How to Improve the Drafting of Health Profiles
Abstract
1. Introduction
- To evaluate the concordance of health profiles outlined on the same case record with the CIRS tool, by ward doctors and a team of epidemiologists in charge of controls;
- To evaluate the effectiveness of training interventions aimed at improving the drafting of the CIRS.
2. Materials and Methods
2.1. Study Setting and Participants
2.2. Cumulative Illness Rating Scale (CIRS)
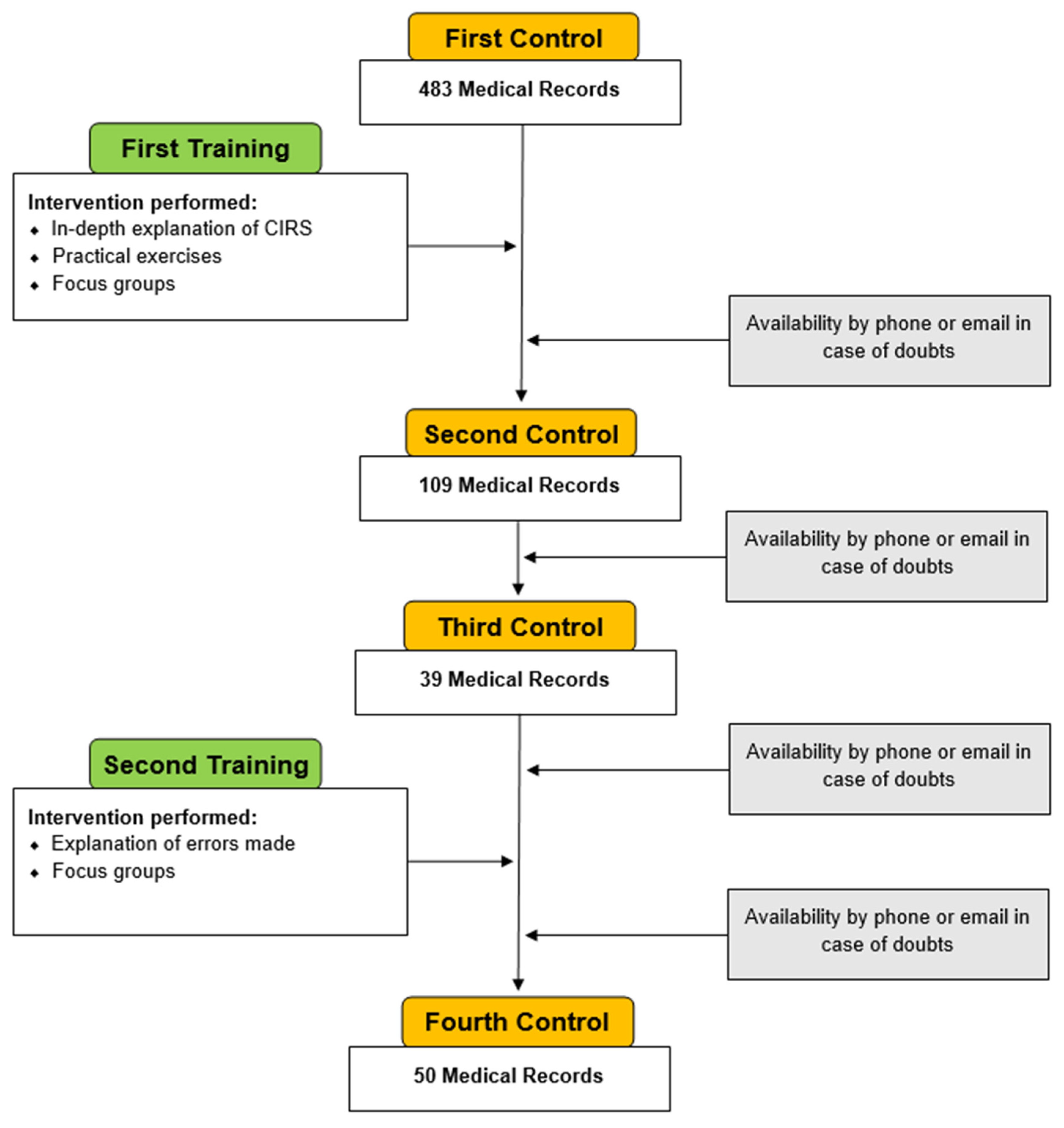
2.3. Study Design and Project
2.4. Data Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Constitution of WHO: Principles. Available online: https://www.who.int/about/governance/constitution (accessed on 20 September 2021).
- Oleribe, O.O.; Ukwedeh, O.; Burstow, N.J.; Gomaa, A.I.; Sonderup, M.W.; Cook, N.; Waked, I.; Spearman, W.; Taylor-Robinson, S.D. Health: Redefined. Pan. Afr. Med. J. 2018, 30, 292. [Google Scholar] [CrossRef]
- Card, A.J. Moving Beyond the WHO Definition of Health: A New Perspective for an Aging World and the Emerging Era of Value-Based Care. World Med. Health Policy 2017, 9, 127–137. [Google Scholar] [CrossRef]
- Thomson, W. On a mechanical representation of electric, magnetic, and galvanic forces. Camb. Dublin Math. J. 1847, 2, 61–64. [Google Scholar]
- Messina, G.; Forni, S.; Rosadini, D.; Falcone, M.; Collini, F.; Nante, N. Risk adjusted mortality after hip replacement surgery: A retrospective study. Ann. Ist. Super Sanità 2017, 53, 40–45. [Google Scholar]
- Messina, G.; Forni, S.; Collini, F.; Galdo, A.; Di Fabrizio, V.; Nante, N. Short-term adjusted outcomes for heart failure. Heart Int. 2015, 10, e1–e5. [Google Scholar] [CrossRef]
- Krawczyk-Suszek, M.; Kleinrok, A. Health-Related Quality of Life (HRQoL) of People over 65 Years of Age. Int. J. Environ. Res. Public Health 2022, 19, 625. [Google Scholar] [CrossRef]
- Dakin, H.; Abel, L.; Burns, R.; Yang, Y. Review and critical appraisal of studies mapping from quality of life or clinical measures to EQ-5D: An online database and application of the MAPS statement. Health Qual Life Outcomes 2018, 16, 31. [Google Scholar] [CrossRef]
- Buchholz, I.; Janssen, M.F.; Kohlmann, T.; Feng, Y.-S. A Systematic Review of Studies Comparing the Measurement Properties of the Three-Level and Five-Level Versions of the EQ-5D. Pharmacoeconomics 2018, 36, 645–661. [Google Scholar] [CrossRef]
- Fineschi, D.; Acciai, S.; Napolitani, M.; Scarafuggi, G.; Messina, G.; Guarducci, G.; Nante, N. Game of Mirrors: Health Profiles in Patient and Physician perceptions. Int. J. Environ. Res. Public Health 2022, 19, 1201. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yu, Z.; Zhang, X.; Wu, M.; Lin, S.; Zhu, Y.; Xu, Z.; You, L.; Wei, L.; Tang, M.; et al. Association between social health status and health-related quality of life among community-dwelling elderly in Zhejiang. Health Qual Life Outcomes 2020, 18, 110. [Google Scholar] [CrossRef]
- Van der Meulen, M.; Zamanipoor Najafabadi, A.H.; Lobatto, D.J.; Andela, C.D.; Vliet Vlieland, T.P.M.; Pereira, A.M.; van Furth, W.R.; Biermasz, N.R. SF-12 or SF-36 in pituitary disease? Toward concise and comprehensive patient-reported outcomes measurements. Endocrine 2020, 70, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, Y.; Rajaa, S.; Rehman, T. Diagnostic accuracy of various forms of geriatric depression scale for screening of depression among older adults: Systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 87, 104002. [Google Scholar] [CrossRef]
- Miller, M.D.; Paradis, C.F.; Houck, P.R.; Mazumdar, S.; Stack, J.A.; Rifai, A.H.; Mulsant, B.; Reynolds, C.F., III. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992, 41, 237–248. [Google Scholar] [CrossRef]
- Thomas, S.J.; Perez, J.L.; Lockhart, S.P.; Hariharan, S.; Kitchin, N.; Bailey, R.; Liau, K.; Lagkadinou, E.; Türeci, Ö.; Şahin, U.; et al. 1558O COVID-19 vaccine in participants (ptcpts) with cancer: Subgroup analysis of efficacy/safety from a global phase III randomized trial of the BNT162b2 (tozinameran) mRNA vaccine. Ann. Oncol. 2021, 32, S1129–S1163. [Google Scholar] [CrossRef]
- Decreto Ministeriale 2 Aprile 2015 n° 70. Regolamento Recante Definizione Degli Standard Qualitativi, Strutturali, Tecnologici e Quantitativi Relativi All’ Assistenza Ospedaliera. Available online: https://www.gazzettaufficiale.it/eli/gu/2015/06/04/127/sg/pdf (accessed on 10 January 2022).
- Hall, S.F.; Rochon, P.A.; Streiner, D.L.; Paszat, L.F.; Groome, P.A.; Rohlan, S.L. Measuring comorbidity in patients with head and neck cancer. Laryngoscope 2002, 112, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Roeker, L.E.; Lamanna, N.; Allan, J.N.; Leslie, L.; Pagel, J.M.; Patel, K.; Osterborg, A.; Wojenski, D.; Kamdar, M.; et al. Outcomes of COVID-19 in patients with CLL: A multicenter international experience. Blood 2020, 136, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Iezzoni, L.I. Risk Adjustment for Measuring Healthcare Outcomes; Health Administration Press: Chicago, IL, USA, 1997. [Google Scholar]
- Arcà, M.; Fusco, D.; Barone, A.P.; Perucci, C.A. Introduction to risk adjustment methods in comparative evaluation of outcomes. Epidemiol. Prev. 2006, 30, 5–47. [Google Scholar] [PubMed]
- Lizarondo, L.; Grimmer, K.; Kumar, S. Assisting allied health in performance evaluation: A systematic review. BMC Health Serv. Res. 2014, 14, 572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quercioli, C.; Nisticò, F.; Troiano, G.; Maccari, M.; Messina, G.; Barducci, M.; Carriero, G.; Golinelli, D.; Nante, N. Developing a new predictor of health expenditure: Preliminary results from a primary healthcare setting. Public Health 2018, 163, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hudon, C.; Fortin, M.; Vanasse, A. Cumulative Illness Rating Scale was a reliable and valid index in a family practice context. J. Clin. Epidemiol. 2005, 58, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Steenbakkers, K.; Hudon, C.; Poitras, M.E.; Almirall, J.; van den Akker, M. The electronic Cumulative Illness Rating Scale: A reliable and valid tool to assess multi-morbidity in primary care. J. Eval. Clin. Pract. 2011, 17, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Salvi, F.; Miller, M.D.; Grilli, A.; Giorgi, R.; Towers, A.L.; Morichi, V.; Spazzafumo, L.; Mancinelli, L.; Espinosa, E.; Rappelli, A.; et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J. Am. Geriatr. Soc. 2008, 56, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Lydersen, S. Cohen’s kappa—A measure of agreement between observers. Tidsskr. Den Nor. Laegeforening Tidsskr. Prakt. Med. Ny Raekke 2018, 138. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.F.; Groome, P.A.; Streiner, D.L.; Rochon, P.A. Interrater reliability of measurements of comorbid illness should be reported. J. Clin. Epidemiol. 2006, 59, 926–933. [Google Scholar] [CrossRef]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Corbi, G.; Ambrosino, I.; Massari, M.; De Lucia, O.; Simplicio, S.; Dragone, M.; Paolisso, G.; Piccioni, M.; Ferrara, N.; Campobasso, C.P. The potential impact of multidimesional geriatric assessment in the social security system. Aging Clin. Exp. Res. 2018, 30, 1225–1232. [Google Scholar] [CrossRef]
- Martin, R.S.; Hayes, B.J.; Hutchinson, A.; Yates, P.; Lim, W.K. Implementation of ‘Goals of Patient Care’ medical treatment orders in residential aged care facilities: Protocol for a randomised controlled trial. BMJ Open 2017, 7, e013909. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moorhouse, P.; Theou, O.; Fay, S.; McMillan, M.; Moffatt, H.; Rockwood, K. Treatment in a Geriatric Day Hospital improve individualized outcome measures using Goal Attainment Scaling. BMC Geriatr. 2017, 17, 9. [Google Scholar] [CrossRef][Green Version]
- Ariza-Vega, P.; Mora-Traverso, M.; Ortiz-Piña, M.; Ashe, M.C.; Kristensen, M.T. Translation, inter-rater reliability, agreement, and internal consistency of the Spanish version of the cumulated ambulation score in patients after hip fracture. Disabil. Rehabil. 2020, 42, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Daiber, A.; Michal, M.; Kuntic, M.; Lieb, K.; Beutel, M.; Münzel, T. Smoking and Neuropsychiatric Disease—Associations and Underlying Mechanisms. Int. J. Mol. Sci. 2021, 22, 7272. [Google Scholar] [CrossRef] [PubMed]
- Kooij, L.; Groen, W.G.; van Harten, W.H. Barriers and Facilitators Affecting Patient Portal Implementation from an Organizational Perspective: Qualitative Study. J. Med. Internet Res. 2018, 20, e183. [Google Scholar] [CrossRef] [PubMed]
- Mayo, E. The Social Problems of an Industrial Civilisation; Routledge & Kegan Paul: London, UK, 1949. [Google Scholar]
- Anderson, J.; Singh, J. A Case Study of Using Telehealth in a Rural Healthcare Facility to Expand Services and Protect the Health and Safety of Patients and Staff. Healthcare 2021, 9, 736. [Google Scholar] [CrossRef]
- Tappen, R.M.; Wolf, D.G.; Rahemi, Z.; Engstrom, G.; Rojido, C.; Shutes, J.M.; Ouslander, J.G. Barriers and Facilitators to Implementing a Change Initiative in Long-Term Care Using the INTERACT® Quality Improvement Program. Health Care Manag. 2017, 36, 219–230. [Google Scholar] [CrossRef]
- Davis, D.A.; Thomson, M.A.; Oxam, A.D.; Haynes, R.B. Changing physician performance: A systematic review of the effect of continuing medical education strategies. JAMA 1995, 274, 700–705. [Google Scholar] [CrossRef]
- Moussa, L.; Garcia-Cardenas, V.; Benrimoj, S.I. Change Facilitation Strategies Used in the Implementation of Innovations in Healthcare Practice: A Systematic Review. J. Chang. Manag. 2019, 19, 283–301. [Google Scholar] [CrossRef]
- Locke, E.A.; Shaw, K.N.; Saari, L.M.; Latham, G.P. Goal setting task performance: 1969–1980. Psychol. Bull. 1981, 90, 125. [Google Scholar] [CrossRef]
- Klein, H.J.; Whitener, E.M.; Ilgen, D.R. The role of goal specificity in the goal-setting process. Motiv. Emot. 1990, 14, 179–193. [Google Scholar] [CrossRef]





| CATEGORY | Agreement | Kappa | 95% C.I. | p Value |
|---|---|---|---|---|
| HEART | 86% | 0.49 | 0.4116–0.5684 | <0.01 |
| BLOOD PRESSURE | 42.4% | 0.25 | 0.2108–0.2892 | <0.01 |
| VASCULAR | 8.3% | −0.001 | −0.0206–0.0186 | 0.55 |
| RESPIRATORY | 75.5% | 0.12 | 0.0612–0.1788 | <0.01 |
| SENSE ORGANS | 66.2% | 0.23 | 0.1516–0.3084 | <0.01 |
| UPPER G.I. | 93.0% | 0.40 | 0.3216–0.4784 | <0.01 |
| LOWER G.I. | 93.3% | 0.34 | 0.2616–0.4184 | <0.01 |
| HEPATIC-PANCREATIC | 87.9% | −0.03 | −0.1084–0.0484 | 0.8 |
| RENAL | 94.9% | 0.31 | 0.212–0.408 | <0.01 |
| GENITOURINARY | 74.5% | 0.33 | 0.2516–0.4084 | <0.01 |
| MUSCULOSKELETAL | 20.38% | −0.01 | −0.0296–0.0096 | 0.6 |
| NEUROLOGICAL | 94.6% | 0.17 | 0.0916–0.2484 | <0.01 |
| ENDOCRINE | 66.6% | 0.33 | 0.2516–0.4084 | <0.01 |
| PSYCHIATRIC | 71.7% | 0.30 | 0.2412–0.3588 | <0.01 |
| TOTAL CIRS | 9.5% | 0.03 | 0.0104–0.0496 | 0.04 |
| SEVERITY INDEX | 0.3% | 0.00 | 0 | 0.3 |
| COMORBIDITY INDEX | 14.7% | 0.00 | −0.0392–0.0392 | 0.2 |
| CATEGORY | Agreement | Kappa | 95% C.I. | p Value |
|---|---|---|---|---|
| HEART | 93.7% | 0.81 | 0.6728–0.9472 | <0.01 |
| BLOOD PRESSURE | 84.2% | 0.74 | 0.5832–0.8968 | <0.01 |
| VASCULAR | 44.2% | 0.24 | 0.1224–0.3576 | <0.01 |
| RESPIRATORY | 80.0% | 0.48 | 0.3428–0.6172 | <0.01 |
| SENSE ORGANS | 76,8% | 0.59 | 0.4332–0.7468 | <0.01 |
| UPPER G.I. | 94.7% | 0.65 | 0.4932–0.8068 | <0.01 |
| LOWER G.I. | 93.7% | 0.65 | 0.5128–0.7872 | <0.01 |
| HEPATIC-PANCREATIC | 91.6% | 0.47 | 0.3132–0.6268 | <0.01 |
| RENAL | 96.8% | 0.49 | 0.3332–0.6468 | <0.01 |
| GENITOURINARY | 95.7% | 0.86 | 0.7228–0.9972 | <0.01 |
| MUSCULOSKELETAL | 82.1% | 0.68 | 0.5232–0.8368 | <0.01 |
| NEUROLOGICAL | 94.7% | 0.71 | 0.5924–0.8276 | <0.01 |
| ENDOCRINE | 80.0% | 0.67 | 0.5328–0.8072 | <0.01 |
| PSYCHIATRIC | 89.5% | 0.50 | 0.3432–0.6568 | <0.01 |
| TOTAL CIRS | 21.1% | 0.15 | 0.0912–0.2088 | <0.01 |
| SEVERITY INDEX | 24.8% | 0.18 | 0.1212–0.2388 | <0.01 |
| COMORBIDITY INDEX | 43.1% | 0.30 | 0.2216–0.3784 | <0.01 |
| CATEGORY | Agreement | Kappa | 95% C.I. | p Value |
|---|---|---|---|---|
| HEART | 87.5% | 0.64 | 0.4048–0.8752 | <0.01 |
| BLOOD PRESSURE | 75.0% | 0.63 | 0.3948–0.8652 | <0.01 |
| VASCULAR | 56.3% | 0.33 | 0.1732–0.4868 | <0.01 |
| RESPIRATORY | 71.9% | 0.21 | −0.0056–0.4256 | 0.03 |
| SENSE ORGANS | 56.3% | 0.22 | −0.0152–0.4552 | 0.03 |
| UPPER G.I. | 71.9% | 0.41 | 0.1356–0.6844 | <0.01 |
| LOWER G.I. | 71.9% | 0.35 | 0.1932–0.5068 | <0.01 |
| HEPATIC-PANCREATIC | 84.4% | 0.43 | 0.1360–0.7240 | <0.01 |
| RENAL | 87.5% | 0.52 | 0.3044–0.7356 | <0.01 |
| GENITOURINARY | 62.5% | 0.32 | 0.1044–0.5356 | <0.01 |
| MUSCULOSKELETAL | 34.4% | 0.03 | −0.1660–0.2260 | 0.04 |
| NEUROLOGICAL | 93.8% | 0.64 | 0.4048–0.8752 | <0.01 |
| ENDOCRINE | 65.7% | 0.43 | 0.2340–0.6260 | <0.01 |
| PSYCHIATRIC | 75.0% | 0.44 | 0.2244–0.6556 | <0.01 |
| TOTAL CIRS | 21.9% | 0.16 | 0.0620–0.2580 | <0.01 |
| SEVERITY INDEX | 18.8% | 0.13 | 0.0516–0.2084 | <0.01 |
| COMORBIDITY INDEX | 21.9% | 0.06 | −0.0772–0.1972 | 0.2 |
| CATEGORY | Agreement | Kappa | 95% C.I | p |
|---|---|---|---|---|
| HEART | 98.0% | 0.94 | 0.7244–1.1556 | <0.01 |
| BLOOD PRESSURE | 98.0% | 0.97 | 0.7740–1.1660 | <0.01 |
| VASCULAR | 94.0% | 0.74 | 0.5048–0.9752 | <0.01 |
| RESPIRATORY | 98.0% | 0.82 | 0.5848–1.0552 | <0.01 |
| SENSE ORGANS | 92.0% | 0.77 | 0.4956–1.0444 | <0.01 |
| UPPER G.I. | 100% | 1.0 | 0.7844–1.2156 | <0.01 |
| LOWER G.I. | 98.0% | 0.67 | 0.4740–0.8660 | <0.01 |
| HEPATIC-PANCREATIC | 98.0% | 0.88 | 0.6056–1.1544 | <0.01 |
| RENAL | 100% | 1.0 | 0.7256–1.2744 | <0.01 |
| GENITOURINARY | 86.0% | 0.59 | 0.3940–0.7860 | <0.01 |
| MUSCULOSKELETAL | 88.0% | 0.78 | 0.5252–1.0348 | <0.01 |
| NEUROLOGICAL | 98.0% | 0.67 | 0.4740–0.8660 | <0.01 |
| ENDOCRINE | 88.0% | 0.75 | 0.5344–0.9656 | <0.01 |
| PSYCHIATRIC | 96.0% | 0.74 | 0.5440–0.9360 | <0.01 |
| TOTAL CIRS | 80.4% | 0.70 | 0.6020–0.27980 | <0.01 |
| SEVERITY INDEX | 82.1% | 0.73 | 0.6320–0.8280 | <0.01 |
| COMORBIDITY INDEX | 89.3% | 0.83 | 0.7124–0.9476 | <0.01 |
| CATEGORY | First Control | Second Control | Third Control | Fourth Control |
|---|---|---|---|---|
| HEART | 86% | 93.7% | 87.5% | 98.0% |
| BLOOD PRESSURE | 42.4% | 84.2% | 75.0% | 98.0% |
| VASCULAR | 8.3% | 44.2% | 56.3% | 94.0% |
| RESPIRATORY | 75.5% | 80.0% | 71.9% | 98.0% |
| SENSE ORGANS | 66.2% | 76.8% | 56.3% | 92.0% |
| UPPER G.I. | 93.0% | 94.7% | 71.9% | 100% |
| LOWER G.I. | 93.3% | 93.7% | 71.9% | 98.0% |
| HEPATIC-PANCREATIC | 87.9% | 91.6% | 84.4% | 98.0% |
| RENAL | 94.9% | 96.8% | 87.5% | 100% |
| GENITOURINARY | 74.5% | 95.7% | 62.5% | 86.0% |
| MUSCULOSKELETAL | 20.38% | 82.1% | 34.4% | 88.0% |
| NEUROLOGICAL | 94.6% | 94.7% | 93.8% | 98.0% |
| ENDOCRINE | 66.6% | 80.0% | 65.7% | 88.0% |
| PSYCHIATRIC | 71.7% | 89.5% | 75.0% | 96.0% |
| TOTAL CIRS | 9.5% | 21.1% | 21.9% | 80.4% |
| SEVERITY INDEX | 0.3% | 24.8% | 18.8% | 82.1% |
| COMORBIDITY INDEX | 14.7% | 43.1% | 21.9% | 89.3% |
| MEAN | 58.79 ± 33.84 | 75.69 ± 24.78 | 62.16 ± 23.46 | 93.16 ± 6.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napolitani, M.; Guarducci, G.; Abinova, G.; Messina, G.; Nante, N. How to Improve the Drafting of Health Profiles. Int. J. Environ. Res. Public Health 2022, 19, 3452. https://doi.org/10.3390/ijerph19063452
Napolitani M, Guarducci G, Abinova G, Messina G, Nante N. How to Improve the Drafting of Health Profiles. International Journal of Environmental Research and Public Health. 2022; 19(6):3452. https://doi.org/10.3390/ijerph19063452
Chicago/Turabian StyleNapolitani, Margherita, Giovanni Guarducci, Gulnara Abinova, Gabriele Messina, and Nicola Nante. 2022. "How to Improve the Drafting of Health Profiles" International Journal of Environmental Research and Public Health 19, no. 6: 3452. https://doi.org/10.3390/ijerph19063452
APA StyleNapolitani, M., Guarducci, G., Abinova, G., Messina, G., & Nante, N. (2022). How to Improve the Drafting of Health Profiles. International Journal of Environmental Research and Public Health, 19(6), 3452. https://doi.org/10.3390/ijerph19063452







