Neuroendocrine Determinants of Polycystic Ovary Syndrome
Abstract
:1. Introduction
2. Methods
3. GnRH Secretion in PCOS Patients
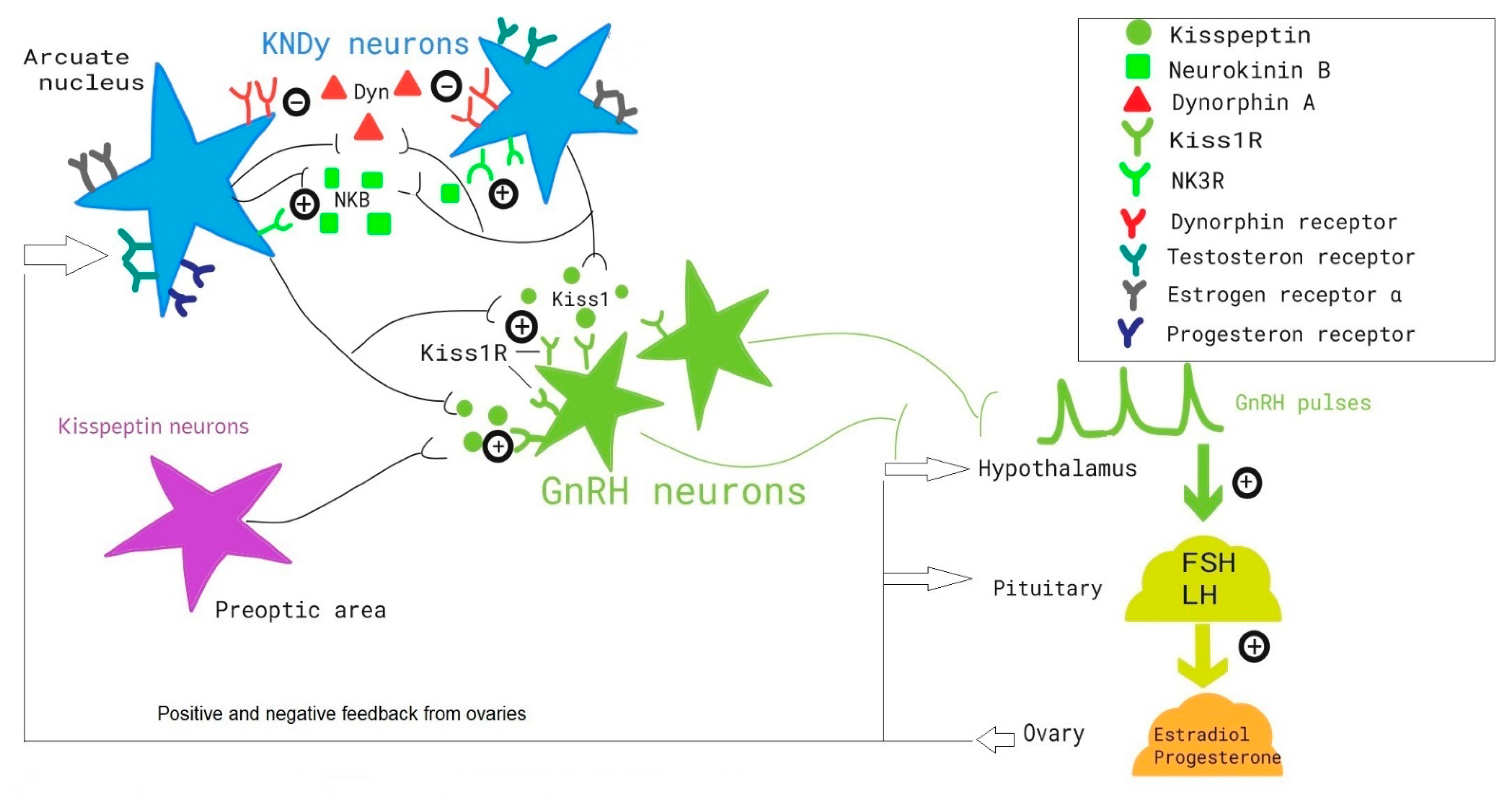
4. KNDy Neurons
5. Kisspeptin and PCOS
6. Neurokinin B and PCOS
7. Kisspeptin and NKB Analogs in PCOS
8. Other Neurohormones and Adipokines in PCOS (Phoenixin-14, Galanin, GLP-1)
9. Neurotransmitters in PCOS
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Pal, L.; Seli, E. Speroff’s Clinical Gynecologic Endocrinology and Infertility, 9th ed.; Department of Obstetrics, Gynecology and reproductive Sciences, Yale School of Medicine: New Haven, CT, USA, 2019. [Google Scholar]
- Franks, S.; McCarthy, M.I.; Hardy, K. Development of polycystic ovary syndrome: Involvement of genetic and environmental factors. Int. J. Androl. 2006, 29, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Piperi, C.; Korkolopoulou, P.; Kandaraki, E.; Levidou, G.; Papalois, A.; Patsouris, E.; Papavassiliou, A.G. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. J. Mol. Med. 2007, 85, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, F.; Hatala, D.A.; Speroff, L. Adrenal and ovarian steroid hormone responses to gonadotropin-releasing hormone agonist treatment in polycystic ovary syndrome. Am. J. Obstet. Gynecol. 1991, 165, 535–545. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487. [Google Scholar] [CrossRef] [Green Version]
- McCartney, C.R.; Eagleson, C.A.; Marshall, J.C. Regulation of gonadotropin secretion: Implications for polycystic ovary syndrome. Semin. Reprod. Med. 2002, 20, 317–325. [Google Scholar] [CrossRef]
- Burt Solorzano, C.M.; Beller, J.P.; Abshire, M.Y.; Collins, J.S.; McCartney, C.R.; Marshall, J.C. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids 2012, 77, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Blank, S.K.; McCartney, C.R.; Chhabra, S.; Helm, K.D.; Eagleson, C.A.; Chang, R.J.; Marshall, J.C. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls—implications for regulation of pubertal maturation. J. Clin. Endocrinol. Metab. 2009, 94, 2360–2366. [Google Scholar] [CrossRef]
- Franks, S.; Mason, H.; Willis, D. Follicular dynamics in the polycystic ovary syndrome. Mol. Cell Endocrinol. 2000, 163, 49–52. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update. 2016, 22, 709–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, S.; Stark, J.; Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update 2008, 14, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin. Endocrinol. 2018, 89, 251–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Naftolin, F.; Tolis, G. Neuroendocrine regulation of the menstrual cycle. Clin. Obstet. Gynecol. 1978, 21, 17–29. [Google Scholar] [CrossRef]
- Knobil, E. The neuroendocrine control of the menstrual cycle. Recent Prog. Horm Res. 1980, 36, 53–88. [Google Scholar] [CrossRef]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.L.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef] [Green Version]
- Nagae, M.; Uenoyama, Y.; Okamoto, S.; Tsuchida, H.; Ikegami, K.; Goto, T.; Majarune, S.; Nakamura, S.; Sanbo, M.; Hirabayashi, M.; et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc. Natl. Acad. Sci. USA 2021, 118, e2009156118. [Google Scholar] [CrossRef]
- Goodman, R.L.; Lehman, M.; Smith, J.; Coolen, L.; De Oliveira, C.V.R.; Shirazi, M.R.J.; Pereira, A.; Iqbal, J.; Caraty, A.; Ciofi, P.; et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007, 148, 5752–5760. [Google Scholar] [CrossRef]
- Lehman, M.N.; Coolen, L.M.; Goodman, R.L. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010, 151, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; De Tassigny, X.D.A.; Doran, J.; College, W.H. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruka, K.A.; Burger, L.L.; Moenter, S.M. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology 2013, 154, 2761–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Croft, S.; Boehm, U.; Herbison, A.E. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 2013, 154, 2750–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weems, P.W.; Coolen, L.; Hileman, S.M.; Hardy, S.; McCosh, R.; Goodman, R.L.; Lehman, M.N. Evidence That Dynorphin Acts Upon KNDy and GnRH Neurons During GnRH Pulse Termination in the Ewe. Endocrinology 2018, 159, 3187–3199. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; Theodosis, D.T. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 1992, 50, 283–298. [Google Scholar] [CrossRef]
- Matsuda, F.; Nakatsukasa, K.; Suetomi, Y.; Naniwa, Y.; Ito, D.; Inoue, N.; Wakabayashi, Y.; Okamura, H.; Maeda, K.-I.; Uenoyama, Y.; et al. The luteinising hormone surge-generating system is functional in male goats as in females: Involvement of kisspeptin neurones in the medial preoptic area. J. Neuroendocrinol. 2015, 27, 57–65. [Google Scholar] [CrossRef]
- Goodman, R.L.; Coolen, L.M.; Anderson, G.M.; Hardy, S.L.; Valent, M.; Connors, J.M.; Fitzgerald, M.E.; Lehman, M.N. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 2004, 145, 2959–2967. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, M.V.; Servili, A.; Molés, G.; Gueguen, M.M.; Carrillo, M.; Kah, O.; Felip, A. Actions of sex steroids on kisspeptin expression and other reproduction-related genes in the brain of the teleost fish European sea bass. J. Exp. Biol. 2016, 219, 3353–3365. [Google Scholar] [CrossRef] [Green Version]
- Walters, K.A.; Gilchrist, R.B.; Ledger, W.L.; Teede, H.J.; Handelsman, D.J.; Campbell, R.E. New Perspectives on the Pathogenesis of PCOS: Neuroendocrine Origins. Trends Endocrinol. Metab. 2018, 29, 841–852. [Google Scholar] [CrossRef]
- Coyle, C.; Campbell, R.E. Pathological pulses in PCOS. Mol. Cell Endocrinol. 2019, 498, 110561. [Google Scholar] [CrossRef] [PubMed]
- Panidis, D.; Rousso, D.; Koliakos, G.; Kourtis, A.; Katsikis, I.; Farmakiotis, D.; Votsi, E.; DiamantiKandarakis, E. Plasma metastin levels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil. Steril. 2006, 85, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Ding, X.; Zhu, J. Kisspeptin and Polycystic Ovary Syndrome. Front. Endocrinol 2019, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Phylactou, M.; Clarke, S.A.; Patel, B.; Baggaley, C.; Jayasena, C.N.; Kelsey, T.W.; Comninos, A.N.; Dhillo, W.S.; Abbara, A. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2021, 95, 239–252. [Google Scholar] [CrossRef]
- Liu, J.; Qu, T.; Li, Z.; Yu, L.; Zhang, S.; Yuan, D.; Wu, H. Serum kisspeptin levels in polycystic ovary syndrome: A meta-analysis. J. Obstet. Gynaecol. Res. 2021, 47, 2157–2165. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Ornat, L.; López-Baena, M.T.; Santabárbara, J.; Savirón-Cornudella, R.; Pérez-Roncero, G.R. Circulating kisspeptin and anti-müllerian hormone levels, and insulin resistance in women with polycystic ovary syndrome: A systematic review, meta-analysis, and meta-regression. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 85–98. [Google Scholar] [CrossRef]
- Khan, S.H.; Chaudhry, N. Beyond GnRH, LH and FSH: The role of kisspeptin on hypothalalmic-pituitary gonadal (HPG) axis pathology and diagnostic consideration. J. Pak. Med. Assoc. 2021, 71, 1862–1869. [Google Scholar] [CrossRef]
- Esparza, L.A.; Schafer, D.; Ho, B.S.; Thackray, V.G.; Kauffman, A.S. Hyperactive LH Pulses and Elevated Kisspeptin and NKB Gene Expression in the Arcuate Nucleus of a PCOS Mouse Model. Endocrinology 2020, 161, bqaa018. [Google Scholar] [CrossRef]
- Katulski, K.; Podfigurna, A.; Czyzyk, A.; Meczekalski, B.; Genazzani, A.D. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine 2018, 61, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Han, S.; Tian, W.; Zhao, M.; Zhang, H. Effects of kisspeptin on pathogenesis and energy metabolism in polycystic ovarian syndrome (PCOS). Gynecol. Endocrinol. 2019, 35, 807–810. [Google Scholar] [CrossRef]
- Rashad, N.M.; Al-sayed, R.M.; Yousef, M.S.; Saraya, Y.S. Kisspeptin and body weight homeostasis in relation to phenotypic features of polycystic ovary syndrome; metabolic regulation of reproduction. Diabetes Metab. Syndr. 2019, 13, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, M.T. Polycystic ovarian syndrome: Diagnosis and management. Clin. Med. Res. 2004, 2, 13–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trent, M.; Gordon, C.M. Diagnosis and Management of Polycystic Ovary Syndrome in Adolescents. Pediatrics 2020, 145 (Suppl. 2), S210–S218. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.P. GnRHs and GnRH receptors. Anim Reprod Sci. 2005, 88, 5–28. [Google Scholar] [CrossRef]
- Dhillo, W.S. Kisspeptin, Neurokinin B and New Players in Reproduction. Semin. Reprod. Med. 2019, 37, 107–108. [Google Scholar] [CrossRef]
- Hunjan, T.; Abbara, A. Clinical Translational Studies of Kisspeptin and Neurokinin, B. Semin. Reprod. Med. 2019, 37, 119–124. [Google Scholar] [CrossRef]
- Rance, N.E.; Young, W.S. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 1991, 128, 2239–2247. [Google Scholar] [CrossRef]
- Szeliga, A.; Podfigurna, A.; Bala, G.; Meczekalski, B. Kisspeptin and neurokinin B analogs use in gynecological endocrinology: Where do we stand? J. Endocrinol. Investig. 2020, 43, 555–561. [Google Scholar] [CrossRef]
- Osuka, S.; Iwase, A.; Nakahara, T.; Kondo, M.; Saito, A.; Bayasula; Nakamura, T.; Takikawa, S.; Goto, M.; Kotani, T.; et al. Kisspeptin in the Hypothalamus of 2 Rat Models of Polycystic Ovary Syndrome. Endocrinology 2017, 158, 367–377. [Google Scholar] [CrossRef] [Green Version]
- George, J.T.; Kakkar, R.; Marshall, J.; Scott, M.L.; Finkelman, R.D.; Ho, T.W.; Veldhuis, J.; Skorupskaite, K.; Anderson, R.A.; McIntosh, S.; et al. Neurokinin B Receptor Antagonism in Women with Polycystic Ovary Syndrome: A Randomized, Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 4313–4321. [Google Scholar] [CrossRef] [Green Version]
- Blasco, V.; Pinto, F.M.; Fernández-Atucha, A.; Prados, N.; Tena-Sempere, M.; Fernández-Sánchez, M.; Candenas, L. Altered expression of the kisspeptin/KISS1R and neurokinin B/NK3R systems in mural granulosa and cumulus cells of patients with polycystic ovarian syndrome. J. Assist. Reprod. Genet. 2019, 36, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cortés, M.E.; Carrera, B.; Rioseco, H.; Pablo del Río, J.; Vigil, P. The Role of Kisspeptin in the Onset of Puberty and in the Ovulatory Mechanism: A Mini-review. J. Pediatr. Adolesc. Gynecol. 2015, 28, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, C.; Abbara, A.; Comninos, A.; Nijher, G.M.; Christopoulos, G.; Narayanaswamy, S.; Izzi-Engbeaya, C.; Sridharan, M.; Mason, A.; Warwick, J.; et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J. Clin. Investig. 2014, 124, 3667–3677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbara, A.; Jayasena, C.; Christopoulos, G.; Narayanaswamy, S.; Izzi-Engbeaya, C.; Nijher, G.M.K.; Comninos, A.; Peters, D.; Buckley, A.; Ratnasabapathy, R.; et al. Efficacy of Kisspeptin-54 to Trigger Oocyte Maturation in Women at High Risk of Ovarian Hyperstimulation Syndrome (OHSS) During In Vitro Fertilization (IVF) Therapy. J. Clin. Endocrinol. Metab. 2015, 100, 3322–3331. [Google Scholar] [CrossRef]
- Romero-Ruiz, A.; Skorupskaite, K.; Gaytan, F.; Torres, E.; Perdices-Lopez, C.; Mannaerts, B.M.; Qi, S.; Leon, S.; Manfredi-Lozano, M.; Lopez-Rodriguez, C.; et al. Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome. Hum. Reprod. 2019, 34, 2495–2512. [Google Scholar] [CrossRef]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update. 2014, 20, 485–500. [Google Scholar] [CrossRef] [Green Version]
- Skorupskaite, K.; George, J.T.; Veldhuis, J.D.; Millar, R.P.; Anderson, R.A. Kisspeptin and neurokinin B interactions in modulating gonadotropin secretion in women with polycystic ovary syndrome. Hum. Reprod. 2020, 35, 1421–1431. [Google Scholar] [CrossRef]
- Prinz, P.; Scharner, S.; Friedrich, T.; Schalla, M.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem. Biophys. Res. Commun. 2017, 493, 195–201. [Google Scholar] [CrossRef]
- Yosten, G.L.C.; Lyu, R.-M.; Hsueh, A.J.; Avsian-Kretchmer, O.; Chang, J.-K.; Tullock, C.; Dun, S.L.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef]
- Billert, M.; Kołodziejski, P.A.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Phoenixin-14 stimulates proliferation and insulin secretion in insulin producing INS-1E cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118533. [Google Scholar] [CrossRef]
- Nguyen, X.P.; Nakamura, T.; Osuka, S.; Bayasula, B.; Nakanishi, N.; Kasahara, Y.; Muraoka, A.; Hayashi, S.; Nagai, T.; Murase, T.; et al. Effect of the neuropeptide phoenixin and its receptor GPR173 during folliculogenesis. Reproduction 2019, 158, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kalamon, N.; Błaszczyk, K.; Szlaga, A.; Billert, M.; Skrzypski, M.; Pawlicki, P.; Wójtowicz, E.G.; Balak, M.K.; Blasiak, A.; Rak, A. Levels of the neuropeptide phoenixin-14 and its receptor GRP173 in the hypothalamus, ovary and periovarian adipose tissue in rat model of polycystic ovary syndrome. Biochem. Biophys. Res. Commun. 2020, 528, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Altinkaya, S.O. Galanin and glypican-4 levels depending on metabolic and cardiovascular risk factors in patients with polycystic ovary syndrome. Arch. Endocrinol Metab. 2021, 65, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, B.; Radzikowska, M.; Wasilewska-Dziubińska, E.; Kapliński, A.; Roguski, K.; Płonowski, A. Neuropeptide Y, leptin, galanin and insulin in women with polycystic ovary syndrome. Gynecol. Endocrinol. 1999, 13, 344–351. [Google Scholar] [CrossRef]
- Rasmussen, C.B.; Lindenberg, S. The Effect of Liraglutide on Weight Loss in Women with Polycystic Ovary Syndrome: An Observational Study. Front. Endocrinol. 2014, 5, 140. [Google Scholar] [CrossRef] [Green Version]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [Green Version]
- Lamos, E.M.; Malek, R.; Davis, S.N. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome. Expert Rev. Clin. Pharmacol. 2017, 10, 401–408. [Google Scholar] [CrossRef]
- Silva, M.S.; Desroziers, E.; Hessler, S.; Prescott, M.; Coyle, C.; Herbison, A.E.; Campbell, R.E. Activation of arcuate nucleus GABA neurons promotes luteinizing hormone secretion and reproductive dysfunction: Implications for polycystic ovary syndrome. EBioMedicine 2019, 44, 582–596. [Google Scholar] [CrossRef] [Green Version]
- Kawwass, J.F.; Sanders, K.M.; Loucks, T.L.; Rohan, L.C.; Berga, S.L. Increased cerebrospinal fluid levels of GABA, testosterone and estradiol in women with polycystic ovary syndrome. Hum. Reprod. 2017, 32, 1450–1456. [Google Scholar] [CrossRef]
- Ruddenklau, A.; Campbell, R.E. Neuroendocrine Impairments of Polycystic Ovary Syndrome. Endocrinology 2019, 160, 2230–2242. [Google Scholar] [CrossRef]
- Chaudhari, N.; Dawalbhakta, M.; Nampoothiri, L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod. Biol. Endocrinol. 2018, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.; Yen, S.C. beta-Endorphin stimulates the secretion of insulin and glucagon in humans. J. Clin. Endocrinol. Metab. 1981, 52, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Kiałka, M.; Milewicz, T.; Spałkowska, M.; Krzyczkowska-Sendrakowska, M.; Wasyl, B.; Pełka, A.; Krzysiek, J. β-endorphins Plasma Level is Higher in Lean Polycystic Ovary Syndrome (PCOS) Women. Exp. Clin. Endocrinol. Diabetes 2016, 124, 55–60. [Google Scholar] [CrossRef]
- Guido, M.; Romualdi, D.; Lanzone, A. Role of opioid antagonists in the treatment of women with glucoregulation abnormalities. Curr. Pharm. Des. 2006, 12, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Duleba, A.J.; El Shahat, O.; Ibrahim, M.E.; Salem, A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum. Reprod. 2008, 23, 2564–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares, R.; Hernández, D.; Morán, C.; Chavira, R.; Cárdenas, M.; Domínguez, R.; Morales-Ledesma, L. Unilateral or bilateral vagotomy induces ovulation in both ovaries of rats with polycystic ovarian syndrome. Reprod. Biol. Endocrinol. 2013, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Linares, R.; Acuña, X.N.; Rosas, G.; Vieyra, E.; Ramírez, D.A.; Chaparro, A.; Espinoza, J.A.; Domínguez, R.; Morales-Ledesma, L. Participation of the Cholinergic System in the Development of Polycystic Ovary Syndrome. Molecules 2021, 26, 5506. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, A.; Lester, W.; Stuart, S.P.; Dokras, A. Risk of depression and other mental health disorders in women with polycystic ovary syndrome: A longitudinal study. Fertil. Steril. 2009, 91, 207–212. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeliga, A.; Rudnicka, E.; Maciejewska-Jeske, M.; Kucharski, M.; Kostrzak, A.; Hajbos, M.; Niwczyk, O.; Smolarczyk, R.; Meczekalski, B. Neuroendocrine Determinants of Polycystic Ovary Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 3089. https://doi.org/10.3390/ijerph19053089
Szeliga A, Rudnicka E, Maciejewska-Jeske M, Kucharski M, Kostrzak A, Hajbos M, Niwczyk O, Smolarczyk R, Meczekalski B. Neuroendocrine Determinants of Polycystic Ovary Syndrome. International Journal of Environmental Research and Public Health. 2022; 19(5):3089. https://doi.org/10.3390/ijerph19053089
Chicago/Turabian StyleSzeliga, Anna, Ewa Rudnicka, Marzena Maciejewska-Jeske, Marek Kucharski, Anna Kostrzak, Marta Hajbos, Olga Niwczyk, Roman Smolarczyk, and Blazej Meczekalski. 2022. "Neuroendocrine Determinants of Polycystic Ovary Syndrome" International Journal of Environmental Research and Public Health 19, no. 5: 3089. https://doi.org/10.3390/ijerph19053089





