Short-Term Effects of Low-Fat Chocolate Milk on Delayed Onset Muscle Soreness and Performance in Players on a Women’s University Badminton Team
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
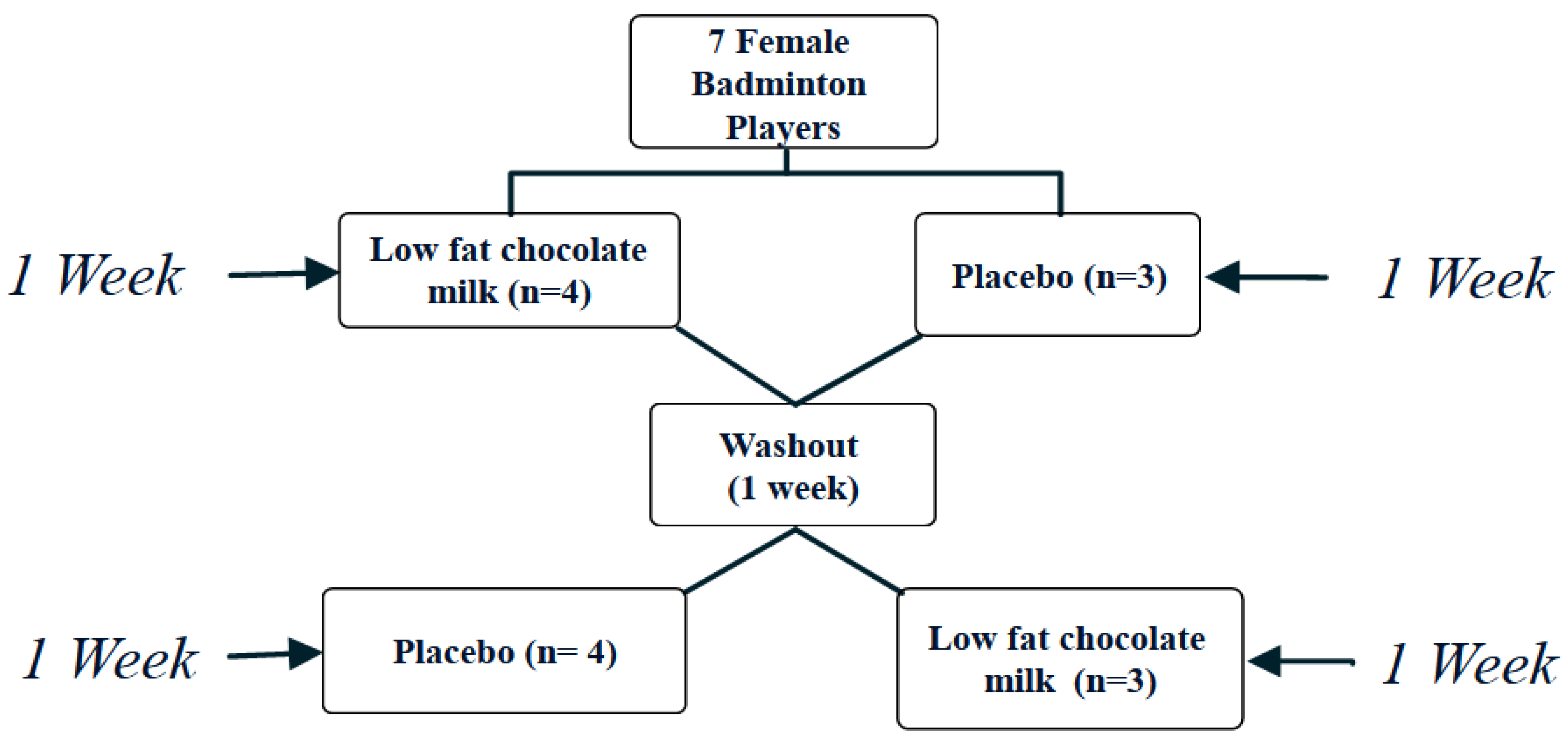
2.2. Study Design
2.3. Anthropometrics
2.4. Performance Indicators
2.5. Delay Onset Muscle Soreness
2.6. Training and Intervention Protocol
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Practical Applications
References
- Liddle, S.; Murphy, M.; Bleakley, W. And Doubles Badminton: A Heart. J. Hum. Mov. Stud. 1996, 30, 159–176. [Google Scholar]
- Phomsoupha, M.; Laffaye, G. The science of badminton: Game characteristics, anthropometry, physiology, visual fitness and biomechanics. Sports Med. 2015, 45, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Khanna, G.; Malik, V.; Sachdeva, S.; Arif, M.; Mandal, M. Physiological analysis to quantify training load in badminton. Br. J. Sports Med. 1997, 31, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Abian-Vicen, J.; Castanedo, A.; Abian, P.; Sampedro, J. Temporal and notational comparison of badminton matches between men’s singles and women’s singles. Int. J. Perform. Anal. Sport 2013, 13, 310–320. [Google Scholar] [CrossRef]
- Cabello, D.; Padial, P.; Lees, A.; Rivas, F. Temporal and Physiological Characteristics of Elite Women’s and Men’s Singles Badminton. Int. J. Appl. Sports Sci. 2004, 16, 1–12. [Google Scholar]
- Faude, O.; Meyer, T.; Rosenberger, F.; Fries, M.; Huber, G.; Kindermann, W. Physiological characteristics of badminton match play. Eur. J. Appl. Physiol. 2007, 100, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lees, A. Science and the major racket sports: A review. J. Sports Sci. 2003, 21, 707–732. [Google Scholar] [CrossRef]
- Manrique, D.C.; Gonzalez-Badillo, J. Analysis of the characteristics of competitive badminton. Br. J. Sports Med. 2003, 37, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Phomsoupha, M.; Laffaye, G. Injuries in badminton: A review. Sci. Sports 2020, 35, 189–199. [Google Scholar] [CrossRef]
- Chang, W.-D.; Chang, N.-J.; Lin, H.-Y.; Wu, J.-H. Effects of Acupuncture on Delayed-Onset Muscle Soreness: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2020, 2020, 5864057. [Google Scholar] [CrossRef]
- Lin, R.Z. Neuromuscular Fatigue following a Singles Badminton Match. Master’s Thesis, Edith Cowan University, Joondalup, Australia, 2014. [Google Scholar]
- Barnett, A. Using recovery modalities between training sessions in elite athletes. Sports Med. 2006, 36, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Moghadam, B.H.; Jo, E.; Tinsley, G.M.; Stratton, M.T.; Ashtary-Larky, D.; Eskandari, M.; Wong, A. Comparison of whole egg v. egg white ingestion during 12 weeks of resistance training on skeletal muscle regulatory markers in resistance-trained men. Br. J. Nutr. 2020, 124, 1035–1043. [Google Scholar] [CrossRef]
- Bagheri, R.; Moghadam, B.H.; Ashtary-Larky, D.; Forbes, S.C.; Candow, D.G.; Galpin, A.J.; Eskandari, M.; Kreider, R.B.; Wong, A. Whole egg vs. egg white ingestion during 12 weeks of resistance training in trained young males: A randomized controlled trial. J. Strength Cond. Res. 2021, 35, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Pourabbas, M.; Bagheri, R.; Hooshmand Moghadam, B.; Willoughby, D.S.; Candow, D.G.; Elliott, B.T.; Forbes, S.C.; Ashtary-Larky, D.; Eskandari, M.; Wong, A. Strategic ingestion of high-protein dairy milk during a resistance training program increases lean mass, strength, and power in trained young males. Nutrients 2021, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Moghadam, B.H.; Candow, D.G.; Elliott, B.T.; Wong, A.; Ashtary-Larky, D.; Forbes, S.C.; Rashidlamir, A. Effects of Icelandic yogurt consumption and resistance training in healthy untrained older males. Br. J. Nutr. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.R.; Johnston, J.D.; Tecklenburg, S.; Mickleborough, T.D.; Fly, A.D.; Stager, J.M. Chocolate milk as a post-exercise recovery aid. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 78–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilson, S.F.; Saunders, M.J.; Moran, C.W.; Moore, R.W.; Womack, C.J.; Todd, M.K. Effects of chocolate milk consumption on markers of muscle recovery following soccer training: A randomized cross-over study. J. Int. Soc. Sports Nutr. 2010, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockburn, E.; Hayes, P.R.; French, D.N.; Stevenson, E.; St Clair Gibson, A. Acute milk-based protein–CHO supplementation attenuates exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2008, 33, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, K.; Bishop, P.; Pritchett, R.; Green, M.; Katica, C. Acute effects of chocolate milk and a commercial recovery beverage on postexercise recovery indices and endurance cycling performance. Appl. Physiol. Nutr. Metab. 2009, 34, 1017–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockburn, E.; Stevenson, E.; Hayes, P.R.; Robson-Ansley, P.; Howatson, G. Effect of milk-based carbohydrate-protein supplement timing on the attenuation of exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2010, 35, 270–277. [Google Scholar] [CrossRef]
- Amiri, M.; Ghiasvand, R.; Kaviani, M.; Forbes, S.C.; Salehi-Abargouei, A. Chocolate milk for recovery from exercise: A systematic review and meta-analysis of controlled clinical trials. Eur. J. Clin. Nutr. 2019, 73, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcantara, J.M.; Sanchez-Delgado, G.; Martinez-Tellez, B.; Labayen, I.; Ruiz, J.R. Impact of cow’s milk intake on exercise performance and recovery of muscle function: A systematic review. J. Int. Soc. Sports Nutr. 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloby Nielsen, L.L.; Tandrup Lambert, M.N.; Jeppesen, P.B. The effect of ingesting carbohydrate and proteins on athletic performance: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2020, 12, 1483. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.A.; Fuller, B. The effectiveness of chocolate milk as a post-climbing recovery aid. J. Sports Med. Phys. Fit. 2015, 55, 1438–1444. [Google Scholar]
- Mallari, M.F.T.; Nana, A.; Pinthong, M.; Kongkum, S.; Chaunchaiyakul, R.; Valleser, C.W. Effect of ad libitum intake of lactose-free milk on subsequent performance of collegiate badminton athletes. Ger. J. Exerc. Sport Res. 2019, 49, 266–274. [Google Scholar] [CrossRef]
- Kirk, B.; Mitchell, J.; Jackson, M.; Amirabdollahian, F.; Alizadehkhaiyat, O.; Clifford, T. A2 milk enhances dynamic muscle function following repeated sprint exercise, a possible ergogenic aid for A1-protein intolerant athletes? Nutrients 2017, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Papacosta, E.; Nassis, G.P.; Gleeson, M. Effects of acute postexercise chocolate milk consumption during intensive judo training on the recovery of salivary hormones, salivary SIgA, mood state, muscle soreness, and judo-related performance. Appl. Physiol. Nutr. Metab. 2015, 40, 1116–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunn, W.R.; Pasiakos, S.M.; Colletto, M.R.; Karfonta, K.E.; Carbone, J.W.; Anderson, J.M.; Rodriguez, N.R. Chocolate milk and endurance exercise recovery: Protein balance, glycogen, and performance. Med. Sci. Sports Exerc. 2012, 44, 682–691. [Google Scholar] [CrossRef]
- Thomas, K.; Morris, P.; Stevenson, E. Improved endurance capacity following chocolate milk consumption compared with 2 commercially available sport drinks. Appl. Physiol. Nutr. Metab. 2009, 34, 78–82. [Google Scholar] [CrossRef]
- Wadey, C.; Perkins, I.; Potter, J.A. Chocolate Milk Improves Post-Exercise recovery in Tennis Players. Rev. Press 2018, 2, 77–83. [Google Scholar]
- Kucab, M.; Bellissimo, N.; Prusky, C.; Brett, N.R.; de Zepetnek, J.O.T. Effects of a high-intensity interval training session and chocolate milk on appetite and cognitive performance in youth aged 9–13 years. Eur. J. Clin. Nutr. 2021, 75, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Haff, G.G.; Dumke, C. Laboratory Manual for Exercise Physiology, 2E.; Human Kinetics: Champaign, IL, USA, 2019. [Google Scholar]
- De Andrade, V.L.; Santiago, P.; Kalva Filho, C.A.; Campos, E.Z.; Papoti, M. Reproducibility of Running Anaerobic Sprint Test for soccer players. J. Sports Med. Phys. Fit. 2014, 56, 34–38. [Google Scholar]
- Paterson, S.; McMaster, D.T.; Cronin, J. Assessing change of direction ability in badminton athletes. Strength Cond. J. 2016, 38, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Palao, J.M.; Valdes, D. Testing protocol for monitoring upper-body strength using medicine balls. J. Hum. Sport Exerc. 2013, 8, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, R.; Forbes, S.C.; Candow, D.G.; Wong, A. Effects of branched-chain amino acid supplementation and resistance training in postmenopausal women. Exp. Gerontol. 2021, 144, 111185. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Rashidlamir, A.; Motevalli, M.S.; Elliott, B.T.; Mehrabani, J.; Wong, A. Effects of upper-body, lower-body, or combined resistance training on the ratio of follistatin and myostatin in middle-aged men. Eur. J. Appl. Physiol. 2019, 119, 1921–1931. [Google Scholar] [CrossRef]
- Haff, G.G.; Triplett, N.T. Essentials of Strength Training and Conditioning, 4th ed.; Human kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Lau, W.Y.; Muthalib, M.; Nosaka, K. Visual analog scale and pressure pain threshold for delayed onset muscle soreness assessment. J. Musculoskelet. Pain 2013, 21, 320–326. [Google Scholar] [CrossRef]
- Bishop, P.A.; Jones, E.; Woods, A.K. Recovery from training: A brief review: Brief review. J. Strength Cond. Res. 2008, 22, 1015–1024. [Google Scholar] [CrossRef]
- Burke, L. Fasting and recovery from exercise. Br. J. Sports Med. 2010, 44, 502–508. [Google Scholar] [CrossRef]
- Pritchett, K.; Pritchett, R. Chocolate milk: A post-exercise recovery beverage for endurance sports. In Acute Topics in Sport Nutrition; Karger Publishers: Basel, Switzerland, 2012; Volume 59, pp. 127–134. [Google Scholar]
- Burke, L.M.; van Loon, L.J.; Hawley, J.A. Postexercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2017, 122, 1055–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.M.; Bailey, S.P.; Woods, J.A.; Galiano, F.J.; Hamilton, M.T.; Bartoli, W.P. Effects of carbohydrate feedings on plasma free tryptophan and branched-chain amino acids during prolonged cycling. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Hickson, R.; Rennie, M.; Conlee, R.; Winder, W.; Holloszy, J. Effects of increased plasma fatty acids on glycogen utilization and endurance. J. Appl. Physiol. 1977, 43, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Ferguson-Stegall, L.; McCleave, E.; Ding, Z.; Doerner, P.G., III; Liu, Y.; Wang, B.; Healy, M.; Kleinert, M.; Dessard, B.; Lassiter, D.G. Aerobic exercise training adaptations are increased by postexercise carbohydrate-protein supplementation. J. Nutr. Metab. 2011, 2011, 623182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brechtel, K.; Dahl, D.; Machann, J.; Bachmann, O.; Wenzel, I.; Maier, T.; Claussen, C.; Häring, H.; Jacob, S.; Schick, F. Fast elevation of the intramyocellular lipid content in the presence of circulating free fatty acids and hyperinsulinemia: A dynamic 1H-MRS study. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2001, 45, 179–183. [Google Scholar] [CrossRef]
- Ekblom, B.; Astrand, P.-O.; Saltin, B.; Stenberg, J.; Wallström, B. Effect of training on circulatory response to exercise. J. Appl. Physiol. 1968, 24, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Saltin, B.; Blomqvist, G.; Mitchell, J.H.; Johnson, R.L., Jr.; Wildenthal, K.; Chapman, C.B.; Frenkel, E.; Norton, W.; Siperstein, M.; Suki, W. A longitudinal study of adaptive changes in oxygen transport and body composition. Circulation 1968, 38, VII-1–VII-78. [Google Scholar] [CrossRef]
- Valentine, R.J.; Saunders, M.J.; Todd, M.K.; Laurent, T.G.S. Influence of carbohydrate-protein beverage on cycling endurance and indices of muscle disruption. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 363–378. [Google Scholar] [CrossRef]
- Ivy, J.; Lee, M.; Brozinick, J., Jr.; Reed, M. Muscle glycogen storage after different amounts of carbohydrate ingestion. J. Appl. Physiol. 1988, 65, 2018–2023. [Google Scholar] [CrossRef]
- Halson, S.L.; Lancaster, G.I.; Achten, J.; Gleeson, M.; Jeukendrup, A.E. Effects of carbohydrate supplementation on performance and carbohydrate oxidation after intensified cycling training. J. Appl. Physiol. 2004, 97, 1245–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Russo, I.; Della Gatta, P.A.; Garnham, A.; Porter, J.; Burke, L.M.; Costa, R.J. Assessing Overall Exercise Recovery Processes Using Carbohydrate and Carbohydrate-Protein Containing Recovery Beverages. Front. Physiol. 2021, 12, 50. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, K.F. Acute Effects of Low-Fat Chocolate Milk Intake on Muscle Recovery following Lower Extremity Exhausting Exercise. Bachelor’s Thesis, Hong Kong Baptist University, Hong Kong, China, 2014. [Google Scholar]
- Ivy, J.; Portman, R. The Performance Zone: Your Nutrition Action Plan for Greater Endurance & Sports Performance; Basic Health Publications, Inc.: Laguna Beach, CA, USA, 2004. [Google Scholar]
- Roy, B.D. Milk: The new sports drink? A Review. J. Int. Soc. Sports Nutr. 2008, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, S.B.; Tarnopolsky, M.A.; MacDonald, M.J.; MacDonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef]
- Ferguson-Stegall, L.; McCleave, E.L.; Ding, Z.; Doerner, P.G., III; Wang, B.; Liao, Y.-H.; Kammer, L.; Liu, Y.; Hwang, J.; Dessard, B.M. Postexercise carbohydrate–protein supplementation improves subsequent exercise performance and intracellular signaling for protein synthesis. J. Strength Cond. Res. 2011, 25, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hurst, C.; Dismore, L.; Stevenson, E.; Sayer, A.A.; Aspray, T. Feasibility and acceptability of a milk and resistance exercise intervention to improve muscle function in community-dwelling older adults (MIlkMAN): Pilot study. PLoS ONE 2020, 15, e0235952. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, A.U.; Wong, T.S.; Bandegan, A.; Lemon, P.W. Cycling time trial performance 4 hours after glycogen-lowering exercise is similarly enhanced by recovery nondairy chocolate beverages versus chocolate Milk. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Maughan, R.J.; Shirreffs, S.M.; Watson, P. Effects of milk ingestion on prolonged exercise capacity in young, healthy men. Nutrition 2008, 24, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Love, T.D.; Maughan, R.J.; Shirreffs, S.M. A comparison of the effects of milk and a carbohydrate-electrolyte drink on the restoration of fluid balance and exercise capacity in a hot, humid environment. Eur. J. Appl. Physiol. 2008, 104, 633–642. [Google Scholar] [CrossRef] [PubMed]
| Energy (Kcal) | 370 |
| Fat (g) | 7.5 |
| Protein (g) | 16 |
| Carbohydrate (g) | 59.5 |
| Calcium (mg) | 600 |
| Phosphorus (mg) | 500 |
| Age (y) | 23.14 ± 1.5 years |
| Height (cm) | 163.8 ± 4.1 cm |
| Body mass (kg) | 58.7 ± 0.9 kg |
| Body mass index (kg/m2) | 21.9 ± 3.5 kg/m2 |
| TTE | Aerobic Power | Max Anaerobic Power | Min Anaerobic Power | Ave Anaerobic Power | Agility | Explo Power Upper Body | Max Hand Strength | Rel Explo Power Lower Body | Max Explo Power Lower Body | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
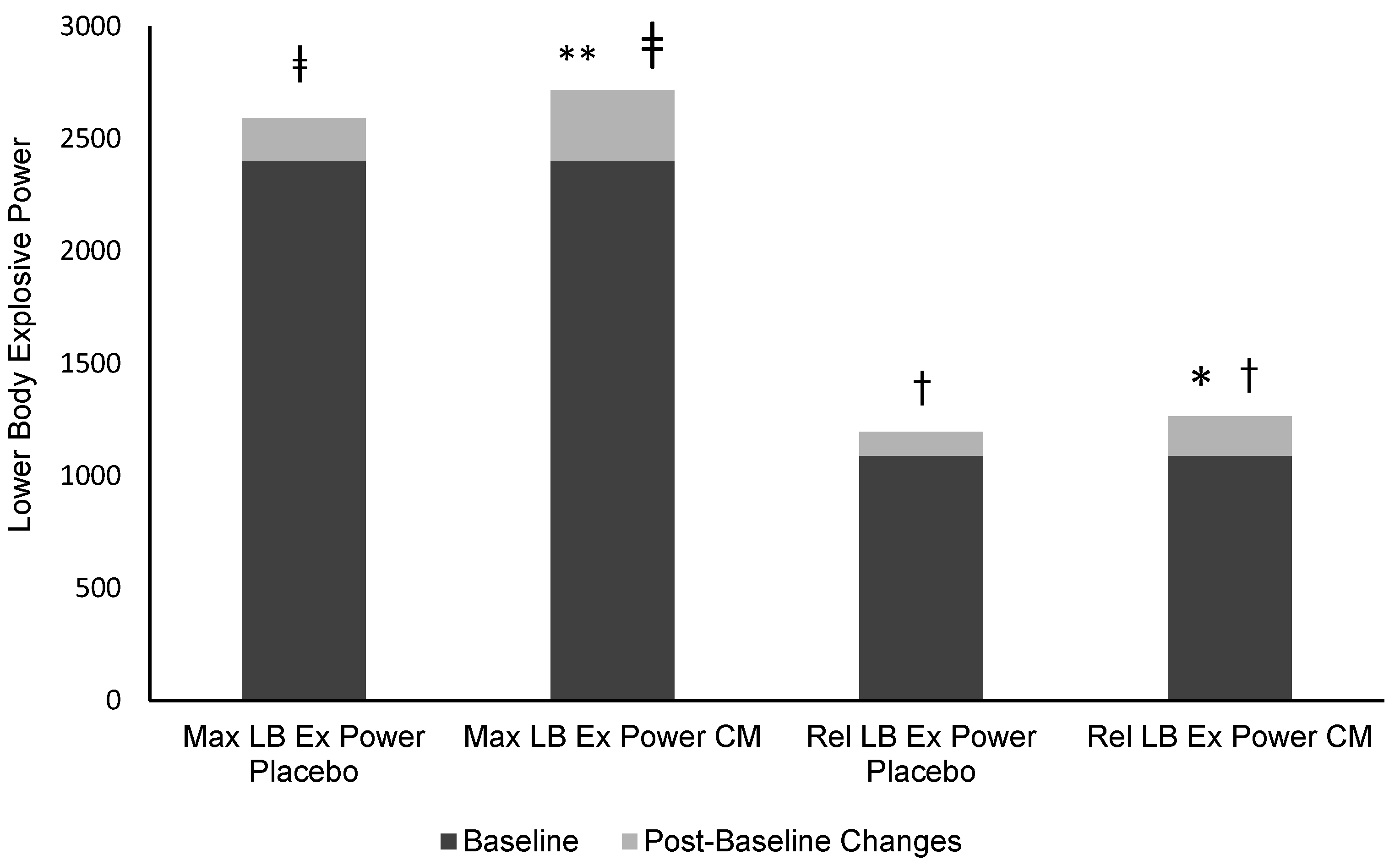
| Pre-test (Baseline) | 262.8 ± 39 | 38.6 ± 0.32 | 266.1 ± 56.4 | 134.5 ± 28.4 | 195 ± 43.9 | 14.8 ± 1.1 | 4.4 ± 0.5 | 22.8 ± 4.7 | 1089.6 ± 258.6 | 2402.1 ± 508.6 | |
| Post-test Placebo | 301.4 ±38.4 | 38.9 ± 0.32 | 234.1 ± 37.4 | 141.4 ± 32.6 | 184.01± 30.3 | 14.3 ± 0.35 | 5 ± 0.36 | 24 ± 4.8 | 1196 ± 264.7 * | 2593 ± 514 * | |
| Post-test LFCM | 325.7 ± 67 * | 39.1 ± 0.56 * | 252.5 ± 64.6 | 159 ± 35.5 * | 200.1 ± 41.1 | 14.2 ± 0.38 | 5.1 ± 0.31 * | 25.5 ± 3.9 | 1265.7 ± 304.6 *# | 2716.5 ± 583.8 *# | |
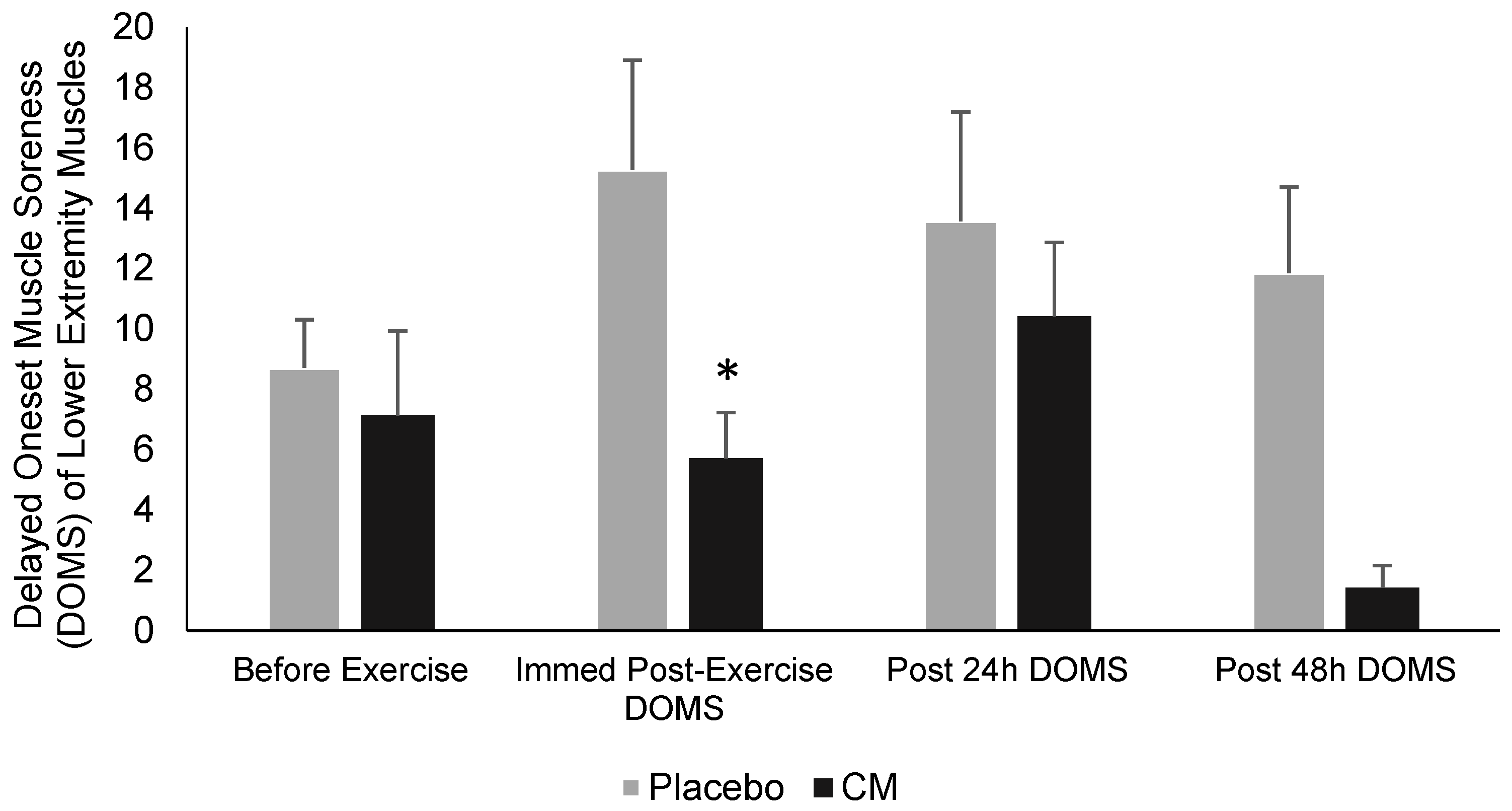
| RPE | DOMS (Upper Body) | DOMS (Lower Body) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Exercise | IP | Post 24 h | Post 48 h | Before Exercise | IP | Post 24 h | Post 48 h | |||
| Week Placebo | 11.8 ± 0.69 | 3.5 ± 0.77 | 2.18 ± 0.75 | 0 | 0 | 8.71 ± 1.6 | 15.28 ± 3.63 | 13.57 ± 3.62 | 11.85 ± 2.85 | |
| Week LFCM | 10.5 ± 0.78 * | 0 | 6.42 ± 1.8 | 0 | 0 | 7.14 ± 2.8 | 5.71 ± 1.52 * | 10.42 ± 2.45 | 1.42 ± 0.74 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molaeikhaletabadi, M.; Bagheri, R.; Hemmatinafar, M.; Nemati, J.; Wong, A.; Nordvall, M.; Namazifard, M.; Suzuki, K. Short-Term Effects of Low-Fat Chocolate Milk on Delayed Onset Muscle Soreness and Performance in Players on a Women’s University Badminton Team. Int. J. Environ. Res. Public Health 2022, 19, 3677. https://doi.org/10.3390/ijerph19063677
Molaeikhaletabadi M, Bagheri R, Hemmatinafar M, Nemati J, Wong A, Nordvall M, Namazifard M, Suzuki K. Short-Term Effects of Low-Fat Chocolate Milk on Delayed Onset Muscle Soreness and Performance in Players on a Women’s University Badminton Team. International Journal of Environmental Research and Public Health. 2022; 19(6):3677. https://doi.org/10.3390/ijerph19063677
Chicago/Turabian StyleMolaeikhaletabadi, Maryam, Reza Bagheri, Mohammad Hemmatinafar, Javad Nemati, Alexei Wong, Michael Nordvall, Maryam Namazifard, and Katsuhiko Suzuki. 2022. "Short-Term Effects of Low-Fat Chocolate Milk on Delayed Onset Muscle Soreness and Performance in Players on a Women’s University Badminton Team" International Journal of Environmental Research and Public Health 19, no. 6: 3677. https://doi.org/10.3390/ijerph19063677









