Phytoplankton Composition and Ecological Status of Lakes with Cyanobacteria Dominance
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Physicochemical Characteristics
3.2. Phytoplankton Assemblages and Dominant Species
3.3. Phytoplankton-Based Indices and Ecological Status of the Studied Lakes
4. Discussion
4.1. Phytoplankton Composition
4.2. Phytoplankton-Based Indices and Ecological Status of the Studied Lakes
5. Conclusions
- Using three indices basing on phytoplankton data—the Q, PSI and PMPL—we assessed the ecological status of three small and shallow lakes located within the Lublin Plane, Eastern Poland. With one exception, all three indices pointed at a status that was below the boundary for good status recommended by the European Commission. According to the PSI and PMPL indices, the status was worse than using the Hungarian method—the Q index.
- We claim that the best index that can be used for small lakes is the Polish (PMPL) index. This is due to the fact that small lakes, which undergo eutrophication at a faster pace mainly because of their geomorphological conditions, usually have a high concentration of cyanobacteria, especially in the summer period. The Polish index (PMPL) takes into account the whole cyanobacterial community in lakes as one of the components for calculating the index, thus providing information about the current ecological status of a given lake.
- The dominant group of all the studied lakes was filamentous cyanobacteria, both heterocytous and non-heterocytous (Aphanizomenon, Planktothrix, Limnothrix and Planktolyngbya species), which is a typical group in nutrient-rich lakes.
- The results we obtained also showed the usefulness of the tested indices for small and shallow freshwaters.
- The functional approach (functional groups—FGs; or morpho-functional groups—MFGs) seemed to express a diversity of physicochemical conditions in water bodies and the real condition of the studied lakes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosén, G. Phytoplankton indicators and their relations to certain chemical and physical factors. Limnologica 1981, 13, 263–290. [Google Scholar]
- Kolkwitz, R.; Marsson, M. Grundsätze für die biologische Beurteilung des Wassers nach seiner Flora und Fauna. Mitt, aus d. Kgl. Pürfungsanstalt für Wasserversorgung u. Abwasserbeseit. Berl. 1902, 1, 33–72. [Google Scholar]
- Kolkwitz, R.; Marsson, M. Ökologie der pflanzlichen Saprobien. Ber. Dt. Botan. Ges. 1908, 26, 505–519. [Google Scholar]
- Kolkwitz, R.; Marsson, M. Ökologie der tierischen Saprobien. Int. Revue Ges. Hydrobiol. 1909, 2, 126–152. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Kilham, S.S. Phosphate and silicate growth and uptake kinetics of the diatoms Asterionella formosa and Cyclotella meneghiniana in batch and semicontinuous culture 1. J. Phycol. 1976, 12, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.S. Phytoplankton assemblages and their periodicity in stratifying lake systems. Holartic Ecol. 1980, 3, 141–159. [Google Scholar] [CrossRef]
- Hörnström, E. Trophic characterisation of lakes by means of qualitative phytoplankton analysis. Limnologica 1981, 13, 246–261. [Google Scholar]
- Rott, H.E. Phytoplankton as biological parameter for the trophic characterization of lakes. Verh. Int. Ver. Limnol. 1984, 22, 1078–1085. [Google Scholar] [CrossRef]
- Trifonova, I.S. Phytoplankton composition and biomass structure in relation to trophic gradient in some temperate and subarctic lakes of north-western Russia and the Prebaltic. Hydrobiologia 1998, 369/370, 99–108. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.L.M.; Kruk, C.; Nasseli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Padisák, J.; Borics, G.; Grigorszky, I.; Soróczki-Pintér, É. Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: The assemblage index. Hydrobiologia 2006, 553, 1–14. [Google Scholar] [CrossRef]
- Mieleitner, J.; Borsuk, M.; Bürgi, H.-R.; Reichert, P. Identifying functional groups of phytoplankton using data from three lakes of different trophic state. Aquat. Sci. 2008, 70, 30–46. [Google Scholar] [CrossRef] [Green Version]
- Becker, V.; Caputo, L.; Ordóñez, J.; Marcé, R.; Armengol, J.; Crossetti, L.O.; Huszar, V.L.M. Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res. 2009, 44, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Becker, V.; Huszar, V.L.M.; Crossetti, L.O. Responses of phytoplankton functional groups to the mixing regime in a deep subtropical reservoir. Hydrobiologia 2009, 628, 137–151. [Google Scholar] [CrossRef]
- Pasztaleniec, A.; Poniewozik, M. Phytoplankton based assessment of the ecological status of four shallow lakes (Eastern Poland) according to Water Framework Directive–a comparison of approaches. Limnol.-Ecol. Manag. Inland Waters 2010, 40, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Lenard, T.; Ejankowski, W.; Poniewozik, M. Does an increase in water colour intensity affect the lake trophic status and phytoplankton metrics? Knowl. Manag. Aquat. Ecosyst. 2018, 419, 46. [Google Scholar] [CrossRef]
- Salmaso, N.; Padisák, J. Morpho-functional groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 2007, 578, 97–112. [Google Scholar] [CrossRef]
- Kruk, C.; Huszar, V.L.; Peeters, E.T.; Bonilla, S.; Costa, L.; Lürling, M.; Reynolds, C.S.; Scheffer, M. A morphological classification capturing functional variation in phytoplankton. Freshw. Biol. 2010, 55, 614–627. [Google Scholar] [CrossRef]
- EC Parliament and Council, 2000. Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Communities 2000, L327, 1–72. [Google Scholar]
- Mischke, U.; Riedmüller, U.; Hoehn, E.; Schönfelder, I.; Nixdorf, B. Description of the German system for phytoplankton-based assessment of lakes for implementation of the EU Water Framework Directive (WFD). In Gewässerreport (Nr. 10): Bewertung von Seen Mittels Phytoplankton zur Umsetzung der EU-Wasserrahmenrichtlinie; Univ. Cottbus: Berlin, Germany, 2008. [Google Scholar]
- Hutorowicz, A.; Pasztaleniec, A. Phytoplankton metric of ecological status assessment for Polish Lakes and its performance along nutrient gradients. Pol. J. Ecol. 2014, 62, 525–540. [Google Scholar] [CrossRef]
- Marchetto, A.; Padedda, B.M.; Mariani, M.A.; Luglie, A.; Sechi, N. A numerical index for evaluating phytoplankton response to changes in nutrient levels in deep mediterranean reservoirs. J. Limnol. 2009, 68, 106–121. [Google Scholar] [CrossRef] [Green Version]
- Noges, P.; Mischke, U.; Laugaste, R.; Solimini, A.G. Analysis of changes over 44 years in the phytoplankton of Lake Võrtsjärv (Estonia): The effect of nutrients, climate and the investigator on phytoplankton-based water quality indices. Hydrobiologia 2010, 646, 33–48. [Google Scholar] [CrossRef]
- Brettum, P. Algen als Indikatoren für die Gewässerqualität in Norwegischen Binnenseen; Norsk Institutt for Vannforskning (NIVA): Oslo, Norwegian, 1989; p. 102. [Google Scholar]
- Oertli, B.; Cereghino, R.; Hull, A.; Miracle, R. Pond conservation: From science to practice. Hydrobiologia 2009, 634, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar] [CrossRef]
- Elmberg, J.; Nummi, P.; Poysa, H.; Sjoberg, K. Relationships between species number, lake size and resource diversity in assemblages of breeding waterfowl. J. Biogeogr. 1994, 21, 75–84. [Google Scholar] [CrossRef]
- Scheffer, M.; Van Geest, G.J.; Zimmer, K.; Jeppesen, E.; Søndergaard, M.; Butler, M.G.; Hanson, M.A.; Declerck, S.; De Meester, L. Small habitat size and isolation can promote species richness: Second-order effects on biodiversity in shallow lakes and ponds. Oikos 2006, 112, 227–231. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54 Pt 2, 2298–2314. [Google Scholar] [CrossRef] [Green Version]
- Poniewozik, M.; Juráň, J. Extremely high diversity of euglenophytes in a small pond in eastern Poland. Plant Ecol. Evol. 2018, 151, 18–34. [Google Scholar] [CrossRef]
- Harasimiuk, M.; Michalczyk, Z.; Turczyński, M. Jeziora łęczyńsko-włodawskie. In Biblioteka Monitoringu Środowiska; Studia Ośrodka Dokumentacji Fizjograficznej: Lublin, Poland, 1998; p. 176. [Google Scholar]
- Wilgat, T.; Michalczyk, Z.; Turczyński, M.; Wojciechowski, K. The Łęczna-Włodawa Lakes. Studia Ośr. Dok. Fizjogr. PAN 1991, 19, 23–140. [Google Scholar]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Fizyczno-Chemiczne Badanie Wody i Ścieków; Arkady: Warszawa, Poland, 1999. [Google Scholar]
- Nusch, E.A. Comparison of different methods for chlorophyll and phaeopigment determination. Arch. Hydrobiol Beih. Ergebn. Limnol. 1980, 14, 14–36. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes 1. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Utermöhl, H. Zur Vervollkommung der quantitative Phytoplankton-Methodik. Mitt. Int. Verein. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Ettl, H. Chlorophyta I. Phytomonadina. In Subwasserflora Von Mitteleuropa; Journal of Basic Microbiology: Weinheim, Germany, 1983; p. 9. [Google Scholar]
- Hindák, F. Colour Atlas of Cyanophytes; VEDA, Publishing House of the Slovak Academy of Sciences: Bratislava, Slovakia, 2008; p. 205. [Google Scholar]
- Mathes, J.; Plambeck, G.; Schaumburg, J. Das Typisierungssystem für stehende Gewässer in Deutschland mit Wasserflächen ab 0, 5 km² zur Umsetzung der Wasserrahmenrichtlinie. Implementierung der EU-WRRL in Deutschland: Ausgewählte Bewertungsmethoden und Defizite. Aktuelle Reihe 2002, 5, 15–23. [Google Scholar]
- Kolada, A. The effect of lake morphology on aquatic vegetation development and changes under the influence of eutrophication. Ecol. Indic. 2014, 38, 282–293. [Google Scholar] [CrossRef]
- Sugier, P.; Lorens, B.; Chmiel, S.; Turczyński, M. The influence of Ceratophyllum demersum L. and Stratiotes aloides L. on richness and diversity of aquatic vegetation in the lakes of mid-eastern Poland. Hydrobiologia 2010, 656, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Sender, J. Impact of the Drainage System on Water Vegetation of the Lowland Lakes (Eastern Poland). Turk. J. Fish. Aquat. Sci. 2018, 18, 611–622. [Google Scholar] [CrossRef]
- Radwan, S.; Kornijów, R. Hydrobiological and hydrochemical characteristics of surface waters. In Środowisko Przyrodnicze w Strefie Oddziaływania Kanału Wieprz-Krzna; Radwan, S., Ed.; AR, TWWP: Lublin, Poland, 1991; pp. 47–58. [Google Scholar]
- Janiec, B. Wpływ Kanału Wieprz-Krzna na przenoszenie zanieczyszczeń do środowiska wodnego. In Środowisko Przyrodnicze w Strefie Oddziaływania Kanału Wieprz-Krzna; AR w Lublinie, TWWP: Lublin, Poland, 1994; pp. 59–68. [Google Scholar]
- Michalczyk, Z. Problems of water conditions protection and the environmental monitoring in the Łęczna-Włodawa Lakeland. In Nature and Landscape Monitoring System in the West Polesie Region; Chmielewski, T.J., Sławiński, C., Eds.; PZN Press: Lublin, Poland, 2009; pp. 152–159. [Google Scholar]
- Garnier, J.; Billen, G.; Sanchez, N.; Leporcq, B. Ecological functioning of the Marne reservoir (upper Seine basin, France). Regul. Rivers Res. Manag. Int. J. Devoted River Res. Manag. 2000, 16, 51–71. [Google Scholar] [CrossRef]
- Kimmel, K.; Kull, A.; Salm, J.O.; Mander, Ü. The status, conservation and sustainable use of Estonian wetlands. Wetl. Ecol. Manag. 2010, 18, 375–395. [Google Scholar] [CrossRef]
- Clarkson, B.R.; Ausseil, A.G.E.; Gerbeaux, P. Wetland ecosystem services. In Ecosystem services in New Zealand: Conditions and Trends; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 192–202. [Google Scholar]
- Reynolds, C.S. The response of phytoplankton communities to changing lake environments. Swiss J. Hydrol. 1987, 49, 220–236. [Google Scholar] [CrossRef]
- Mehnert, G.; Leunert, F.; Cirés, S.; Jöhnk, K.D.; Rücker, J.; Nixdorf, B.; Wiedner, C. Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. J. Plankton Res. 2010, 32, 1009–1021. [Google Scholar] [CrossRef]
- Cirés, S.; Ballot, A. A review of the phylogeny, ecology and toxin production of bloom-forming Aphanizomenon spp. and related species within the Nostocales (Cyanobacteria). Harmful Algae 2016, 54, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef] [PubMed]
- De Nobel, W.T.; Snoep, J.L.; Westerhoff, H.V.; Mur, L.R. Interaction of nitrogen fixation and phosphorus limitation in Aphanizomenon flos-aquae (Cyanophyceae) 1. J. Phycol. 1997, 33, 794–799. [Google Scholar] [CrossRef]
- Scott, J.T.; McCarthy, M.J. Response to comment: Nitrogen fixation has not offset declines in the Lake 227 nitrogen pool and shows that nitrogen control deserves consideration in aquatic ecosystems. Limnol. Oceanogr. 2011, 56, 1548–1550. [Google Scholar] [CrossRef]
- Degerholm, J.; Gundersen, K.; Bergman, B.; Söderbäck, E. Phosphorus-limited growth dynamics in two Baltic Sea cyanobacteria, Nodularia sp. and Aphanizomenon sp. FEMS Microbiol. Ecol. 2006, 58, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kangro, K.; Nõges, P. Seasonal development of Planktothrix agardhii Anagnostidis et Komarek and Limnothrix redekei (Van Goor) Meffert in a sharply stratified hypertrophic lake. Algol. Stud. 2003, 109, 267–280. [Google Scholar] [CrossRef]
- Janse, I.; Kardinaal, W.E.A.; Agterveld, M.K.V.; Meima, M.; Visser, P.M.; Zwart, G. Contrasting microcystin production and cyanobacterial population dynamics in two Planktothrix-dominated freshwater lakes. Environ. Microbiol. 2005, 7, 1514–1524. [Google Scholar] [CrossRef]
- Nixdorf, B.; Mischke, U.; Rücker, J. Phytoplankton assemblages and steady state in deep and shallow eutrophic lakes—An approach to differentiate the habitat properties of Oscillatoriales. In Phytoplankton and Equilibrium Concept: The Ecology of Steady-State Assemblages; Springer: Dordrecht, The Netherlands, 2003; pp. 111–121. [Google Scholar]
- Foy, R.H.; Gibson, C.E.; Smith, R.V. The influence of daylength, light intensity and temperature on the growth rates of planktonic blue-green algae. Br. Phycol. J. 1976, 11, 151–163. [Google Scholar] [CrossRef]
- Post, A.F.; Loogman, J.G.; Mur, L.R. Regulation of growth and photosynthesis by Oscillatoria agardhii grown with a lightldark cycle. FEMS Microbiol. Ecol. 1985, 31, 97–102. [Google Scholar] [CrossRef]
- Davis, P.A.; Walsby, A.E. Comparison of measured growth rates with those calculated from rates of photosynthesis in Planktothrix spp. isolated from Blelham Tarn, English Lake District. New Phycol. 2002, 156, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Budzyńska, A.; Gołdyn, R.; Zagajewski, P.; Dondajewska, R.; Kowalczewska-Madura, K. The dynamics of a Planktothrix agardhii population in a shallow dimictic lake. Oceanol. Hydrobiol. Stud. 2009, 38, 7–12. [Google Scholar]
- Davis, P.A.; Dent, M.; Parker, J.; Reynolds, C.S.; Walsby, A.E. The annual cycle of growth rate and biomass change in Planktothrix spp. in Blelham Tarn, English Lake District. Freshw. Biol. 2003, 48, 852–867. [Google Scholar] [CrossRef]
- Halstvedt, C.B.; Rohrlack, T.; Andersen, T.; Skulberg, O.; Edvardsen, B. Seasonal dynamics and depth distribution of Planktothrix spp. in Lake Steinsfjorden (Norway) related to environmental factors. J. Plankton Res. 2007, 29, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Kokociński, M.; Stefaniak, K.; Mankiewicz-Boczek, J.; Izydorczyk, K.; Soininen, J. The ecology of the invasive cyanobacterium Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) in two hypereutrophic lakes dominated by Planktothrix agardhii (Oscillatoriales, Cyanophyta). Eur. J. Phycol. 2010, 45, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Rücker, J.; Wiedner, C.; Zippel, P. Factors controlling the dominance of Planktothrix agardhii and Limnothrix redekei in eutrophic shallow lakes. In Shallow Lakes’ 95; Springer: Dordrecht, The Netherlands, 1997; pp. 107–115. [Google Scholar]
- Mischke, U. Cyanobacteria associations in shallow polytrophic lakes: Influence of environmental factors. Acta Oecologica 2003, 24, S11–S23. [Google Scholar] [CrossRef]
- Briand, J.F.; Robillot, C.; Quiblier-Lloberas, C.; Humbert, J.F.; Couté, A.; Bernard, C. Environmental context of Cylindrospermopsis raciborskii (Cyanobacteria) blooms in a shallow pond in France. Water Res. 2002, 36, 3183–3192. [Google Scholar] [CrossRef]
- Rojo, C.; Cobelas, M.A. Population dynamics of Limnothrix redekei, Oscillatoria lanceaeformis, Planktothrix agardhii and Pseudanabaena limnetica (cyanobacteria) in a shallow hypertrophic lake (Spain). In Nutrient Dynamics and Biological Structure in Shallow Freshwater and Brackish Lakes; Springer: Dordrecht, The Netherlands, 1994; pp. 165–171. [Google Scholar]
- Stević, F.; Mihaljević, M.; Špoljarić, D. Changes of phytoplankton functional groups in a floodplain lake associated with hydrological perturbations. Hydrobiologia 2013, 709, 143–158. [Google Scholar] [CrossRef]
- Berger, C.; Sweers, H.E. The IJsselmeer and its phytoplankton—with special attention to the suitability of the lake as a habitat for Oscillatoria agardhii Gom. J. Plankton Res. 1988, 10, 579–599. [Google Scholar] [CrossRef]
- Nabout, J.C.; Nogueira, I.D.S. Spatial and Temporal Dynamics of Phytoplankton Functional Group in a Blocked Valley; Brazil, 1919. [Google Scholar]
- Gemelgo, M.C.P.; Mucci, J.L.N.; Navas-Pereira, D. Population dynamics: Seasonal variation of phytoplankton functional groups in Brazilian reservoirs (Billings and Guarapiranga, São Paulo). Braz. J. Biol. 2009, 69, 1001–1013. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, L.G.; Barbosa, P.M.M.; Barbosa, F.A.R. Vertical distribution of phytoplankton functional groups in a tropical shallow lake: Driving forces on a diel scale. Acta Limnol. Bras. 2011, 23, 63–73. [Google Scholar] [CrossRef]
- da Costa, M.R.A.; Attayde, J.L.; Becker, V. Effects of water level reduction on the dynamics of phytoplankton functional groups in tropical semi-arid shallow lakes. Hydrobiologia 2016, 778, 75–89. [Google Scholar] [CrossRef]
- Padisák, J.; Borics, G.; Fehér, G.; Grigorszky, I.; Oldal, I.; Schmidt, A.; Zámbóné-Doma, Z. Dominant species, functional assemblages and frequency of equilibrium phases in late summer phytoplankton assemblages in Hungarian small shallow lakes. Hydrobiologia 2003, 502, 157–168. [Google Scholar] [CrossRef]
- Moss, B.; Stephen, D.; Alvarez, C.; Becares, E.; Bund, W.V.D.; Collings, S.E.; Van Donk, E.; De Eyto, E.; Feldmann, T.; Fernández-Aláez, C.; et al. The determination of ecological status in shallow lakes—A tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquatic Conservation: Mar. Freshw. Ecosyst. 2003, 13, 507–549. [Google Scholar] [CrossRef]
- Penning, W.E.; Dudley, B.; Mjelde, M.; Hellsten, S.; Hanganu, J.; Kolada, A.; van den Berg, M.; Poikane, S.; Phillips, G.; Willby, N.; et al. Using aquatic macrophyte community indices to define the ecological status of European lakes. Aquat. Ecol. 2008, 42, 253–264. [Google Scholar] [CrossRef]
- Bennion, H.; Kelly, M.G.; Juggins, S.; Yallop, M.L.; Burgess, A.; Jamieson, J.; Krokowski, J. Assessment of ecological status in UK lakes using benthic diatoms. Freshw. Sci. 2014, 33, 639–654. [Google Scholar] [CrossRef] [Green Version]
- Poikane, S.; Kelly, M.; Cantonati, M. Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Sci. Total Environ. 2016, 568, 603–613. [Google Scholar] [CrossRef]
- Poikane, S.; Portielje, R.; Denys, L.; Elferts, D.; Kelly, M.; Kolada, A.; Mäemets, H.; Phillips, G.; Søndergaard, M.; Willby, N.; et al. Macrophyte assessment in European lakes: Diverse approaches but convergent views of ‘good’ ecological status. Ecol. Indic. 2018, 94, 185–197. [Google Scholar] [CrossRef]
- Dembowska, E.A.; Napiórkowski, P.; Mieszczankin, T.; Józefowicz, S. Planktonic indices in the evaluation of the ecological status and the trophic state of the longest lake in Poland. Ecol. Indic. 2015, 56, 15–22. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Dembowska, E.A. Phytoplankton dynamics in relation to physicochemical conditions in large, stratified Lake Charzykowskie (Northern Poland). Oceanol. Hydrobiol. Stud. 2017, 46, 260–270. [Google Scholar] [CrossRef]
- Solis, M.; Pawlik-Skowrońska, B.; Kalinowska, R. Seasonal changes of phytoplankton and cyanobacteria/cyanotoxin risk in two shallow morphologically altered lakes: Effects of water level manipulation (Wieprz-Krzna Canal System, Eastern Poland). Ecol. Indic. 2016, 66, 103–112. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Brettum, P. Performance of a new phytoplankton composition metric along a eutrophication gradient in Nordic lakes. Hydrobiologia 2009, 633, 75–82. [Google Scholar] [CrossRef]
- Katsiapi, M.; Moustaka-Gouni, M.; Sommer, U. Assessing ecological water quality of freshwaters: PhyCoI—A new phytoplankton community Index. Ecol. Inform. 2016, 31, 22–29. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]


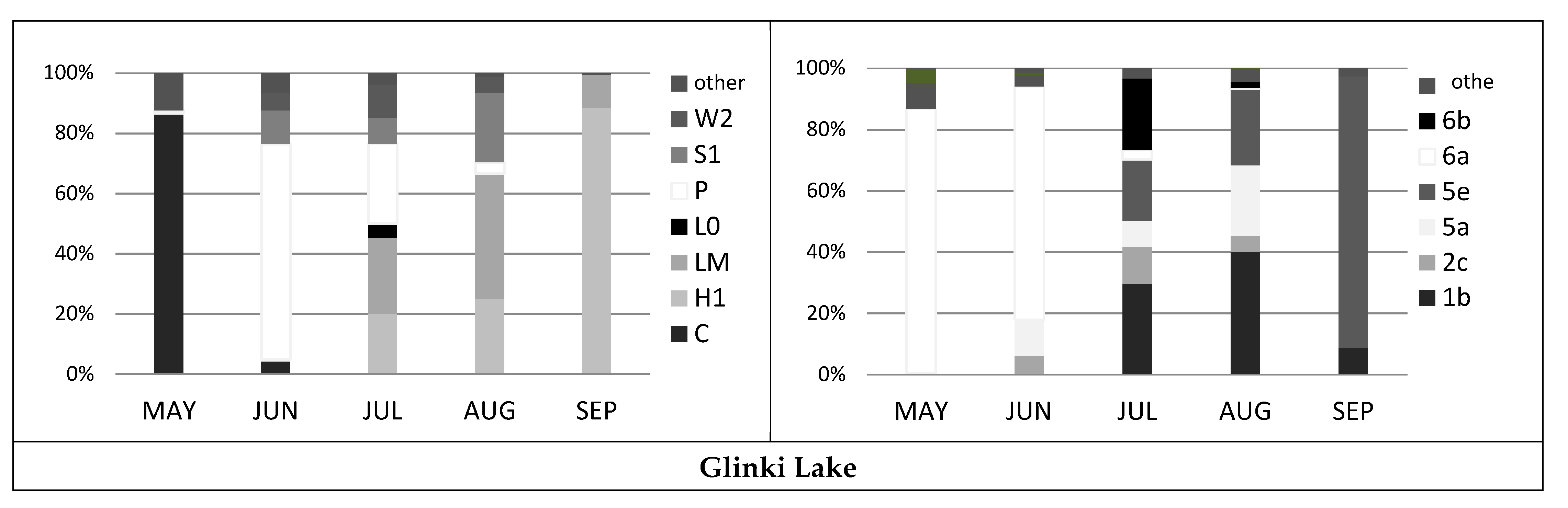

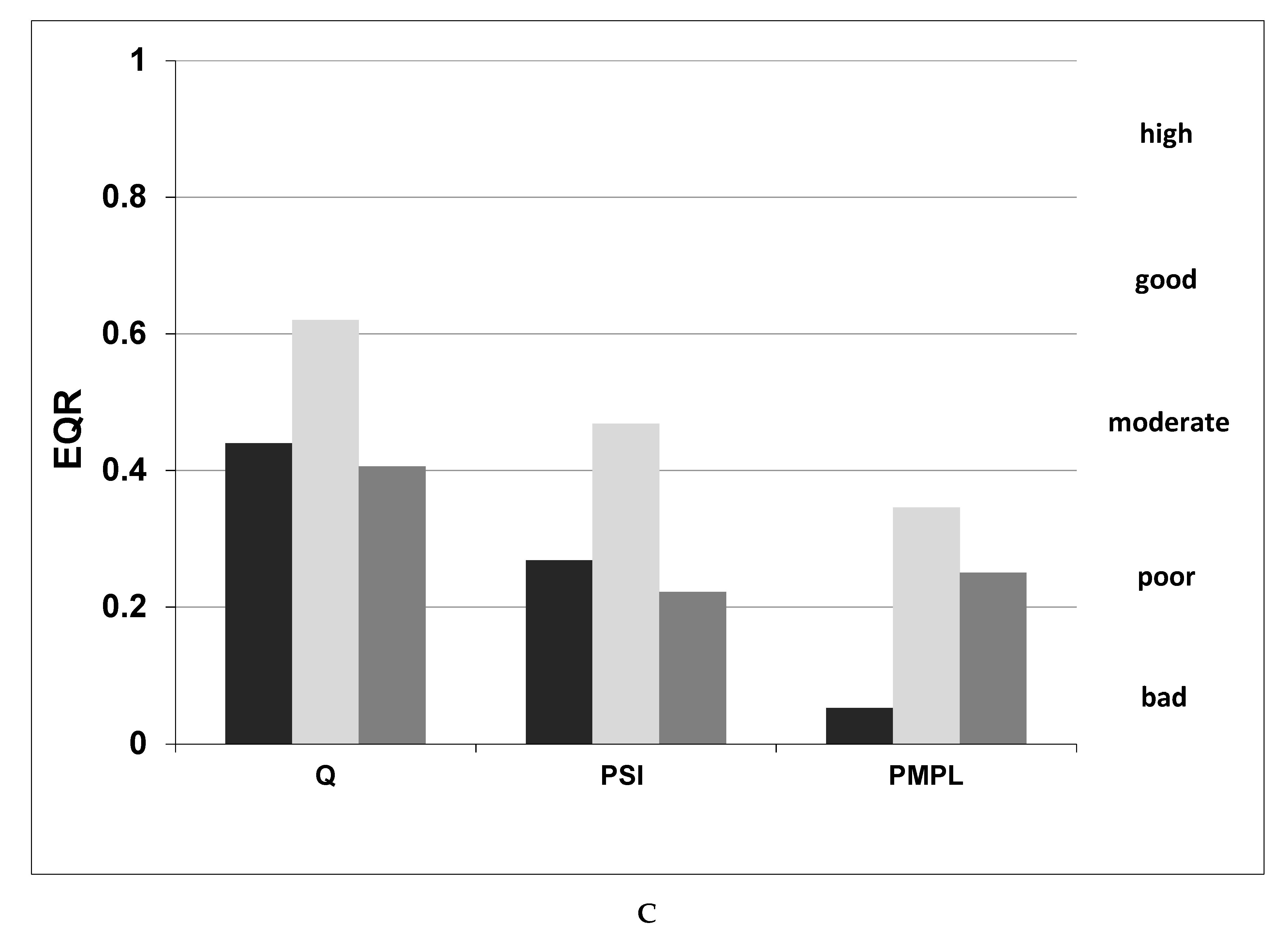
| Morphorogical Data According to Harasimiuk et al. (1998) | Gumienek | Czarne | Glinki |
|---|---|---|---|
| Area (ha) | 8.5 | 24.8 | 40.9 |
| Maximum depth (m) | 7.8 | 10.3 | 8.8 |
| Mean depth (m) | 3.8 | 3.7 | 2.8 |
| Volume (thousands m3) | 307 | 915 | 1342 |
| Catchment area (ha) | 21.5 | -* | 159.7 |
| Physicochemical data (mean values and SD–Standard Deviations (±)) | |||
| Secchi Disk–SD (m) | 1.5 (±0.28) | 2.9 (±0.81) | 0.6 (±0.16) |
| Zeu (m) | 2.92 (±0.61) | 4.38 (±0.79) | 1.77 (±0.23) |
| Water colour (mg Pt L−1) | n.d. | n.d. | 234 (±49.47) |
| pH | 8.4 (±0.09) | 8.2 (±0.10) | 8.1 (±0.51) |
| Conductivity (μS cm−1) | 296 (±28.60) | 234 (±20.10) | 246 (±5.85) |
| Temperature of epilimnion water (°C) | 22.9 (±2.95) | 21.9 (±2.94) | 21.88 (±1.98) |
| P-PO4 (mg L−1) | 0.015 (±0.002) | 0.014 (±0.004) | 0.007 (±0.002) |
| TP (mg L−1) | 0.111 (±0.005) | 0.039 (±0.014) | 0.035 (±0.016) |
| N-NH4 (mg L−1) | 0.26 (±0.042) | 0.24 (±0.042) | 0.69 (±0.300) |
| N-NO3 (mg L−1) | 0.72 (±0.490) | 0.68 (±0.360) | 0.49 (±0.190) |
| TN (mg L−1) | 2.53 (±0.590) | 2.14 (±0.670) | 3.81 (±0.490) |
| Biological data (mean values) | |||
| Total abundance of phytoplankton (N 103 L−1) | 24436 (±17577) | 2637 (±1369) | 5779 (±31.44) |
| Total biomass of phytoplankton (mg L−1) | 20.38 (±12.38) | 4.74 (±1.44) | 8.23 (±1.96) |
| Chlorophyll a (μg L−1) | 20.7 (±6.99) | 10.2 (±7.98) | 66.1 (±28.10) |
| Data for Q index calculation (Padisák et al., 2006) | Gumienek | Czarne | Glinki |
| Type of lake | type 7 | type 7 | type 7 |
| Hydro-geographical features | calcareous | calcareous | calcareous |
| Persistency of water | persistent | persistent | persistent |
| Data for PSI index calculation | |||
| VQ—ratio of volume of lake to catchment area | 1.43 | <1.5 | 0.84 |
| LAWA lake type [40] | 13—lowlands, stratified, VQ < 1.5 | 13—lowlands, stratified, VQ < 1.5 | 13—lowlands, stratified, VQ < 1.5 |
| Data for PMPL index calculation | |||
| Maximum chlorophyll value (μg L−1) | 30.7 | 24.5 | 113.1 |
| Mean value of Cyanoprokaryota biomass (mg L−1) | 11.85 | 2.75 | 2.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poniewozik, M.; Lenard, T. Phytoplankton Composition and Ecological Status of Lakes with Cyanobacteria Dominance. Int. J. Environ. Res. Public Health 2022, 19, 3832. https://doi.org/10.3390/ijerph19073832
Poniewozik M, Lenard T. Phytoplankton Composition and Ecological Status of Lakes with Cyanobacteria Dominance. International Journal of Environmental Research and Public Health. 2022; 19(7):3832. https://doi.org/10.3390/ijerph19073832
Chicago/Turabian StylePoniewozik, Małgorzata, and Tomasz Lenard. 2022. "Phytoplankton Composition and Ecological Status of Lakes with Cyanobacteria Dominance" International Journal of Environmental Research and Public Health 19, no. 7: 3832. https://doi.org/10.3390/ijerph19073832
APA StylePoniewozik, M., & Lenard, T. (2022). Phytoplankton Composition and Ecological Status of Lakes with Cyanobacteria Dominance. International Journal of Environmental Research and Public Health, 19(7), 3832. https://doi.org/10.3390/ijerph19073832






