Effects of Δ9-Tetrahydrocannibinol (THC) on Obesity at Different Stages of Life: A Literature Review
Abstract
1. Introduction
2. Background and Epidemiology of Obesity
2.1. Metabolic Pathways to Obesity
2.2. Epidemiology of Obesity
3. Cannabinoids and Their Mechanism of Action
3.1. The Endocannabinoid System
3.2. Phytocannabinoids and Synthetic Cannabinoids
4. Δ9-Tetrahydrocannabinol (THC)
5. Effects on Obesity across Development: Δ9-Tetrahydrocannabinol (THC)
5.1. Effects of Δ9-Tetrahydrocannabinol (THC) on Adults and Obesity
5.2. Effects of Δ9-Tetrahydrocannabinol (THC) on Adolescents and Obesity
5.3. Effects of Δ9-Tetrahydrocannabinol (THC) on Prenatal/Perinatal and Obesity
Impact of Low Birthweight on Obesity
6. Cannabidiol
General Effects of Cannabidiol (CBD) on Obesity Risks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuardi, A.W. History of cannabis as a medicine: A review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Carliner, H.; Brown, Q.L.; Sarvet, A.L.; Hasin, D.S. Cannabis use, attitudes, and legal status in the U.S.: A review. Prev. Med. 2017, 104, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Substance Abuse and Mental Health Services Administration. Results from the 2019 National Survey on Drug Use and Health: Summary of National Findings; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2019. [Google Scholar]
- Lloyd, D.; Miech, R.; O’Malley, P.; Bachman, J.; Schulenberg, J.; Patrick, M. Monitoring the Future National Survey Results on Drug Use, 1975−2020: Overview, Key Findings on Adolescent Drug Use; Institute for Social Research, The University of Michigan: Ann Arbor, MI, USA, 2020. [Google Scholar]
- Hurd, Y.L.; Manzoni, O.J.; Pletnikov, M.V.; Lee, F.S.; Bhattacharyya, S.; Melis, M. Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects. J. Neurosci. 2019, 39, 8250–8258. [Google Scholar] [CrossRef]
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Myers, M.G.; Olson, D. Central nervous system control of metabolism. Nature 2012, 491, 357–363. [Google Scholar] [CrossRef]
- Gayle, D.A.; Desai, M.; Casillas, E.; Beloosesky, R.; Ross, M.G. Gender-specific orexigenic and anorexigenic mechanisms in rats. Life Sci. 2006, 79, 1531–1536. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef]
- Betley, J.N.; Cao, Z.F.H.; Ritola, K.D.; Sternson, S.M. Parallel, Redundant Circuit Organization for Homeostatic Control of Feeding Behavior. Cell 2013, 155, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Tsaousidou, E.; Paeger, L.; Belgardt, B.F.; Pal, M.; Wunderlich, C.M.; Brönneke, H.; Collienne, U.; Hampel, B.; Wunderlich, F.T.; Schmidt-Supprian, M.; et al. Distinct Roles for JNK and IKK Activation in Agouti-Related Peptide Neurons in the Development of Obesity and Insulin Resistance. Cell Rep. 2014, 9, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lin, L.; Xu, P.; Saito, K.; Wei, Q.; Meadows, A.G.; Bongmba, O.Y.N.; Pradhan, G.; Zheng, H.; Xu, Y.; et al. Neuronal Deletion of Ghrelin Receptor Almost Completely Prevents Diet-Induced Obesity. Diabetes 2016, 65, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.B.; Wunderlich, C.M.; Hess, S.; Paehler, M.; Mesaros, A.; Koralov, S.; Kleinridders, A.; Husch, A.; Münzberg, H.; Hampel, B.; et al. Enhanced Stat3 Activation in POMC Neurons Provokes Negative Feedback Inhibition of Leptin and InsulinSignaling in Obesity. J. Neurosci. 2009, 29, 11582–11593. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, A.A.; Ravussin, Y.; Leibel, R.L.; LeDuc, C.A. Energy homeostasis in leptin deficient Lepob/ob mice. PLoS ONE 2017, 12, e0189784. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.D.L.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Consumption of a Fat-Rich Diet Activates a Proinflammatory Response and Induces Insulin Resistance in the Hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.C.; Coope, A.; Morari, J.; Cintra, D.E.; Roman, E.A.; Pauli, J.R.; Romanatto, T.; Carvalheira, J.B.; Oliveira, A.L.R.; Saad, M.J.; et al. High-Fat Diet Induces Apoptosis of Hypothalamic Neurons. PLoS ONE 2009, 4, e5045. [Google Scholar] [CrossRef] [PubMed]
- García-Cáceres, C.; Yi, C.-X.; Tschöp, M.H. Hypothalamic Astrocytes in Obesity. Endocrinol. Metab. Clin. N. Am. 2013, 42, 57–66. [Google Scholar] [CrossRef]
- Velloso, L.A.; Araujo, E.P.; de Souza, C.T. Diet-Induced Inflammation of the Hypothalamus in Obesity. Neuroimmunomodulation 2008, 15, 189–193. [Google Scholar] [CrossRef]
- Hruby, A.; Manson, J.E.; Qi, L.; Malik, V.S.; Rimm, E.B.; Sun, Q.; Willett, W.C.; Hu, F.B. Determinants and Consequences of Obesity. Am. J. Public Health 2016, 106, 1656–1662. [Google Scholar] [CrossRef]
- De Schutter, A.; Lavie, C.J.; Milani, R.V. The Impact of Obesity on Risk Factors and Prevalence and Prognosis of Coronary Heart Disease—The Obesity Paradox. Prog. Cardiovasc. Dis. 2014, 56, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef]
- Robison, L.S.; Gannon, O.; Thomas, M.; Salinero, A.; Abi-Ghanem, C.; Poitelon, Y.; Belin, S.; Zuloaga, K. Role of sex and high-fat diet in metabolic and hypothalamic disturbances in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 285. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.A.; Wirths, O. Focusing the amyloid cascade hypothesis on N-truncated Abeta peptides as drug targets against Alzheimer’s disease. Acta Neuropathol. 2014, 127, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Rosario, E.; Elteriefi, R.; Pike, C. Sex-specific effects of high fat diet on indices of metabolic syndrome in 3xTg-AD mice: Implications for Alzheimer’s disease. PLoS ONE 2013, 8, e78554. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles, J.; Jiménez, A.; Vilaplana, E.; Montal, V.; Carmona-Iragui, M.; Pané, A.; Alcolea, D.; Videla, L.; Casajoana, A.; Clarimón, J.; et al. Obesity and Alzheimer’s disease, does the obesity paradox really exist? A magnetic resonance imaging study. Oncotarget 2018, 9, 34691–34698. [Google Scholar] [CrossRef]
- Anstey, K.J.; Cherbuin, N.; Budge, M.; Young, J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes. Rev. 2011, 12, e426–e437. [Google Scholar] [CrossRef]
- Karaca, U.; Schram, M.; Houben, A.; Muris, D.; Stehouwer, C. Microvascular dysfunction as a link between obesity, insulin resistance and hypertension. Diabetes Res. Clin. Pract. 2014, 103, 382–387. [Google Scholar] [CrossRef]
- Folli, F.; Okada, T.; Perego, C.; Gunton, J.; Liew, C.; Akiyama, M.; D’Amico, A.; La Rosa, S.; Placidi, C.; Lupi, R.; et al. Altered insulin receptor signalling and β-cell cycle dynamics in type 2 diabetes mellitus. PLoS ONE 2011, 6, e28050. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and Increased β-Cell Apoptosis in Humans With Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Yoon, K.H.; Ko, S.H.; Cho, J.H.; Lee, J.M.; Ahn, Y.B.; Song, K.H.; Yoo, S.J.; Kang, M.I.; Cha, B.Y.; Lee, K.W.; et al. Selective β-Cell Loss and α-Cell Expansion in Patients with Type 2 Diabetes Mellitus in Korea. J. Clin. Endocrinol. Metab. 2003, 88, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.-C.; Bai, C.; Sun, C.; Chen, C. Prevalence of metabolically healthy obesity and its impacts on incidences of hypertension, diabetes and the metabolic syndrome in Taiwan. Asia Pac. J. Clin. Nutr. 2012, 21, 227–233. [Google Scholar] [PubMed]
- Pataky, Z.; Bobbioni-Harsch, E.; Golay, A. Open questions about metabolically normal obesity. Int. J. Obes. 2010, 34, S18–S23. [Google Scholar] [CrossRef]
- Bell, J.A.; Kivimaki, M.; Hamer, M. Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes. Rev. 2014, 15, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, G.; Duval, S.; Jacobs, D.R.; Silventoinen, K. Comparison of Body Mass Index, Waist Circumference, and Waist/Hip Ratio in Predicting Incident Diabetes: A Meta-Analysis. Epidemiol. Rev. 2007, 29, 115–128. [Google Scholar] [CrossRef]
- Hadaegh, F.; Bozorgmanesh, M.; Safarkhani, M.; Khalili, D.; Azizi, F. Predictability of body mass index for diabetes: Affected by the presence of metabolic syndrome? BMC Public Health 2011, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Brüning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]
- Emanuela, F.; Grazia, M.; Marco, D.R.; Paola, L.M.; Giorgio, F.; Marco, B. Inflammation as a Link between Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012, 2012, 476380. [Google Scholar] [CrossRef]
- Boura-Halfon, S.; Zick, Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E581–E591. [Google Scholar] [CrossRef]
- De Felice, F.G.; Benedict, C. A Key Role of Insulin Receptors in Memory. Diabetes 2015, 64, 3653–3655. [Google Scholar] [CrossRef]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Grillo, C.A.; Piroli, G.G.; Lawrence, R.C.; Wrighten, S.A.; Green, A.J.; Wilson, S.P.; Sakai, R.R.; Kelly, S.J.; Wilson, M.A.; Mott, D.D.; et al. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes 2015, 64, 3927–3936. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.; Bray, G.; Hong, Y.; Stern, J.; Pi-Sunyer, F.; Eckel, R. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [PubMed]
- Carbone, S.; Lavie, C.J.; Arena, R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin. Proc. 2017, 92, 266–279. [Google Scholar] [CrossRef]
- Abed, H.S.; Wittert, G.; Leong, D.; Shirazi, M.; Bahrami, B.; Middledrop, M.; Lorimer, M.; Lau, D.; Antic, N.; Brooks, A.; et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: A randomized clinical trial. JAMA 2013, 310, 2050–2060. [Google Scholar] [CrossRef]
- Brown, C.D.; Higgins, M.; Donato, K.A.; Rohde, F.C.; Garrison, R.; Obarzanek, E.; Ernst, N.D.; Horan, M. Body Mass Index and the Prevalence of Hypertension and Dyslipidemia. Obes. Res. 2000, 8, 605–619. [Google Scholar] [CrossRef]
- Poirier, P.; Lemieux, I.; Mauriege, P.; Dewaily, E.; Blanchet, C.; Bergeron, J.; Despres, J. Impact of Waist Circumference on the Relationship Between Blood Pressure and Insulin: The Quebec Health Survey. Hypertension 2005, 45, 363–367. [Google Scholar] [CrossRef]
- Antonios, T.F.T.; Singer, D.R.J.; Markandu, N.D.; Mortimer, P.S.; MacGregor, G.A. Structural Skin Capillary Rarefaction in Essential Hypertension. Hypertension 1999, 33, 998–1001. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Crocq, M.-A. History of cannabis and the endocannabinoid system. Dialog Clin. Neurosci. 2020, 22, 223–228. [Google Scholar]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- de Fonseca, F.; Del Arco, I.; Bermudez-Silva, F.; Bilbao, A.; Cippitelli, A.; Navarro, M. The Endocannabinoid System: Physiology and Pharmacology. Alcohol Alcohol. 2005, 40, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L.S.; Da Silva, R.T.; Lima, D.; Sampaio, C.L.C.; Iannotti, F.; Mazzarella, E.; Di Marzo, V.; Vieyra, A.; Reis, R.A.M.; Einicker-Lamas, M. The endocannabinoid system in renal cells: Regulation of Na+ transport by CB1 receptors through distinct cell signalling pathways. Br. J. Pharmacol. 2015, 172, 4615–4625. [Google Scholar] [CrossRef]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2016, 10, 294. [Google Scholar] [CrossRef]
- Stella, N.; Schweitzer, P.J.; Piomelli, D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 1997, 388, 773–778. [Google Scholar] [CrossRef]
- Azad, S.C.; Eder, M.; Marsicano, G.; Lutz, B.; Zieglgänsberger, W.; Rammes, G. Activation of the Cannabinoid Receptor Type 1 Decreases Glutamatergic and GABAergic Synaptic Transmission in the Lateral Amygdala of the Mouse. Learn. Mem. 2003, 10, 116–128. [Google Scholar] [CrossRef]
- Moreira, F.A.; Lutz, B. The endocannabinoid system: Emotion, learning and addiction. Addict. Biol. 2008, 13, 196–212. [Google Scholar] [CrossRef]
- Moreira, F.A.; Grieb, M.; Lutz, B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: Focus on anxiety and depression. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Jacob, W.; Yassouridis, A.; Marsicano, G.; Monory, K.; Lutz, B.; Wotjak, C.T. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: Role of glutamatergic transmission. Genes Brain Behav. 2009, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Kruk-Slomka, M.; Dzik, A.; Budzynska, B.; Biala, G. Endocannabinoid System: The Direct and Indirect Involvement in the Memory and Learning Processes—a Short Review. Mol. Neurobiol. 2017, 54, 8332–8347. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, F.A.; Takahashi, R.N. WIN 55212-2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci. Lett. 2006, 397, 88–92. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Racz, I.; Valverde, O.; Otto, M.; Michel, K.; Sarstre, M.; Zimmer, A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 15670–15675. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Cagniard, B.; Yamazaki, M.; Nakayama, J.; Sakimura, K.; Kirino, Y.; Kano, M. Task-specific enhancement of hippocampus-dependent learning in mice deficient in monoacylglycerol lipase, the major hydrolyzing enzyme of the endocannabinoid 2-arachidonoylglycerol. Front. Behav. Neurosci. 2015, 9, 134. [Google Scholar] [CrossRef]
- Varvel, S.; Wise, L.E.; Niyuhire, F.; Cravatt, B.F.; Lichtman, A.H. Inhibition of Fatty-Acid Amide Hydrolase Accelerates Acquisition and Extinction Rates in a Spatial Memory Task. Neuropsychopharmacology 2007, 32, 1032–1041. [Google Scholar] [CrossRef]
- Lumir, H.; Raphael, M. Novel Natural and Synthetic Ligands of the Endocannabinoid System. Curr. Med. Chem. 2010, 17, 1341–1359. [Google Scholar]
- Okine, B.N.; Norris, L.M.; Woodhams, S.; Burston, J.; Patel, A.; Alexander, S.; Barrett, D.; Kendall, D.; Bennett, A.J.; Chapman, V. Lack of effect of chronic pre-treatment with the FAAH inhibitor URB597 on inflammatory pain behaviour: Evidence for plastic changes in the endocannabinoid system. Br. J. Pharmacol. 2012, 167, 627–640. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Wiley, J.W. The Role of the Endocannabinoid System in the Brain–Gut Axis. Gastroenterology 2016, 151, 252–266. [Google Scholar] [CrossRef]
- Kaczocha, M.; Glaser, S.T.; Maher, T.; Clavin, B.; Hamilton, J.; Rourke, J.O.; Rebecchi, M.J.; Puopolo, M.; Owada, Y.; Thanos, P.K. Fatty Acid Binding Protein Deletion Suppresses Inflammatory Pain through Endocannabinoid/N-Acylethanolamine-Dependent Mechanisms. Mol. Pain 2015, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.T.; Ralph, B.; Kaczocha, M.; Sun, J.; Balius, T.; Rizzo, R.; Haj-Dahmane, S.; Ojima, I.; Deutsch, D. Targeting fatty acid binding protein (FABP) anandamide transporters—A novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS ONE 2012, 7, e50968. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.; McIntosh, A.L.; Martin, G.G.; Huang, H.; Landrock, D.; Chung, S.; Landrock, K.K.; Dangott, L.J.; Li, S.; Kaczocha, M.; et al. Fatty Acid Binding Protein-1 (FABP1) and the Human FABP1 T94A Variant: Roles in the Endocannabinoid System and Dyslipidemias. Lipids 2016, 51, 655–676. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Gul, W. Constituents of Cannabis Sativa. In Handbook of Cannabis; Oxford University Press: Oxford, UK, 2014; pp. 3–22. [Google Scholar]
- Gertsch, J.; Pertwee, R.G.; Di Marzo, V. Phytocannabinoids beyond the Cannabis plant—Do they exist? Br. J. Pharmacol. 2010, 160, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S163–S171. [Google Scholar] [CrossRef]
- Moreno-Sanz, G. Can You Pass the Acid Test? Critical Review and Novel Therapeutic Perspectives of Δ9-Tetrahydrocannabinolic Acid, A. Cannabis Cannabinoid Res. 2016, 1, 124–130. [Google Scholar] [CrossRef]
- Fantegrossi, W.E.; Moran, J.; Randominska-Pandya, A.; Prather, P. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ9-THC: Mechanism underlying greater toxicity? Life Sci. 2014, 97, 45–54. [Google Scholar] [CrossRef]
- Mills, B.; Yepes, A.; Nugent, K. Synthetic Cannabinoids. Am. J. Med. Sci. 2015, 350, 59–62. [Google Scholar] [CrossRef]
- Dougherty, D.M.; Mathias, C.; Dawes, M.A.; Furr, R.M.; Charles, N.; Liguori, A.; Shannon, E.E.; Acheson, A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology 2012, 226, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Elkashef, A.; Vocci, F.; Huestis, M.; Haney, M.; Budney, A.; Gruber, A.; El-Guebaly, N. Marijuana Neurobiology and Treatment. Subst. Abuse 2008, 29, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.; Lynskey, M. Long-term Marijuana Use and Cognitive Impairment in Middle Age. JAMA Intern. Med. 2016, 176, 362–363. [Google Scholar] [CrossRef] [PubMed]
- McKetin, R.; Parasu, P.; Cherbuin, N.; Eramudugolla, R.; Anstey, K.J. A longitudinal examination of the relationship between cannabis use and cognitive function in mid-life adults. Drug Alcohol Depend. 2016, 169, 134–140. [Google Scholar] [CrossRef]
- Solowij, N.; Stephens, R.S.; Roffman, R.A.; Babor, T.; Kadden, R.; Miller, M.; Christiansen, K.; McRee, B.; Vendetti, J. Cognitive Functioning of Long-term Heavy Cannabis Users Seeking Treatment. JAMA 2002, 287, 1123–1131. [Google Scholar] [CrossRef]
- Bruijnzeel, A.W.; Knight, P.; Panunzio, S.; Xue, S.; Bruner, M.M.; Wall, S.C.; Pompilus, M.; Febo, M.; Setlow, B. Effects in rats of adolescent exposure to cannabis smoke or THC on emotional behavior and cognitive function in adulthood. Psychopharmacology 2019, 236, 2773–2784. [Google Scholar] [CrossRef]
- Kirschmann, E.K.; McCalley, D.; Edwards, C.; Torregrossa, M. Consequences of Adolescent Exposure to the Cannabinoid Receptor Agonist WIN55,212-2 on Working Memory in Female Rats. Front. Behav. Neurosci. 2017, 11, 137. [Google Scholar] [CrossRef]
- Quinn, H.R.; Matsumoto, I.; Callaghan, P.; Long, L.; Arnold, J.; Gunasekeran, N.; Thompson, M.; Dawson, B.; Mallet, P.; Kashem, M.; et al. Adolescent rats find repeated Δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 2008, 33, 1113–1126. [Google Scholar] [CrossRef]
- Suliman, N.A.; Taib, C.N.M.; Moklas, M.A.M.; Basir, R. Delta-9-Tetrahydrocannabinol ∆9-THC Induce Neurogenesis and Improve Cognitive Performances of Male Sprague Dawley Rats. Neurotox. Res. 2018, 33, 402–411. [Google Scholar] [CrossRef]
- Blaes, S.L.; Orsini, C.A.; Holik, H.M.; Stubbs, T.D.; Ferguson, S.N.; Heshmati, S.C.; Bruner, M.M.; Wall, S.C.; Febo, M.; Bruijnzeel, A.W.; et al. Enhancing effects of acute exposure to cannabis smoke on working memory performance. Neurobiol. Learn. Mem. 2019, 157, 151–162. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Albayram, O.; Draffehn, A.; Michel, K.; Piyanova, A.; Oppenheimer, H.; Dvir-Ginzberg, M.; Racz, I.; Ulas, T.; Imbeault, S.; et al. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat. Med. 2017, 23, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, C.T.; Liguori, A.; Livengood, L.B.; Hart, S.L.; Mussat-Whitlow, B.J.; Lamborn, C.M.; Laurienti, P.J.; Porrino, L.J. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004, 76, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lindgren, J.-E.; Wahlen, A.; Agurell, S.; Hollister, L.; Gillespie, H.K. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin. Pharmacol. Ther. 1980, 28, 409–416. [Google Scholar] [CrossRef]
- Dai, H.; Hao, J. Electronic cigarette and marijuana use among youth in the United States. Addict. Behav. 2017, 66, 48–54. [Google Scholar] [CrossRef]
- Eggers, M.E.; Lee, Y.O.; Jackson, K.; Wiley, J.L.; Porter, L.; Nonnemaker, J.M. Youth use of electronic vapor products and blunts for administering cannabis. Addict. Behav. 2017, 70, 79–82. [Google Scholar] [CrossRef]
- Baglot, S.L.; Hume, C.; Petrie, G.N.; Aukema, R.J.; Lightfoot, S.H.M.; Grace, L.M.; Zhou, R.; Parker, L.; Rho, J.M.; Borgland, S.L.; et al. Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Sci. Rep. 2021, 11, 23990. [Google Scholar] [CrossRef]
- Batalla, A.; Bhattacharyya, S.; Yücel, M.; Fusar-Poli, P.; Crippa, J.A.; Nogué, S.; Torrens, M.; Pujol, J.; Farré, M.; Martin-Santos, R. Structural and Functional Imaging Studies in Chronic Cannabis Users: A Systematic Review of Adolescent and Adult Findings. PLoS ONE 2013, 8, e55821. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Wilkinson, S.T.; D’Souza, D.C. Gone to Pot—A Review of the Association between Cannabis and Psychosis. Front. Psychiatry 2014, 5, 54. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Braley, G.; Blaise, R.; Vendetti, M.; Oliver, S.; Pittman, B.; Ranganthan, M.; Bhakta, S.; Zimolo, Z.; Cooper, T.; et al. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Δ-9-tetrahydrocannabinol in humans. Psychopharmacology 2008, 198, 587–603. [Google Scholar] [CrossRef]
- Mokrysz, C.; Freeman, T.; Korkki, S.; Griffiths, K.; Curran, H.V. Are adolescents more vulnerable to the harmful effects of cannabis than adults? A placebo-controlled study in human males. Transl. Psychiatry 2016, 6, e961. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.M.; Jones, K.H.; Kuhn, C.M.; Wilson, W.A.; Swartzwelder, H.S. Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav. Pharmacol. 2007, 18, 563–569. [Google Scholar] [CrossRef]
- Cha, Y.M.; White, A.M.; Kuhn, C.M.; Wilson, W.A.; Swartzwelder, H. Differential effects of Δ9-THC on learning in adolescent and adult rats. Pharmacol. Biochem. Behav. 2006, 83, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Kittler, J.T.; Grigorenko, E.; Clayton, C.; Zhuang, S.; Bundey, S.; Trower, M.; Wallance, D.; Hampson, R.; Deadwyler, S. Large-scale analysis of gene expression changes during acute and chronic exposure to Δ9-THC in rats. Physiol. Genom. 2000, 3, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Irimia, C.; Polis, I.; Stouffer, D.; Parsons, L. Persistent effects of chronic Δ9-THC exposure on motor impulsivity in rats. Psychopharmacology 2015, 232, 3033–3043. [Google Scholar] [CrossRef]
- Schramm-Sapyta, N.L.; Cha, Y.M.; Chaudhry, S.; Wilson, W.A.; Swartzwelder, H.S.; Kuhn, C.M. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology 2007, 191, 867–877. [Google Scholar] [CrossRef]
- Koch, M.; Varela, L.; Kim, J.G.; Kim, J.D.; Hernández-Nuño, F.; Simonds, S.; Castorena, C.M.; Vianna, C.R.; Elmquist, J.K.; Morozov, Y.; et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 2015, 519, 45–50. [Google Scholar] [CrossRef]
- Tibiriça, E. The multiple functions of the endocannabinoid system: A focus on the regulation of food intake. Diabetol. Metab. Syndr. 2010, 2, 5–6. [Google Scholar] [CrossRef]
- Williams, C.; Kirkham, T.C. Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol. Behav. 2002, 76, 241–250. [Google Scholar] [CrossRef]
- Romero-Zerbo, S.Y.; Bermudez-Silva, S.F. Cannabinoids, eating behaviour, and energy homeostasis. Drug Test Anal. 2014, 6, 52–58. [Google Scholar] [CrossRef]
- Akbas, F.; Gasteyger, C.; Sjödin, A.; Astrup, A.; Larsen, T.M. A critical review of the cannabinoid receptor as a drug target for obesity management. Obes. Rev. 2009, 10, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Farrimond, J.A.; Whalley, B.J.; Williams, C.M. Cannabinol and cannabidiol exert opposing effects on rat feeding patterns. Psychopharmacology 2012, 223, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Miederer, I.; Uebbing, K.; Röhrich, J.; Maus, S.; Bausbacher, N.; Krauter, K.; Weyer-Elberich, V.; Lutz, B.; Schreckenberger, M.; Urban, R. Effects of tetrahydrocannabinol on glucose uptake in the rat brain. Neuropharmacology 2017, 117, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Beatka, M.; Sarvaideo, J. Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr. Physiol. 2016, 7, 1–15. [Google Scholar] [PubMed]
- Lupica, C.R.; Riegel, A.C.; Hoffman, A. Hoffman. Marijuana and cannabinoid regulation of brain reward circuits. Br. J. Pharmacol. 2004, 143, 227–234. [Google Scholar] [CrossRef]
- Cluny, N.L.; Keenan, C.; Reimer, R.; Le Foll, B.; Sharkery, K. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Δ9-Tetrahydrocannabinol. PLoS ONE 2015, 10, e0144270. [Google Scholar] [CrossRef]
- Farrimond, J.A.; Whalley, B.J.; Williams, C.M. A low-Δ9 tetrahydrocannabinol cannabis extract induces hyperphagia in rats. Behav. Pharmacol. 2010, 21, 769–772. [Google Scholar] [CrossRef]
- Koch, J.E. Δ9-THC stimulates food intake in Lewis rats: Effects on chow, high-fat and sweet high-fat diets. Pharmacol. Biochem. Behav. 2001, 68, 539–543. [Google Scholar] [CrossRef]
- Jarrett, M.M.; Limebeer, C.L.; Parker, L.A. Effect of Δ9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol. Behav. 2005, 86, 475–479. [Google Scholar] [CrossRef]
- Mahler, S.V.; Smith, K.S.; Berridge, K. Endocannabinoid Hedonic Hotspot for Sensory Pleasure: Anandamide in Nucleus Accumbens Shell Enhances ‘Liking’ of a Sweet Reward. Neuropsychopharmacology 2007, 32, 2267–2278. [Google Scholar] [CrossRef]
- Dore, R.; Valenza, M.; Wang, X.; Rice, K.C.; Sabino, V.; Cottone, P. The inverse agonist of CB1 receptor SR141716 blocks compulsive eating of palatable food. Addict. Biol. 2014, 19, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Mathes, C.M.; Ferrara, M.; Rowland, N.E. Cannabinoid-1 receptor antagonists reduce caloric intake by decreasing palatable diet selection in a novel dessert protocol in female rats. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R67–R75. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; McLaughlin, P.; Sink, K.; Makriyannis, A.; Parker, L. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiol. Behav. 2007, 91, 383–388. [Google Scholar] [CrossRef]
- Wiley, J.L.; Burston, J.J.; Leggett, D.C.; Alekseeva, O.; Razdan, R.K.; Mahadevan, A.; Martin, B.R. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br. J. Pharmacol. 2005, 145, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, M.S.; Kerr, W.C. Simultaneous Versus Concurrent Use of Alcohol and Cannabis in the National Alcohol Survey. Alcohol. Clin. Exp. Res. 2015, 39, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Hunault, C.C.; Mensinga, T.T.; De Vries, I.; Kelholt-Dijkman, H.H.; Hoek, J.; Kruidenier, M.; Leenders, M.E.C.; Meulenbelt, J. Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology 2008, 201, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Lukas, S.E.; Orozco, S. Ethanol increases plasma Δ9-tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alcohol Depend. 2001, 64, 143–149. [Google Scholar] [CrossRef]
- Nelson, N.G.; Weingarten, M.J.; Law, W.X.; Sangiamo, D.T.; Liang, N.-C. Joint and separate exposure to alcohol and ∆9-tetrahydrocannabinol produced distinct effects on glucose and insulin homeostasis in male rats. Sci. Rep. 2019, 9, 12025. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef]
- Farokhnia, M.; McDiarmid, G.R.; Newmeyer, M.; Munjal, V.; Abulseoud, O.A.; Huestis, M.A.; Leggio, L. Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: A randomized, double-blind, placebo-controlled, human laboratory study. Transl. Psychiatry 2020, 10, 71. [Google Scholar] [CrossRef]
- Hollister, L.E. Health aspects of cannabis. Pharmacol. Rev. 1986, 38, 1. [Google Scholar] [CrossRef]
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gómez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 2020, 583, 603–608. [Google Scholar] [CrossRef] [PubMed]
- García-Cáceres, C.; Fuente-Martín, E.; Argente, J.; Chowen, J.A. Emerging role of glial cells in the control of body weight. Mol. Metab. 2012, 1, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Pilon, C.; Calcagno, A.; Urbanet, R.; Rossato, M.; Milan, G.; Bianchi, K.; Rizzuto, R.; Bernante, P.; Federspil, G.; et al. The Endogenous Cannabinoid System Stimulates Glucose Uptake in Human Fat Cells via Phosphatidylinositol 3-Kinase and Calcium-Dependent Mechanisms. J. Clin. Endocrinol. Metab. 2007, 92, 4810–4819. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Bordicchia, M.; Marcucci, P.; Bedetta, S.; Santini, S.; Giovagnoli, A.; Scappini, L.; Minardi, D.; Muzzonigro, G.; Dessì-Fulgheri, P.; et al. Altered pattern of cannabinoid type 1 receptor expression in adipose tissue of dysmetabolic and overweight patients. Metabolism 2009, 58, 361–367. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sable, S.; Ouwerkerk, R.; Mari, A.; Gharib, A.M.; Walter, M.; Courville, A.; Hall, G.; Chen, K.Y.; Volkow, N.D.; et al. Metabolic Effects of Chronic Cannabis Smoking. Diabetes Care 2013, 36, 2415–2422. [Google Scholar] [CrossRef]
- Wong, A.; Gunasekaran, N.; Hancock, D.; Denyer, G.; Meng, L.; Radford, J.; McGregor, I.; Arnold, J. The Major Plant-derived Cannabinoid Δ9-Tetrahydrocannabinol Promotes Hypertrophy and Macrophage Infiltration in Adipose Tissue. Horm. Metab. Res. 2012, 44, 105–113. [Google Scholar] [CrossRef]
- Brunet, B.; Doucet, C.; Venisse, N.; Hauet, T.; Hébrard, W.; Papet, Y.; Mauco, G.; Mura, P. Validation of Large White Pig as an animal model for the study of cannabinoids metabolism: Application to the study of THC distribution in tissues. Forensic Sci. Int. 2006, 161, 169–174. [Google Scholar] [CrossRef]
- Le Strat, Y.; Le Foll, B. Obesity and Cannabis Use: Results From 2 Representative National Surveys. Am. J. Epidemiol. 2011, 174, 929–933. [Google Scholar] [CrossRef]
- Clark, T.M.; Jones, J.M.; Hall, A.G.; Tabner, S.A.; Kmiec, R.L. Theoretical Explanation for Reduced Body Mass Index and Obesity Rates in Cannabis Users. Cannabis Cannabinoid Res. 2018, 3, 259–271. [Google Scholar] [CrossRef]
- Manning, F.; Mcdonough, J.; Elsmore, T.; Saller, C.; Sodetz, F. Inhibition of Normal Growth by Chronic Administration of Δ9-Tetrahydrocannabinol. Science 1971, 174, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Lavendal, R.; Frost, C. In vivo effects of Cannabis sativa L. extract on blood coagulation, fat and glucose metabolism in normal and streptozocin-induced diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 2006, 3, 1–2. [Google Scholar] [CrossRef][Green Version]
- Gallant, M.; Odei-Addo, F.; Frost, C.L.; Levendal, R.-A. Biological effects of THC and a lipophilic cannabis extract on normal and insulin resistant 3T3-L1 adipocytes. Phytomedicine 2009, 16, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Galve-Roperth, I.; Rueda, D.; Guzman, M. Involvement of Sphingomyelin Hydrolysis and the Mitogen-Activated Protein Kinase Cascade in the Δ9-Tetrahydrocannabinol-Induced Stimulation of Glucose Metabolism in Primary Astrocytes. Mol. Pharmacol. 1998, 54, 834. [Google Scholar] [CrossRef] [PubMed]
- Katsidoni, V.; Kastellakis, A.; Panagis, G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int. J. Neuropsychopharmacol. 2013, 16, 2273–2284. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Rubio-Casillas, A. Biphasic effects of THC in memory and cognition. Eur. J. Clin. Investig. 2018, 48, e12920. [Google Scholar] [CrossRef]
- Ngueta, G.; Ndjaboue, R. Lifetime marijuana use in relation to insulin resistance in lean, overweight, and obese US adults. J. Diabetes 2020, 12, 38–47. [Google Scholar] [CrossRef]
- Pierelli, G.; Stanzione, R.; Forte, M.; Migliarino, S.; Perelli, M.; Volpe, M.; Rubattu, S. Uncoupling Protein 2: A Key Player and a Potential Therapeutic Target in Vascular Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 7348372. [Google Scholar] [CrossRef]
- Rodondi, N.; Pletcher, M.J.; Liu, K.; Hulley, S.B.; Sidney, S. Marijuana Use, Diet, Body Mass Index, and Cardiovascular Risk Factors (from the CARDIA Study). Am. J. Cardiol. 2006, 98, 478–484. [Google Scholar] [CrossRef]
- Haney, M.; Gunderson, E.; Rabkin, J.; Hart, C.; Vosburg, S.; Comer, S.; Foltin, R. Dronabinol and Marijuana in HIV-Positive Marijuana Smokers: Caloric Intake, Mood, and Sleep. J. Acquir. Immune Defic. Syndr. 2007, 45, 545–554. [Google Scholar] [CrossRef]
- Ngueta, G.; Belanger, R.; Laouan-Sidi, E.; Lucas, M. Cannabis use in relation to obesity and insulin resistance in the Inuit population. Obesity 2015, 23, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Penner, E.A.; Buettner, H.; Mittleman, M. The Impact of Marijuana Use on Glucose, Insulin, and Insulin Resistance among US Adults. Am. J. Med. 2013, 126, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Bancks, M.P.; Pletcher, M.J.; Kertesz, S.G.; Sidney, S.; Rana, J.S.; Schreiner, P.J. Marijuana use and risk of prediabetes and diabetes by middle adulthood: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Diabetologia 2015, 58, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Hollister, L.E.; Reaven, G.M. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin. Pharmacol. Ther. 1974, 16, 297–302. [Google Scholar] [CrossRef]
- Foltin, R.W.; Fischman, M.W.; Byrne, M.F. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 1988, 11, 1–14. [Google Scholar] [CrossRef]
- Gerich, M.E.; Isfort, R.; Brimhall, B.; Siegel, C. Medical marijuana for digestive disorders: High time to prescribe? Am. J. Gastroenterol. 2015, 110, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Farhat, T.; Iannotti, R.J.; Simons-Morton, B.G. Overweight, obesity, youth, and health-risk behaviors. Am. J. Prev. Med. 2010, 38, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Verty, A.N.; Evetts, M.J.; Crouch, G.J.; Mcgregor, I.; Stefanidis, A.; Oldfield, B. The Cannabinoid Receptor Agonist THC Attenuates Weight Loss in a Rodent Model of Activity-Based Anorexia. Neuropsychopharmacology 2011, 36, 1349–1358. [Google Scholar] [CrossRef]
- Scherma, M.; Satta, V.; Collu, R.; Boi, M.; Usai, P.; Fratta, W.; Fadda, P. Cannabinoid CB1/CB2 receptor agonists attenuate hyperactivity and body weight loss in a rat model of activity-based anorexia. Br. J. Pharmacol. 2017, 174, 2682–2695. [Google Scholar] [CrossRef]
- Klein, C.; Karanges, E.; Spiro, A.; Wong, A.; Spencer, J.; Huynh, T.; Gunasekaran, N.; Karl, T.; Long, L.; Huang, X. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 2011, 218, 443–457. [Google Scholar] [CrossRef]
- Jarlenski, M.; Koma, J.W.; Zank, J.; Bodnar, L.M.; Bogen, D.L.; Chang, J.C. Trends in perception of risk of regular marijuana use among US pregnant and nonpregnant reproductive-aged women. Am. J. Obstet. Gynecol. 2017, 217, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Brown, Q.L.; Sarvet, A.L.; Shmulewitz, D.; Martins, S.S.; Wall, M.M.; Hasin, D.S. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014. JAMA 2017, 317, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Cowger, V.H.; Pickworth, W.B.; Lordo, R.A.; Peters, E.N. Cigar and Marijuana Blunt Use Among Pregnant and Nonpregnant Women of Reproductive Age in the United States, 2006–2016. Am. J. Public Health 2018, 108, 1073–1075. [Google Scholar] [CrossRef]
- Newnam, K.M. Noteworthy Professional News. Adv. Neonatal Care 2018, 18, 246–247. [Google Scholar] [CrossRef]
- Koren, G.; Cohen, R. The use of cannabis for Hyperemesis Gravidarum (HG). J. Cannabis Res. 2020, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Trezza, V.; Campolongo, P.; Manduca, A.; Morena, M.; Palmery, M.; Vanderschuren, L.J.M.J.; Cuomo, V. Altering endocannabinoid neurotransmission at critical developmental ages: Impact on rodent emotionality and cognitive performance. Front. Behav. Neurosci. 2012, 6, 2. [Google Scholar] [CrossRef]
- Kong, K.L.; Lee, J.; Shisler, S.; Thanos, P.K.; Huestis, M.A.; Hawk, L.; Eiden, R.D. Prenatal Tobacco and Marijuana Co-exposure and Offspring Obesity Development; Children’s Mercy Hospital: Buffalo, NY, USA, 2021; Manuscript in preperation. [Google Scholar]
- Natale, B.V.; Gustin, K.; Lee, K.; Holloway, A.; Laviolette, S.; Natale, D.; Hardy, D. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 2020, 10, 544. [Google Scholar] [CrossRef]
- Rayfield, S.; Plugge, E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J. Epidemiol. Community Health 2017, 71, 162–173. [Google Scholar] [CrossRef]
- Paul, S.E.; Hatoum, A.; Fine, J.; Johnson, E.; Hansen, I.; Karcher, N.; Moreau, A.; Bondy, E.; Qu, Y.; Carter, E.; et al. Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results From the ABCD Study. JAMA Psychiatry 2021, 78, 64–76. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.R.; Flenady, V.J.; Gibbons, K.S.; Kingsbury, A.M.; Hurrion, E.; Mamun, A.A.; Najman, J.M. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr. Res. 2012, 71, 215–219. [Google Scholar] [CrossRef]
- Blázquez, C.; González-Feria, L.; Álvarez, L.; Haro, A.; Casanova, M.L.; Guzmán, M. Cannabinoids Inhibit the Vascular Endothelial Growth Factor Pathway in Gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- Benevenuto, S.G.; Domenico, M.D.; Martins, M.A.G.; Costa, N.D.S.X.; de Souza, A.R.L.; Costa, J.L.; Tavares, M.F.; Dolhnikoff, M.; Veras, M.M. Recreational use of marijuana during pregnancy and negative gestational and fetal outcomes: An experimental study in mice. Toxicology 2016, 376, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Warner, T.D.; Roussos-Ross, D.; Behnke, M. It’s not your mother’s marijuana: Effects on maternal-fetal health and the developing child. Clin. Perinatol. 2014, 41, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, R.J.; Pierce, M.; Hardy, R.; Sattar, N.; Whincup, P.; Ferro, C.; Savage, C.; Kuh, D.; Nitsch, D. Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int. 2013, 84, 1262–1270. [Google Scholar] [CrossRef]
- Friedrich, J.; Khatib, D.; Parsa, K.; Santopietro, A.; Gallicano, G.I. The grass isn’t always greener: The effects of cannabis on embryological development. BMC Pharmacol. Toxicol. 2016, 17, 45. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Casey, P.H.; Bradley, R.H.; Whiteside-Mansell, L.; Barrett, K.; Gossett, J.M.; Simpson, P.M. Evolution of obesity in a low birth weight cohort. J. Perinatol. 2012, 32, 91–96. [Google Scholar] [CrossRef]
- Jornayvaz, F.; Vollenweider, P.; Bochud, M.; Mooser, V.; Waeber, G.; Marques-Vidal, P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: The CoLaus study. Cardiovasc. Diabetol. 2016, 15, 73. [Google Scholar] [CrossRef]
- Mu, M.; Wang, S.-F.; Sheng, J.; Zhao, Y.; Li, H.-Z.; Hu, C.-L.; Tao, F.-B. Birth weight and subsequent blood pressure: A meta-analysis. Arch. Cardiovasc. Dis. 2012, 105, 99–113. [Google Scholar] [CrossRef]
- Mi, D.; Fang, H.; Zhao, Y.; Zhong, L. Birth weight and type 2 diabetes: A meta-analysis. Exp. Ther. Med. 2017, 14, 5313–5320. [Google Scholar] [CrossRef]
- Chaoyang, L.; Maria, S.J.; Michael, I.G. Effects of Low Birth Weight on Insulin Resistance Syndrome in Caucasian and African-American Children. Diabetes Care 2001, 24, 2035–2042. [Google Scholar]
- Bielawiec, P.; Harasim-Symbor, E.; Konstantynowicz-Nowicka, K.; Sztolsztener, K.; Chabowski, A. Chronic Cannabidiol Administration Attenuates Skeletal Muscle De Novo Ceramide Synthesis Pathway and Related Metabolic Effects in a Rat Model of High-Fat Diet-Induced Obesity. Biomolecules 2020, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Callejas, G.; Figueira, R.; Gonçalves, F.; Volpe, F.; Zuardi, A.; Crippa, J.; Hallak, J.; Sbragia, L. Maternal administration of cannabidiol promotes an anti-inflammatory effect on the intestinal wall in a gastroschisis rat model. Braz. J. Med. Biol. Res. 2018, 51, e7132. [Google Scholar] [CrossRef]
- Scopinho, A.A.; Guimarães, F.S.; Corrêa, F.M.; Resstel, L.B. Cannabidiol inhibits the hyperphagia induced by cannabinoid-1 or serotonin-1A receptor agonists. Pharmacol. Biochem. Behav. 2011, 98, 268–272. [Google Scholar] [CrossRef]
- Ignatowska-Jankowska, B.; Jankowski, M.; Swiergiel, A.H. Cannabidiol decreases body weight gain in rats: Involvement of CB2 receptors. Neurosci. Lett. 2011, 490, 82–84. [Google Scholar] [CrossRef]
- Santiago, A.N.; Mori, M.A.; Guimarães, F.S.; Milani, H.; De Oliveira, R.M.W. Effects of Cannabidiol on Diabetes Outcomes and Chronic Cerebral Hypoperfusion Comorbidities in Middle-Aged Rats. Neurotox. Res. 2019, 35, 463–474. [Google Scholar] [CrossRef]
- Wierucka-Rybak, M.; Wolak, M.; Bojanowska, E. The Effects of Leptin in Combination woth a Cannabinoid Receptor 1 Antagonist, AM 251, or Cannabidiol on Food Intake and Body Weight in Rats Fed a High-Fat or a Free-Choice High Sugar Diet. J. Physiol. Pharmacol. 2014, 65, 487–496. [Google Scholar]
- Bi, G.; Galaj, E.; He, Y.; Xi, Z. Cannabidiol inhibits sucrose self-administration by CB1 and CB2 receptor mechanisms in rodents. Addict. Biol. 2020, 25, e12783. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.J.A.; Freeman, T.; Schafer, G.L.; Curran, H.V. Cannabidiol Attenuates the Appetitive Effects of Δ9-Tetrahydrocannabinol in Humans Smoking Their Chosen Cannabis. Neuropsychopharmacology 2010, 35, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Riedel, G.; Fadda, P.; McKillop-Smith, S.; Pertwee, R.G.; Platt, B.; Robinson, L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br. J. Pharmacol. 2009, 156, 1154–1166. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L. Cannabidiol—Recent Advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratu, M.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Moreira, F.; Gomes, F.; Del Bel, E.; Guimaraes, F. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. B 2012, 367, 3364–3378. [Google Scholar] [CrossRef] [PubMed]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; LaPrairie, R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A. Cannabinoids and the Brain; MIT Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Bielawiec, P.; Harasim-Symbor, E.; Chabowski, A. Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front. Endocrinol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Boo, K.; Yu, Y.; Oh, S.; Kim, H.; Jeon, Y.; Bhin, J.; Hwang, D.; Kim, K.; Lee, J.; et al. RORα controls hepatic lipid homeostasis via negative regulation of PPAR gamma transcriptional network. Nat. Commun. 2017, 8, 162. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.; Caldwell, R.; Liou, G. Neuroprotective and Blood-Retinal Barrier-Preserving Effects of Cannabidiol in Experimental Diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Khalil, I.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.-J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G. Neuroprotective effect of (−)Δ9-Tetrahydrocannabinol and Cannabidiol in N-methyl-D-Aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef]
- Pertwee, R.G. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005, 76, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Silva, F.J.; Sanchez-Vera, I.; Suárez, J.; Serrano, A.; Fuentes, E.; Juan-Pico, P.; Nadal, A.; de Fonseca, F.R. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur. J. Pharmacol. 2007, 565, 207–211. [Google Scholar] [CrossRef] [PubMed]
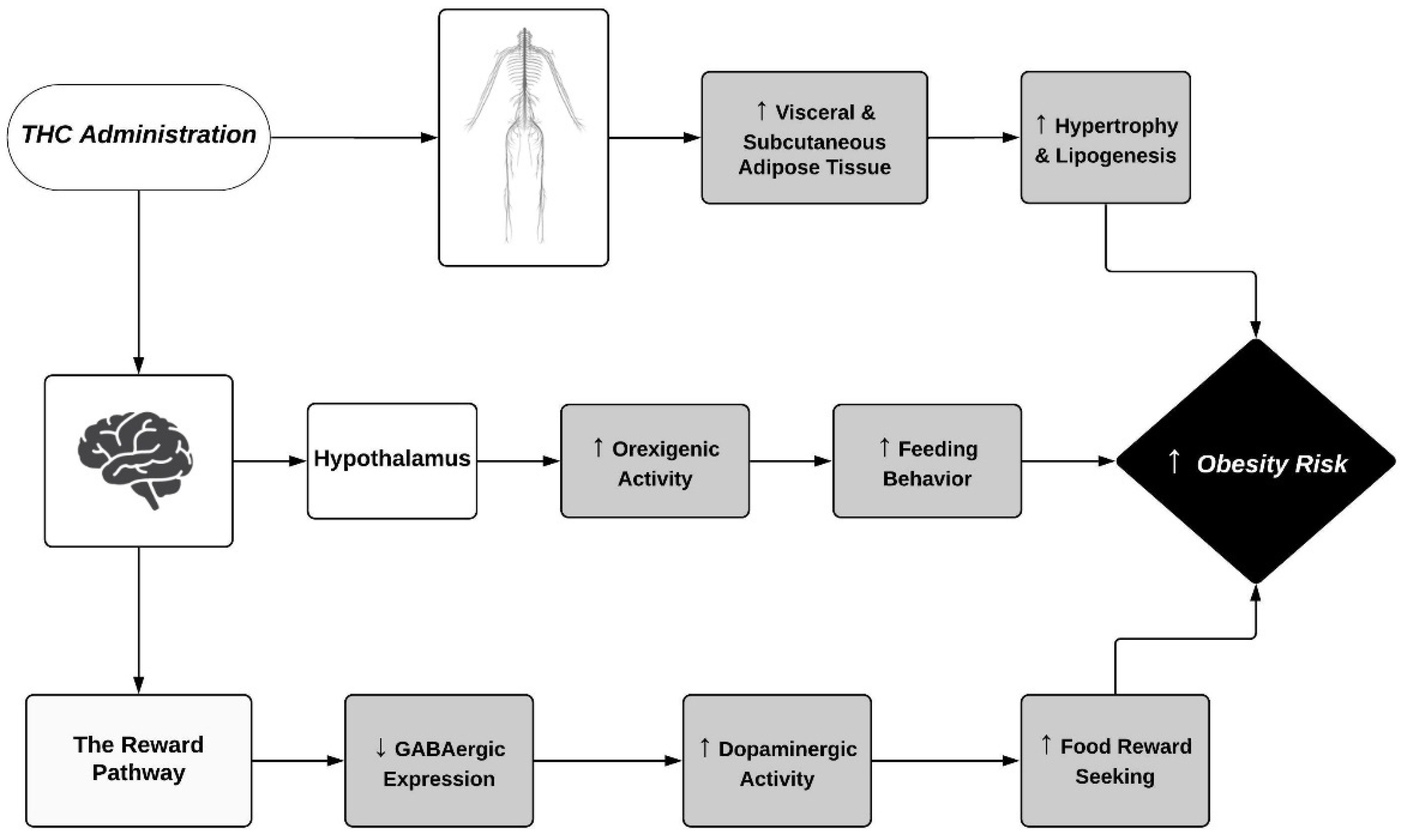
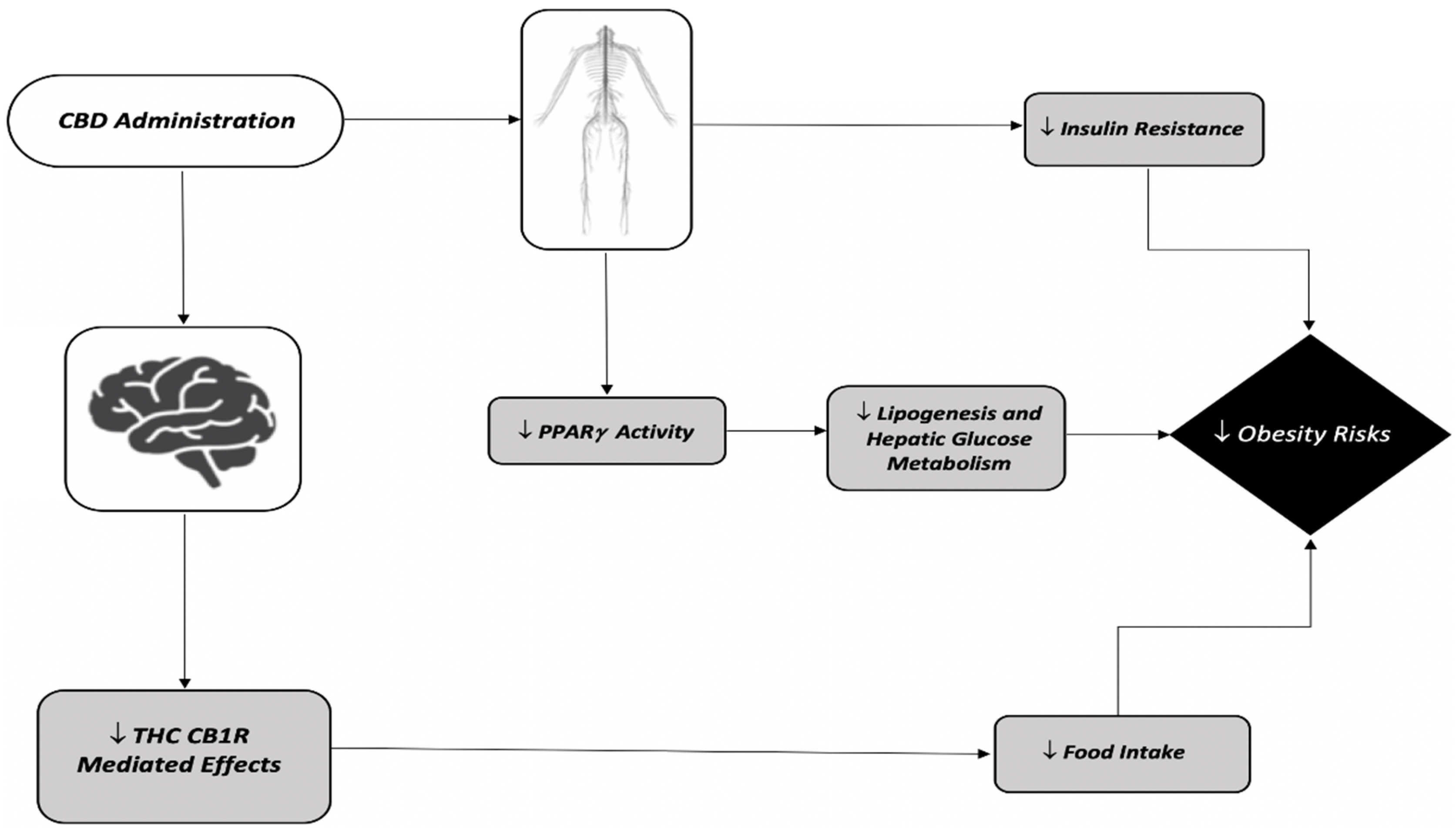
| Species | Regimen | Sex | THC Dose | Route of Administration | Obesity Risk Outcomes | Reference |
|---|---|---|---|---|---|---|
| Rat | Acute | Male | 0.5, 1.0, 2.0, or 4.0 mg/kg | Oral | ↑ food intake, ↓ eating latency | [112] |
| Rat | Acute | Male | 0.5, 1.0, 2.0, or 4.0 mg/kg | Oral | ↑ food intake, ↓ eating latency | [120] |
| Rat | Chronic (30 straight days) | Male | 4.0, 8.0 mg/kg | Intraperitoneally (4.0 mg/kg), Orally (8.0 mg/kg) | ↓ body weight, ↓ food intake | [144] |
| Rat | Acute | Male | 0.5, 1.0, 2.5 mg/kg | Intraperitoneally | ↑ food intake in all diets, ↑ sucrose palatability | [121] |
| Rat | Acute | Male | 2.5, 5.0 mg/kg | Subcutaneous | ↓ energy expenditure | [145] |
| Rat | Acute | Male | 2.5 mg/kg | Intraperitonially | ↑ sucrose palatability, ↑ ingestive responding | [122] |
| Human | Chronic (survey) | Male and female | Self-report | Smoked | ↓ obesity risks | [142] |
| Human | Chronic (meta-analysis) | Male and female | Self-report (users and nonusers) | Smoked | ↑ caloric intake, ↓ body weight, ↓ obesity | [143] |
| Human | Chronic (survey) | Male and female | Self-report (over 15 years: never used, <180 days, 180–1799 days, ≥1800 days) | Smoked | ↑ appetite, ↑ caloric intake, ↑ body weight, ↑ waist size | [152] |
| Human | Acute | Male and female | 2.0%, 3.9% | Smoked | ↑ food intake, ↑ body weight at high doses, ↑ caloric intake from fat | [153] |
| Human | Chronic (survey) | Male and female | Self-report (nonusers and users) | Smoked | ↓ levels of BMI, ↓ % fat mass, ↓ fasting insulin, ↓ insulin resistance | [154] |
| Human | Chronic (survey) | Male and female | Self-report (at least 4 days a week for the past 6 months) | Smoked | ↑ carbohydrate intake, ↑ visceral fat tissue, ↑ blood pressure, ↑ adipose tissue insulin resistance, ↑ leptin and ghrelin, ↓ PYY | [139] |
| Human | Acute | Male and female | 50.6 mg | Oral, smoked, vaporized | ↓ blood insulin concentrations, ↓ GLP-1 levels | [133] |
| Human | Chronic (survey) | Male and female | Self-report | Smoked | ↓ waist circumference, ↓ fasting insulin, ↓ insulin resistance, ↓ HDL-C | [155] |
| Human | Chronic (survey) | Male and female | Self-report (never, former, current use) | Smoked | ↑ rate of prediabetes | [156] |
| Mice | Acute | Male | 1.0, 3.0, 10.0, 30.0, 56.0 mg/kg | Intraperitoneally | ↑ caloric intake | [127] |
| Mice | Acute | Cell cultures | 1 mg/kg | N/A | ↓ in fat content, ↑ in IRS-1/2, ↑ GLUT4 | [146] |
| Species | Regimen | Sex | THC Dose | Route of Administration | Obesity Risk Outcomes | Reference |
|---|---|---|---|---|---|---|
| Rats | Acute | Male | 0.5, 1.0, 2.0 mg/kg | Oral | ↑ total food intake, ↓ eating latency, ↑ total duration of eating | [112] |
| Rats | Acute | Female | 0.1, 0.5, 2.0 mg/kg | Intraperitoneally | ↑ total food intake, ↓ body weight loss, ↓ energy expenditure | [161] |
| Rat | Chronic (16 straight days) | Male | 3, 5, 6, 8, 10 mg/kg | Subcutaneously, Oral | ↓ adiposity, ↓ plasma insulin | [131] |
| Rat | Acute | Male | 0.01, 0.05, 0.1, 0.5, 1.0 mg/kg | Intravenously | ↑ glucose uptake at lower concentrations, ↓ glucose uptake at high blood THC levels | [116] |
| Human | Chronic | Male and Female | Self-report (ranged from never to 40 times or more within a given month) | Smoked | ↑ proportion of being overweight, ↑ likelihood of obesity | [160] |
| Mice | Chronic (28 straight days) | Male | 2, 4 mg/kg | Intraperitonially | ↓ weight gain in DIO mice, ↓ energy intake, ↓ fat mass | [119] |
| Mice | Acute | Male | 10 mg/kg | Intraperitonially | ↓ glucose uptake, ↓ glycolysis, ↓ lactate release | [135] |
| Large white pig | Acute | Male | 0.05, 0.1, 0.2 mg/kg | Intravenously | ↑ levels of THC in fat tissues, ↑ time in fat tissues | [141] |
| Species | Regimen | Sex (Child) | THC Dose | Route of Administration | Obesity Risk Outcomes | References |
|---|---|---|---|---|---|---|
| Rat | Chronic (15.5 straight days) | Male and female | 3 mg/kg | Intraperitoneally | ↓ fetal growth, ↓ expression of GLUT1, ↑ intrauterine growth restriction | [171] |
| Human | Chronic (multiple studies, no specific regimen) | Male and female | Self-report | Smoked | ↑ overweight children, ↑ obesity risks | [172] |
| Human | Chronic (substance use through pregnancy) | Male and female | Self-report (exposure before or after knowledge) | Smoked | ↓ birth weight, ↓ intracranial volume, ↓ white matter volume | [173] |
| Human | Chronic | Male and female | Self-report (during pregnancy, ever regular, lifetime) | Smoked | ↓ birth weight, ↑ preterm birth, ↑ admission to NICU | [174] |
| Human | Chronic (8 straight days) | Cell culture | 0.5–1.5 mg | Intratumorally | ↓ levels of phosphorylated VEGFR-2, ↓ endothelial growth factor expression | [175] |
| Mice | Chronic (12 straight days) | Male and female | 200 mg cigarettes | Smoked | ↓ birth weight | [176] |
| Species | Regimen | Sex | CBD Dose | Route of Administration | Obesity Risk Outcomes | References |
|---|---|---|---|---|---|---|
| Rats | Chronic (14 consecutive days of exposure) | Male | 10 mg/kg | Intraperitoneally | ↓ insulin resistance, ↑ oxidative metabolism of glucose | [186] |
| Rats | Acute | Male and female | 30 mg/kg | Intraperitoneally | ↔ body weight | [187] |
| Rats | Acute | Male | 1, 10, 20 mg/kg | Intraperitoneally | ↓ hyperphagia with CB1 agonist, ↔ food intake | [188] |
| Rats | Chronic (14 consecutive days of exposure) | Male | 2.5, 5 mg/kg | Intraperitoneally | ↓ body weight | [189] |
| Rats | Chronic (30 consecutive days of exposure) | Male | 10 mg/kg | Intraperitoneally | ↓ body weight, ↓ diabetic outcomes | [190] |
| Rat | Acute | Male | 3 mg/kg | Intraperitoneally | ↓ food intake, ↑ body weight | [191] |
| Rats | Acute | Male | 0.044, 0.44, 4.4 mg/kg | Orally, subcutaneously | ↓ food intake | [115] |
| Rats and mice | Chronic (24 consecutive days of exposure) | Male | 20, 40 mg/kg | Intraperitoneally | ↓ sucrose administration | [192] |
| Human | Acute | Male and female | High CBD: low THC; low CBD: high THC | Smoked | ↓ food intake, ↓ hyperphagia | [193] |
| Mice | Acute | Male | 10 mg/kg | Intraperitoneally | ↓ food intake | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fearby, N.; Penman, S.; Thanos, P. Effects of Δ9-Tetrahydrocannibinol (THC) on Obesity at Different Stages of Life: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 3174. https://doi.org/10.3390/ijerph19063174
Fearby N, Penman S, Thanos P. Effects of Δ9-Tetrahydrocannibinol (THC) on Obesity at Different Stages of Life: A Literature Review. International Journal of Environmental Research and Public Health. 2022; 19(6):3174. https://doi.org/10.3390/ijerph19063174
Chicago/Turabian StyleFearby, Nathan, Samantha Penman, and Panayotis Thanos. 2022. "Effects of Δ9-Tetrahydrocannibinol (THC) on Obesity at Different Stages of Life: A Literature Review" International Journal of Environmental Research and Public Health 19, no. 6: 3174. https://doi.org/10.3390/ijerph19063174
APA StyleFearby, N., Penman, S., & Thanos, P. (2022). Effects of Δ9-Tetrahydrocannibinol (THC) on Obesity at Different Stages of Life: A Literature Review. International Journal of Environmental Research and Public Health, 19(6), 3174. https://doi.org/10.3390/ijerph19063174







