pilot studies [34]. This study was anticipated to have 64 participants within 2 months duration.
2.7. Randomisation and Blinding
A randomisation app (RRApp) generated the randomisation sequence. Participants stratification by primigravida and multigravida to minimise the selection bias. A concealed envelope was provided to a non-researcher to reveal the assigned intervention and control group. This study was a single-blinded study, in which the researchers involved were blinded to participant group allocation [35], as it was not feasible and possible to blind the participant due to the nature of the intervention and control conditions.
2.8. Statistical Methods
All analyses were performed utilising the Statistical Package for the Social Sciences version 27.0 (IBM, New York, NY, USA) [36,37]. Data are presented according to normality testing (Shapiro–Wilk test) distribution with mean and standard deviation (SD) or median, interquartile range (IQR) for continuous variables, and counts (percentages) for categorical variables. Baseline characteristics of the participants and the study outcomes were determined using either t-test or Mann–Whitney U test accordingly between two groups.
Analyses for the preliminary effectiveness outcomes were conducted on an intention-to-treat without computation. The generalised estimating equation (GEE) model was employed to manage the missing values in repeated-measure data. GEE has its robust ability to analyse the intervention effect and interaction effect between time and intervention without replacing the missing data [38]. All analyses with a p-value < 0.05 were considered statistically significant.
3. Results
3.1. Participant Characteristics
A total of 26 pregnant women were randomly allocated to the KEPT app group (n = 16) or control group (n = 10), aged 21–39 years. The majority were from lower-income status (92.3%, n = 24/26), with a minority being primigravida (34.6%, n = 9/26), and only one participant had a caesarean section. Less than 20% had provided with PFMT information (14.7%, n = 17/26). There were no statistically significant differences between the two groups in baseline characteristics (Table 3) and baseline outcome measures (Table 4), except UI symptoms severity with p = 0.031. This finding could be due to the imbalance of the intervention group with Stress UI more than Urge UI, whereby the control group has a similar ratio of Stress UI and Urge UI. The intervention group has significantly less severe in its UI symptoms when compared with the control may attenuate the effectiveness of the intervention due to milder severity of the UI.

Table 3.
Baseline characteristics of the intervention vs. control group.

Table 4.
Baseline outcome measures and comparison between intervention and control groups.
3.2. Feasibility of the Study
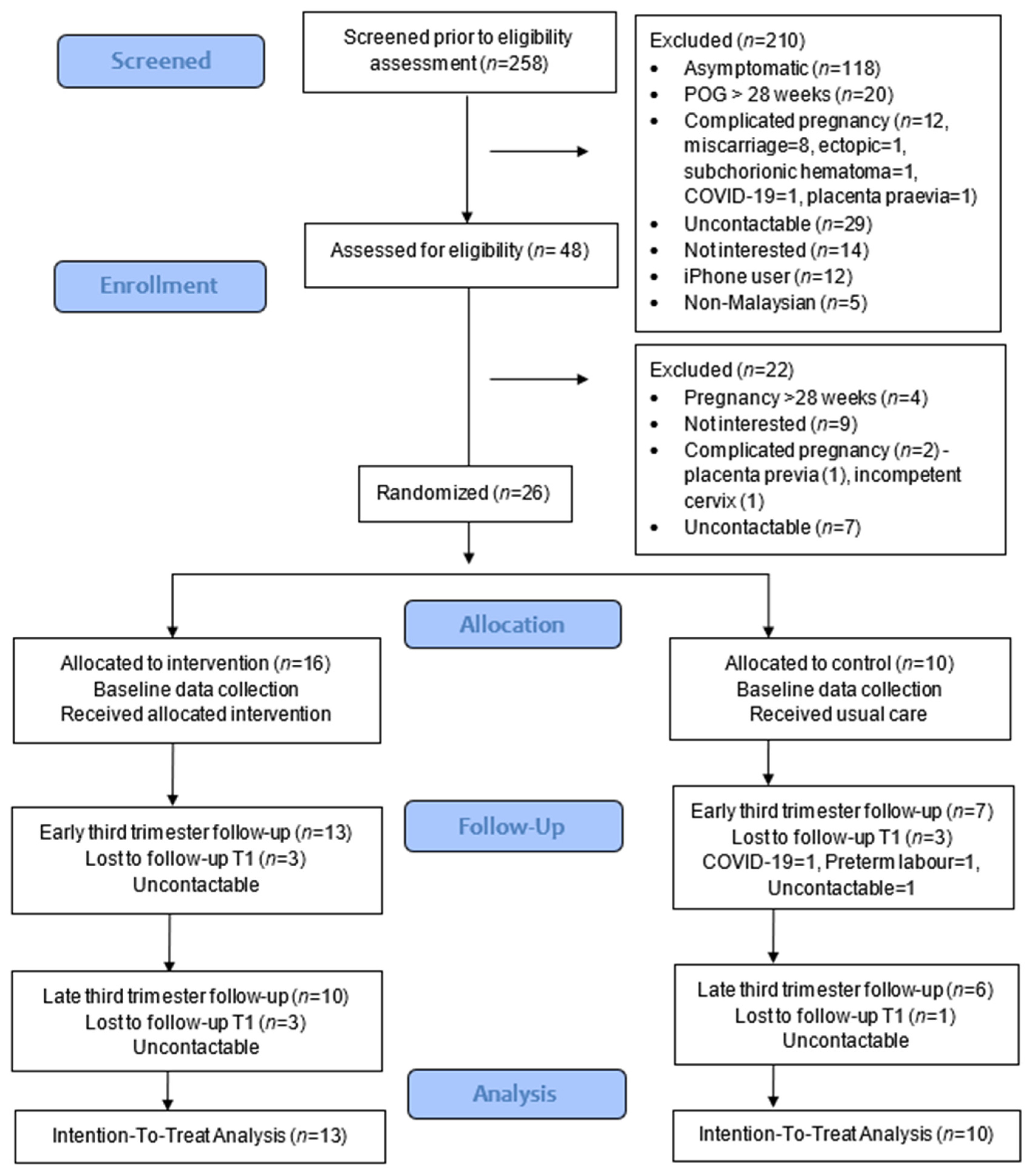
The proportions of respondents who met the eligibility criteria were only 18.6%, with almost half being asymptomatic (45.7%) (Figure 1). The recruitment rate was 54.2%, and the retention rates at 1 month were 81.3% (13/16) for intervention and 70% (7/10) for the control group. At the end of the study, the retention rates were reduced to 62.5% (10/16) for intervention and 60% (6/10) for the control group, and no adverse events were reported in the intervention group. Data collection was feasible using the app and Google Forms.
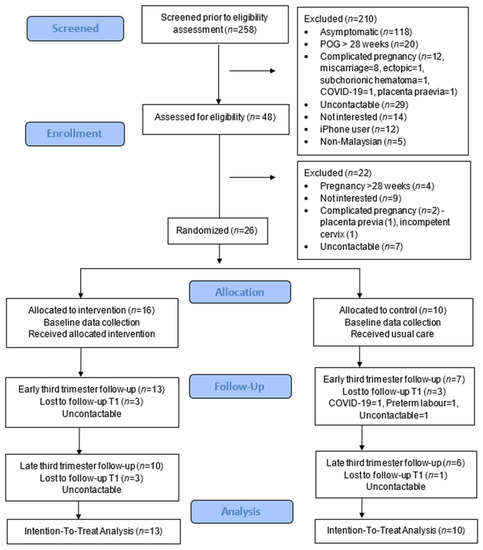
Figure 1.
CONSORT study flowchart.
3.3. Primary Outcome
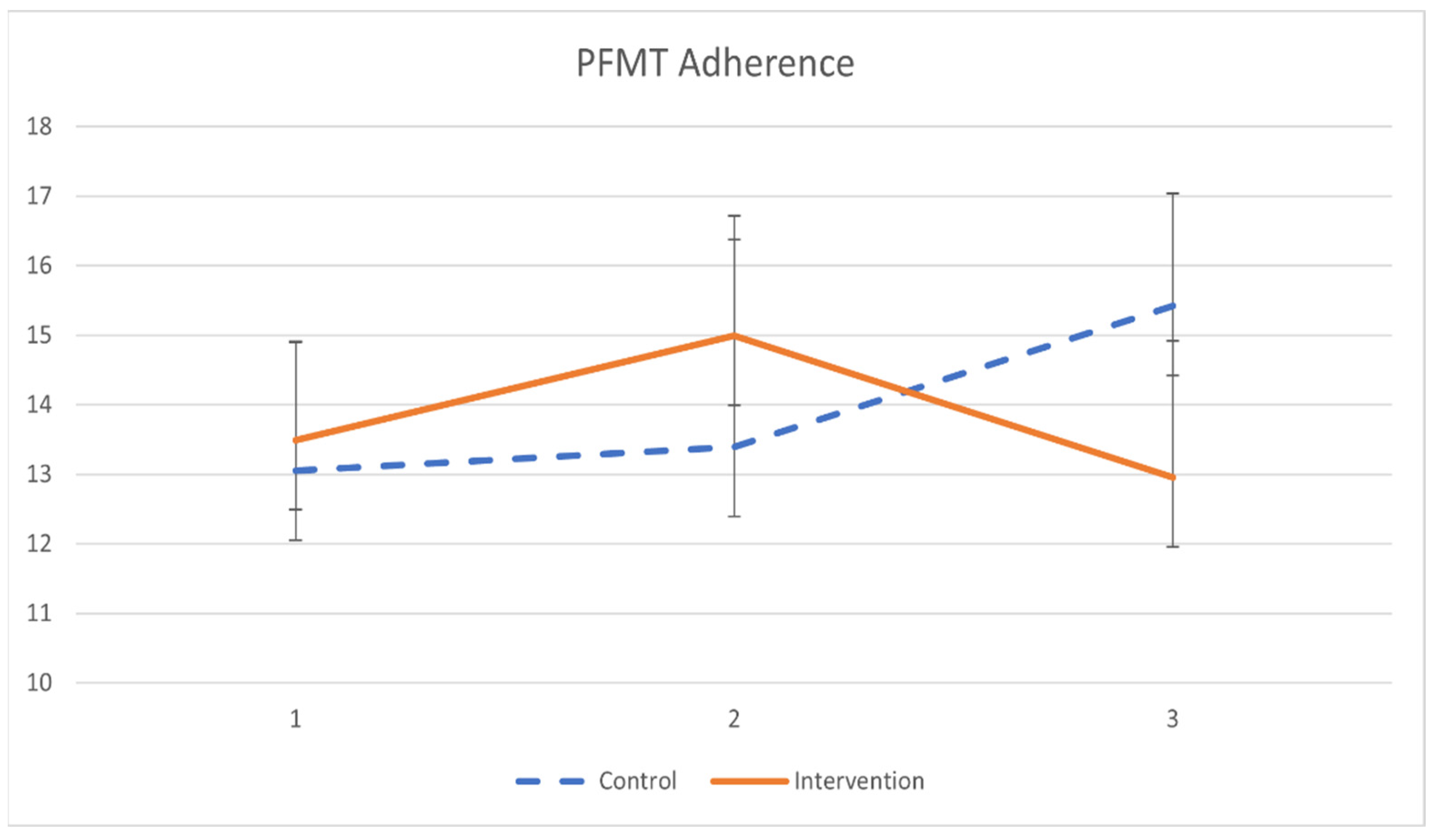
The primary outcomes of pelvic floor muscle training adherence are demonstrated in Table 5 and Figure 2. Participants in the intervention group had minimal significant improvement in PFMT adherence after a 2-month training (β = 0.033, p = 0.019). However, the difference in PFMT adherence between groups was not statistically significant.

Table 5.
The effect of KEPT app on pelvic floor muscle training adherence.
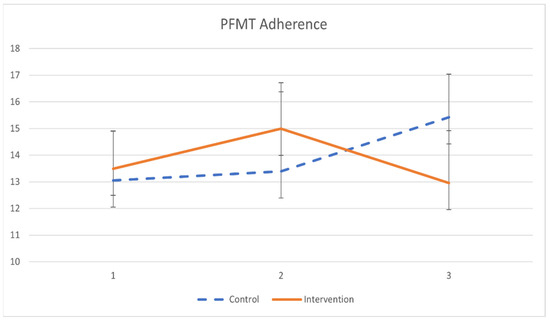
Figure 2.
The mean values and standard errors for PFMT adherence of the two groups across the study.
3.4. Secondary Outcomes
3.4.1. PFMT Knowledge, Attitude, Practice, and Self-Efficacy
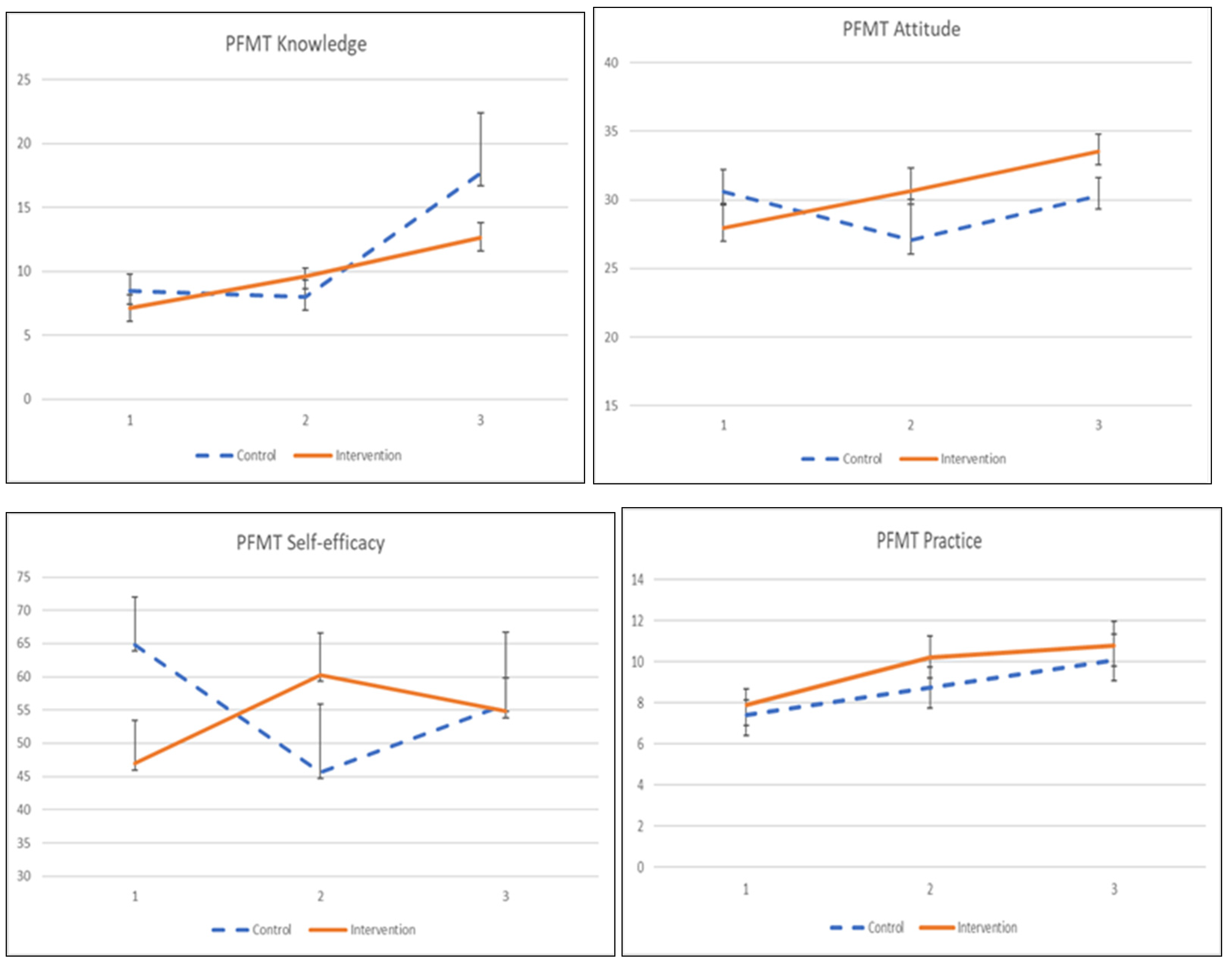
The adherence to the exercise was influenced by its knowledge, attitude, practice, and self-efficacy, as shown in Table 6 and Figure 3. The interaction effects between intervention and time on knowledge, attitude, and self-efficacy were significant. Participants in the KEPT app group indicated significant knowledge and self-efficacy improvement after a 1-month training (β = 2.968, p = 0.011), and β = 6.246, p = 0.038). Meanwhile, the participants demonstrated a significantly improved PFMT attitude at 2 months post-intervention, compared with the control group (β = 5.884, p < 0.034). PFMT practice was found significant only when compared within the KEPT app group at 2 months (β = 2.668, p = 0.018). The differences in PFMT practice between groups were not statistically significant.

Table 6.
The effect of KEPT app on PFMT knowledge, attitude, practice, and self-efficacy.
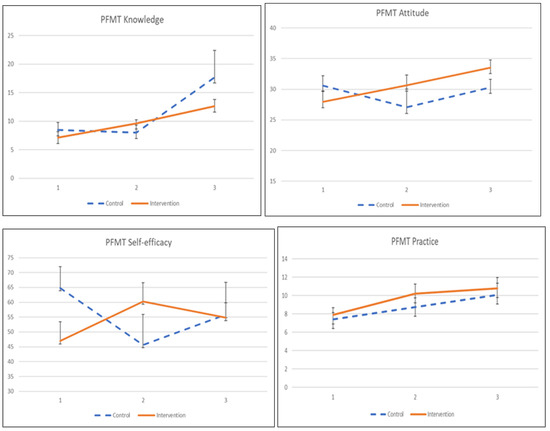
Figure 3.
The mean values and standard errors for PFMT knowledge, attitude, practice, and self-efficacy of the two groups across this pilot RCT study.
3.4.2. Urinary Incontinence and Quality of Life
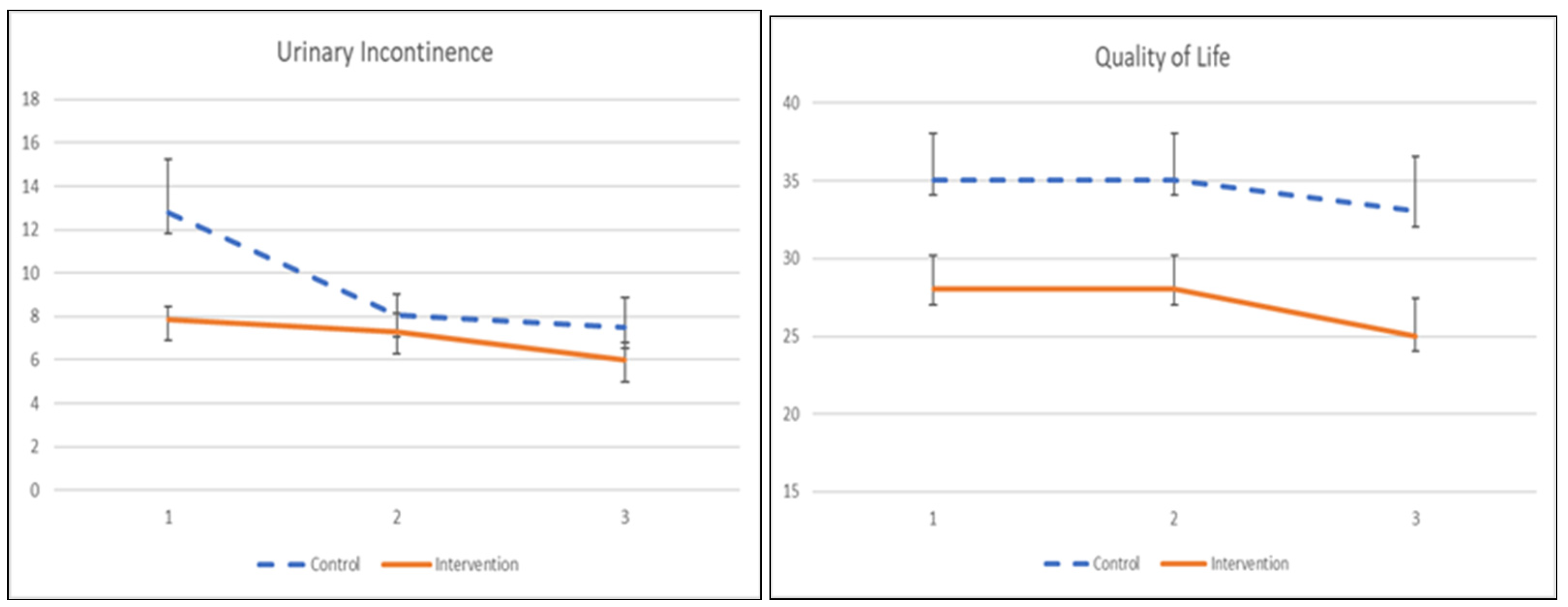
The UI symptom severity and its quality of life to pelvic floor muscle training are demonstrated in Table 7 and Figure 4. Participants receiving the app showed significant improvement in symptom severity after a 1-month training (β = −4.748, p = 0.049) but did not show persistence after 2 months of intervention, as there was no significant treatment effect. The quality of life among participants did not demonstrate any significant improvement at 1 month or 2 months post-intervention.

Table 7.
The effect of KEPT app on urinary incontinence and quality of life.
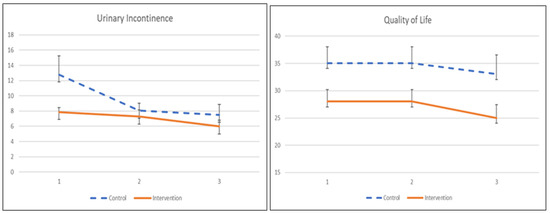
Figure 4.
The mean values and standard errors for urinary incontinence severity and quality of life of the two groups across this pilot RCT study.
4. Discussion
This study was a single-blind, single-centre, pilot feasibility RCT to assist the feasibility of the proposed future full-size RCT and replicating its miniature [27]. This pilot trial was able to identify the potential challenges of recruitment from one single centre, such as poor recruitment and retention rates. The enabling factors to encourage participation were the excellent relationship with the healthcare centre. Their willingness to assist in disseminating the study information was crucial, leading to the participation of the study respondents.
Self-efficacy has been documented to have a modifier effect on the PFMT, whereby women need to perform the exercise independently [39,40]. Pregnant women must understand the anatomical part of pelvic floor muscle and its function and internalise the ability or physical skills to contract the muscles correctly. This app provided them with an educational video from the physiotherapist and notes to further inform them about the exercise. The app improved their self-efficacy (p = 0.038) and knowledge (p = 0.011), compared with the control group in the first month. Hence, this study supports the feasibility of the future RCT conducted in 5 months duration until 2 months post-partum.
Effectiveness was reported with minimum improvement in its adherence and the improvement of the symptoms. The knowledge, attitude, practice, and self-efficacy improved in the intervention group. However, a careful interpretation is crucial, as the findings from this study reveal a risk of type II error due to the study’s small sample size and medium effect size [41].
The various unpredictable restrictions in collecting data due to the COVID-19 pandemic added challenges to this study. This study had applied using e-poster information promoted by the assistance from the healthcare providers in the healthcare centre and extended the duration of the recruitment into three months. A low recruitment rate (54.2%) was expected during the COVID-19 pandemic. This study recruited higher than a previous study using social media, with 20–40% [42]. Few suggested strategies, such as including only the highly motivated and committed participants, may be added to our future full RCT eligibility criteria, to improve the recruitment rate and reduce attrition rates.
Despite an acceptable recruitment rate, this study reported less retention, compared with a recent review from sixteen pilot studies [43]. Aside from pandemic-related issues, other factors such as restriction movement order or perhaps psychological stress and financial stress [44] during the pregnancy may have influenced retention. Therefore, significant changes need to be considered, such as adding incentives for both groups with higher amounts in the control group [45] and screening for psychological distress to assist them.
This study was unable to demonstrate improvement in adherence despite improving the self-efficacy towards PFMT. In contrast, the study using audio-app-based PFMT for 6 months in duration among primigravida demonstrated improved adherence with self-efficacy [46]. The result could be because our pilot study was conducted shorter (2 months) than other studies. Women who have undergone supervised training at or over 8 weeks with weekly appointments adhere more effectively than those with unsupervised training [47]. This could be explained unsupervised training might need external motivations to reinforce engagement. The idea of making exercise a more enjoyable experience, aiming to score points and compete with each other, could modify health outcomes behaviour.
PFMT adherence is crucial to improving muscle strength, increasing urethral closure pressure [48], and shortening the muscle length [49] after repeatedly contracting. This study used a validated questionnaire assessing home-based exercise, which was not explicitly designed for PFMT. Using a validated or adapted PFMT adherence questionnaire [50] may result differently from this study. Therefore, a new adapted and validated adherence questionnaire will be used in future RCT.
This study did not report significant findings in the UI symptoms improvement, similar to another 3-month, home-based PFMT intervention among post-partum study participants [51]. Despite no significant improvement, the urinary symptoms were not worsening, with a significant difference in each group. Further qualitative follow-up study may clarify its clinical significance despite being statistically insignificant from the participants’ perspective.
To our knowledge, this study was the first PFMT mHealth app (interactive version) interventional pilot trial involving antenatal mothers at all parity at a government healthcare clinic. This study has a small sample size, almost similar to a previous PFMT app for nonpregnant women [52].
The limitation of this study was the pilot feasibility in its design, leading to a need to be interpreted with caution for its preliminary effectiveness outcomes due to having a small sample size, single centre, and short duration. Hence, this study was not powered to detect significant changes in adherence in PFMT. Other limitations were that the previous history of UI was not investigated, and the previous muscle tone was not recorded. Both these limitations were the confounder variables in this pilot RCT study. Therefore, in our future effectiveness RCT, these two factors will be assessed and included as the independent variables. Another limitation was that this study applied the sealed envelop for random allocation instead of block randomisation published in the protocol. However, this would not affect the preliminary effectiveness of this pilot RCT.
In the future, the app will be refined to improve the user interface, especially regarding knowledge acquisition and the training timer. The KEPT web will be further improved, to enable pregnant women to communicate with healthcare providers, especially when having doubts about PFMT and UI. Subsequently, for pregnant women with hypertonicity of their pelvic muscles, perhaps an additional interface to explain the other methods of Kegel exercise should be tailored to manage the ‘complicated group’.
5. Conclusions
This pilot study demonstrated the strategies that need to be implemented for the feasibility of our future RCT [53]. Additional incentives and eligibility screening at earlier trimesters (second trimester) may improve recruitment rates. Even though the preliminary effectiveness found significant improvement in knowledge, attitude, and self-efficacy, it did not improve PFMT adherence.
Therefore, this study added another line of evidence to our needs assessment studies [7,54], validation study [19], and formative research [26] in KEPT app development. The data demonstrated that pregnant women (with moderate-to-low income status) in our healthcare clinics are interested in the mHealth app intervention, indicating readiness for the realm of digital health.
Author Contributions
Conceptualisation, A.J.; methodology, A.J.; software, A.J.; writing—original draft preparation, A.J.; writing—review and editing, A.J., C.N.F., N.A.M., R.A.M. and S.M.S.; resources, N.S.; visualisation, A.J.; formal analysis, A.J.; supervision, S.M.S.; funding acquisition, S.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Putra Malaysia, UPM/800-3/3/1/GPB/2018/9668500.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The study approvals have been obtained from Ethics Committee for Research Involving Human Subjects, Universiti Putra Malaysia (JKEUPM-2019-368) Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia (NMRR-19-412-45606) in August 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors would like to thank Prishalini A/P Ramesh@Apparao, Aziemah Sabirah Abdul Halim Anuar, and Darshini A/P Supparao, for the recruitment and data management. The researchers appreciate and acknowledge the National Medical Research Registration committee for their management support. We would like to thank the Director-General of Health Malaysia for his permission to publish this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Jacomo, R.H.; Nascimento, T.R.; Da Siva, M.L.; Salata, M.C.; Alves, A.T.; Da Cruz, P.R.C.; De Sousa, J.B. Exercise regimens other than pelvic floor muscle training cannot increase pelvic muscle strength-a systematic review. J. Bodyw. Mov. Ther. 2020, 24, 568–574. [Google Scholar] [CrossRef]
- Woodley, S.J.; Lawrenson, P.; Boyle, R.; Cody, J.D.; Mørkved, S.; Kernohan, A.; Hay-Smith, E.J.C. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst. Rev. 2020, 2020, 1–216. [Google Scholar]
- Du, Y.; Xu, L.; Ding, L.; Wang, Y.; Wang, Z. The effect of antenatal pelvic floor muscle training on labor and delivery outcomes: A systematic review with meta-analysis. Int. Urogynecol. J. 2015, 26, 1415–1427. [Google Scholar] [CrossRef]
- Ren, S.; Gao, Y.; Yang, Z.; Li, J.; Xuan, R.; Liu, J.; Chen, X.; Thirupathi, A. The Effect of Pelvic Floor Muscle Training on Pelvic Floor Dysfunction in Pregnant and Postpartum Women. Phys. Act. Health 2020, 4, 130–141. [Google Scholar] [CrossRef]
- Hadizadeh-Talasaz, Z.; Sadeghi, R.; Khadivzadeh, T. Effect of pelvic floor muscle training on postpartum sexual function and quality of life: A systematic review and meta-analysis of clinical trials. Taiwan J. Obstet. Gynecol. 2019, 58, 737–747. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.; Apostolidis, A.; Birder, L.; Bliss, D.; Brubaker, L. 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Neurourol. Urodyn. 2018, 37, 2271–2272. [Google Scholar] [CrossRef] [Green Version]
- Jaffar, A.; Mohd-Sidik, S.; Nien, F.C.; Fu, G.Q.; Talib, N.H. Urinary incontinence and its association with pelvic floor muscle exercise among pregnant women attending a primary care clinic in Selangor, Malaysia. PLoS ONE 2020, 15, e0236140. [Google Scholar] [CrossRef]
- Moossdorff-Steinhauser, H.F.A.; Berghmans, B.C.M.; Spaanderman, M.E.A.; Bols, E.M.J. Prevalence, incidence and bothersomeness of urinary incontinence in pregnancy: A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 1633–1652. [Google Scholar] [CrossRef]
- Moossdorff-Steinhauser, H.F.A.; Berghmans, B.C.M.; Spaanderman, M.E.A.; Bols, E.M.J. Urinary incontinence during pregnancy: Prevalence, experience of bother, beliefs, and help-seeking behavior. Int. Urogynecol. J. 2020, 32, 695–701. [Google Scholar] [CrossRef]
- Maeda, N.; Urabe, Y.; Suzuki, Y.; Hirado, D.; Morikawa, M.; Komiya, M.; Mizuta, R.; Naito, K.; Shirakawa, T. Cross-Sectional Study of the Prevalence and Symptoms of Urinary Incontinence among Japanese Older Adults: Associations with Physical Activity, Health-Related Quality of Life, and Well-Being. Int. J. Environ. Res. Public Health 2021, 18, 360. [Google Scholar] [CrossRef]
- Al Kiyumi, M.H.; Al Belushi, Z.I.; Jaju, S.; Al Mahrezi, A.M. Urinary Incontinence Among Omani Women: Prevalence, risk factors and impact on quality of life. Sultan Qaboos Univ. Med. J. 2020, 20, e45–e53. [Google Scholar] [CrossRef] [Green Version]
- Woodley, S.J.; Hay-Smith, E.J.C. Narrative review of pelvic floor muscle training for childbearing women—why, when, what, and how. Int. Urogynecol. J. 2021, 32, 1977–1988. [Google Scholar] [CrossRef]
- Terry, R.; Jarvie, R.; Hay-Smith, J.; Salmon, V.; Pearson, M.; Boddy, K.; MacArthur, C.; Dean, S. “Are you doing your pelvic floor?” An ethnographic exploration of the interaction between women and midwives about pelvic floor muscle exercises (PFME) during pregnancy. Midwifery 2020, 83, 102647. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Jin, Y.; Feng, S. Knowledge, attitude and practice of pelvic floor dysfunction among obstetrical healthcare workers in China: A cross-sectional study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102068. [Google Scholar] [CrossRef]
- Salmon, V.; Hay-Smith, E.J.C.; Jarvie, R.; Dean, S.; Terry, R.; Frawley, H.; Oborn, E.; Bayliss, S.E.; Bick, D.; Davenport, C.; et al. Implementing pelvic floor muscle training in women’s childbearing years: A critical interpretive synthesis of individual, professional, and service issues. Neurourol. Urodyn. 2020, 39, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Tanahashi, T. Health service coverage and its evaluation. Bull. World Health Organ. 1978, 56, 295–303. [Google Scholar]
- Iyawa, G.E.; Dansharif, A.R.; Khan, A. Mobile apps for self-management in pregnancy: A systematic review. Health Technol. 2021, 11, 283–294. [Google Scholar] [CrossRef]
- Rygh, P.; Asklund, I.; Samuelsson, E. Real-world effectiveness of app-based treatment for urinary incontinence: A cohort study. BMJ Open 2021, 11, e040819. [Google Scholar] [CrossRef]
- Jaffar, A.; Mohd-Sidik, S.; Chai Nien, F.; Admodisastro, N.; Abdul Salam, S.N.; Ismail, N.D. Improving Pelvic Floor Muscle Training Adherence Among Pregnant Women: Validation Study. JMIR Hum. Factors 2022, 9, e30989. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef] [Green Version]
- Thabane, L.; Hopewell, S.; Lancaster, G.A.; Bond, C.M.; Coleman, C.L.; Campbell, M.J.; Eldridge, S.M. Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot Feasibility Stud. 2016, 2, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffar, A.; Sidik, S.M.; Foo, C.; Muhammad, N.; Manaf, R.A.; Ismail, S.F.; Suhaili, N. Protocol of a Single-Blind Two-Arm (Waitlist Control) Parallel-Group Randomised Controlled Pilot Feasibility Study for mHealth App among Incontinent Pregnant Women. Int. J. Environ. Res. Public Health 2021, 18, 4792. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Avery, K.; Donovan, J.; Peters, T.J.; Shaw, C.; Gotoh, M.; Abrams, P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol. Urodyn. 2004, 23, 322–330. [Google Scholar] [CrossRef]
- Lim, R.; Liong, M.L.; Lau, Y.K.; Yuen, K.H. Validity, reliability, and responsiveness of the ICIQ-UI SF and ICIQ-LUTSqol in the Malaysian population. Neurourol. Urodyn. 2017, 36, 438–442. [Google Scholar] [CrossRef]
- Jaffar, A.; Sidik, S.M.; Admodisastro, N.; Mansor, E.I.; Fong, L.C. Expert’s Usability Evaluation of the Pelvic Floor Muscle Training mHealth App for Pregnant Women. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 165–173. [Google Scholar] [CrossRef]
- Abbott, J.H. The Distinction Between Randomized Clinical Trials (RCTs) and Preliminary Feasibility and Pilot Studies: What They Are and Are Not. J. Orthop. Sports Phys. Ther. 2014, 44, 555–558. [Google Scholar] [CrossRef]
- Hutchesson, M.J.; Taylor, R.; Shrewsbury, V.A.; Vincze, L.; Campbell, L.E.; Callister, R.; Park, F.; Schumacher, T.L.; Collins, C.E. Be healthe for your heart: A pilot randomized controlled trial evaluating a web-based behavioral intervention to improve the cardiovascular health of women with a history of preeclampsia. Int. J. Environ. Res. Public Health 2020, 17, 5779. [Google Scholar] [CrossRef]
- Newman-Beinart, N.A.; Norton, S.; Dowling, D.; Gavriloff, D.; Vari, C.; Weinman, J.A.; Godfrey, E.L. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: The Exercise Adherence Rating Scale (EARS). Physiotherapy 2017, 103, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Rosediani, M.; Juliawati, M.; Norwati, D. Knowledge, attitude and practice towards pelvic floor muscle exercise among pregnant women attending antenatal clinic in Universiti Sains Malaysia Hospital, Malaysia. Int. Med. J. 2012, 19, 37–38. [Google Scholar]
- Sacomori, C.; Cardoso, F.L.; Porto, I.P.; Negri, N.B. The development and psychometric evaluation of a self-efficacy scale for practicing pelvic floor exercises. Braz. J. Phys. Ther. 2013, 17, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.C.; Davis, L.L.; Kraemer, H.C. The role and interpretation of pilot studies in clinical research. J. Psychiatr. Res. 2011, 45, 626–629. [Google Scholar] [CrossRef] [Green Version]
- Billingham, S.A.; Whitehead, A.L.; Julious, S.A. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med. Res. Methodol. 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Day, S.J.; Altman, D.G. Statistics notes: Blinding in clinical trials and other studies. BMJ 2000, 321, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, G.A.; Barrett, K.C.; Leech, N.L.; Gloeckner, G.W. IBM SPSS for Introductory Statistics: Use and Interpretation; Routledge: London, UK, 2019. [Google Scholar]
- Abu-Bader, S.H. Using Statistical Methods in Social Science Research: With a Complete SPSS Guide; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Ma, Y.; Mazumdar, M.; Memtsoudis, S.G. Beyond repeated-measures analysis of variance: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg. Anesth. Pain Med. 2012, 37, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Firet, L.; Teunissen, T.A.; Kool, R.B.; van Doorn, L.; Aourag, M.; Lagro-Janssen, A.L.; Assendelft, W.J. Women’s adoption of a web-based intervention for stress urinary incontinence: A qualitative study. BMC Health Serv. Res. 2021, 21, 574. [Google Scholar] [CrossRef]
- Hay-Smith, J.; Dean, S.; Burgio, K.; McClurg, D.; Frawley, H.; Dumoulin, C. Pelvic-floor-muscle-training adherence “modifiers”: A review of primary qualitative studies-2011 ICS State-of-the-Science Seminar research paper III of IV. Neurourol. Urodyn. 2015, 34, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef]
- Ali, S.H.; Foreman, J.; Capasso, A.; Jones, A.M.; Tozan, Y.; DiClemente, R.J. Social media as a recruitment platform for a nationwide online survey of COVID-19 knowledge, beliefs, and practices in the United States: Methodology and feasibility analysis. BMC Med. Res. Methodol. 2020, 20, 116. [Google Scholar] [CrossRef]
- Cooper, C.L.; Whitehead, A.; Pottrill, E.; Julious, S.A.; Walters, S.J. Are pilot trials useful for predicting randomisation and attrition rates in definitive studies: A review of publicly funded trials. Clin. Trials 2018, 15, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.R.; Salisbury, A.L.; Uebelacker, L.A.; Abrantes, A.M.; Battle, C.L. Stress, coping and silver linings: How depressed perinatal women experienced the COVID-19 pandemic. J. Affect. Disord. 2022, 298 Pt A, 329–336. [Google Scholar] [CrossRef]
- Robinson, K.A.; Dinglas, V.D.; Sukrithan, V.; Yalamanchilli, R.; Mendez-Tellez, P.A.; Dennison-Himmelfarb, C.; Needham, D.M. Updated systematic review identifies substantial number of retention strategies: Using more strategies retains more study participants. J. Clin. Epidemiol. 2015, 68, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, X.; Luo, J.; Chen, Z.; Feng, S. Effect of app-based audio guidance pelvic floor muscle training on treatment of stress urinary incontinence in primiparas: A randomized controlled trial. Int. J. Nurs. Stud. 2020, 104, 103527. [Google Scholar] [CrossRef] [PubMed]
- Bø, K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J. Urol. 2012, 30, 437–443. [Google Scholar] [CrossRef]
- Zubieta, M.; Carr, R.L.; Drake, M.J.; Bø, K. Influence of voluntary pelvic floor muscle contraction and pelvic floor muscle training on urethral closure pressures: A systematic literature review. Int. Urogynecol. J. 2016, 27, 687–696. [Google Scholar] [CrossRef]
- Braekken, I.H.; Majida, M.; Engh, M.E.; Bø, K.; Brækken, I.H. Test-retest reliability of pelvic floor muscle contraction measured by 4D ultrasound. Neurourol. Urodyn. 2009, 28, 68–73. [Google Scholar] [CrossRef]
- Sacomori, C.; Zomkowski, K.; Porto, I.D.P.; Cardoso, F.L.; Sperandio, F.F. Adherence and effectiveness of a single instruction of pelvic floor exercises: A randomized clinical trial. Int. Urogynecol. J. 2019, 31, 951–959. [Google Scholar] [CrossRef]
- Pt, C.S.; Berghmans, B.; De Bie, R.; Mesters, I.; Cardoso, F.L. Predictors for adherence to a home-based pelvic floor muscle exercise program for treating female urinary incontinence in Brazil. Physiother. Theory Pract. 2018, 36, 186–195. [Google Scholar]
- Araujo, C.C.; Marques, A.D.A.; Juliato, C.R. The Adherence of Home Pelvic Floor Muscles Training Using a Mobile Device Application for Women with Urinary Incontinence: A Randomized Controlled Trial. Female Pelvic Med. Reconstr. Surg. 2020, 26, 697–703. [Google Scholar] [CrossRef]
- Sidik, S.M.; Jaffar, A.; Foo, C.N.; Muhammad, N.A.; Manaf, R.A.; Ismail, S.I.F.; Alagirisamy, P.; Fazlah, A.F.A.; Suli, Z.; Goodyear-Smith, F. KEPT-app trial: A pragmatic, single-blind, parallel, cluster-randomised effectiveness study of pelvic floor muscle training among incontinent pregnant women: Study protocol. BMJ Open 2021, 11, e039076. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, A.; Mohd-Sidik, S.; Abd Manaf, R.; Foo, C.N.; Gan, Q.F.; Saad, H. Quality of life among pregnant women with urinary incontinence: A cross-sectional study in a Malaysian primary care clinic. PLoS ONE 2021, 16, e0250714. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).