Proposal for Handling of Medicine Shortages Based on a Comparison of Retrospective Risk Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Selecting Countries
- First world countries, where drug shortages are not a result of the lack of available financial resources.
- Have a profound pharmaceutical industrial background.
- Publicly available reporting system.
- Reported shortages must be classifiable according to the Anatomical Therapeutic Chemical (ATC) classification system.
- Information is public regarding available substitutes to allow for assessment of severity.
- Discontinued presentations listed separately from current shortages.
2.2. Processing the Databases
2.3. Therapeutic Categories
- C: Cardiovascular system
- L: Antineoplastic and immunomodulating agents
- J: Anti-infectives for systemic use
- N: Nervous system
2.4. Allocation of Risk Assessment in Studied Countries
2.5. Comparison of the Extent of Different Shortages
2.6. Binomial Probability Tests of Proportion of Critical Shortages across ATC Groups
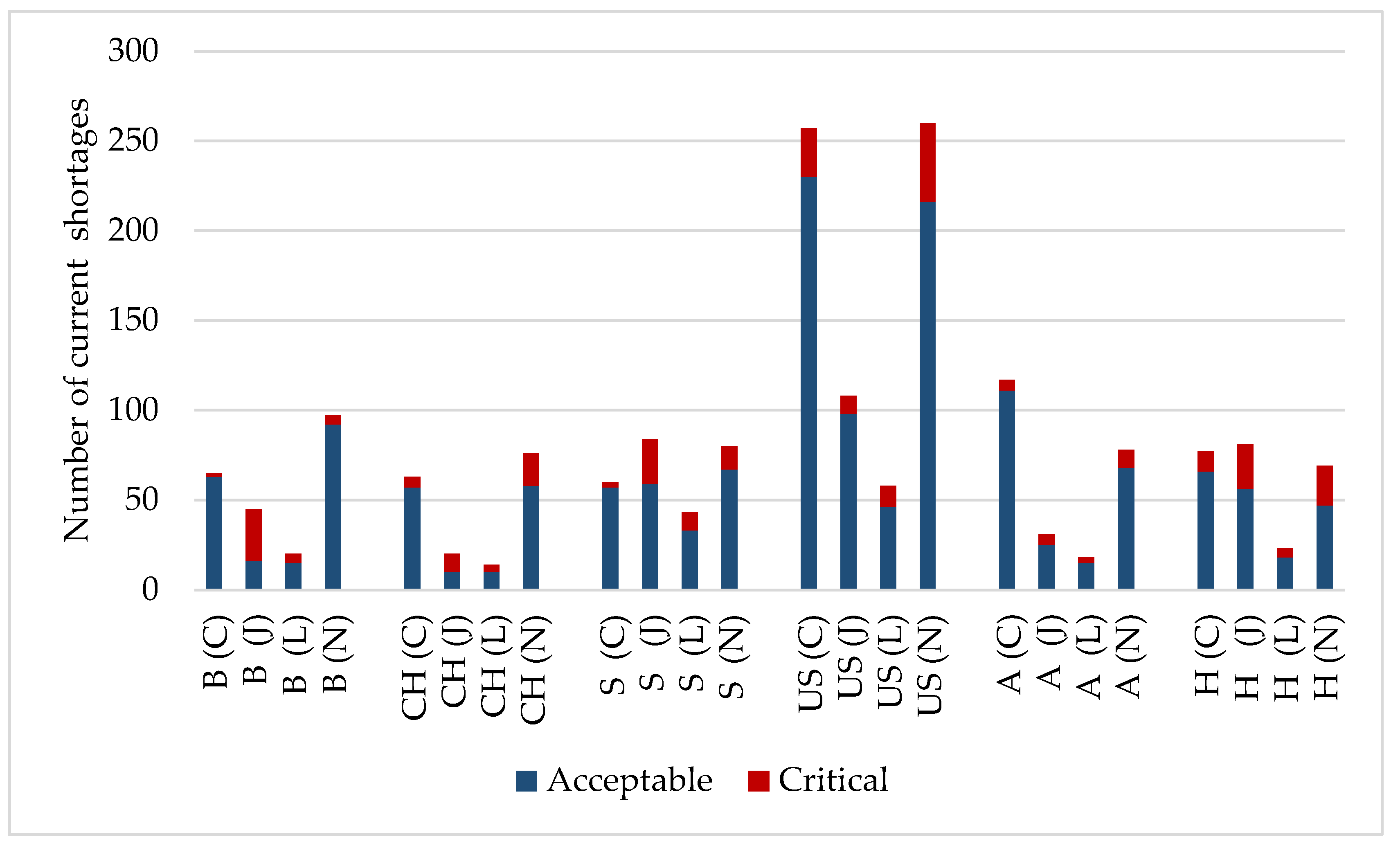
3. Results
- Transforming the data into population-proportionate figures.
- Filtering out critical cases from all shortages (according to criteria in Table 1).
- Comparing critical cases with the WHO Essential Medicine List.
4. Discussion
4.1. Comparison of Shortage Management in Examined Countries
- Compulsory stockpiling
- Measures for essential medicines
- Notification responsibility
- Measures affecting wholesalers
- Export bans
- Emergency imports
4.2. Compulsory Stockpiling
4.3. Measures for Essential Medicines
4.4. Notification Responsibility
4.5. Measures Affecting Wholesalers
4.6. Export Bans
4.7. Emergency Imports
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Weerdt, E.; Simoens, S.; Casteels, M.; Huys, I. Toward a European definition for a drug shortage: A qualitative study. Front. Pharmacol. 2015, 6, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, A.; Manasse, H.R., Jr. Shortages of medicines: A complex global challenge. Bull. World Health Organ. 2012, 90, 158. [Google Scholar] [CrossRef] [PubMed]
- Turbucz, B.; Hankó, B. Overview of the causes and management of drug shortages in the United States and in Hungary. Acta Pharm. Hung. 2020, 90, 170–184. [Google Scholar] [CrossRef]
- Acosta, A.; Vanegas, E.P.; Rovira, J.; Godman, B.; Bochenek, T. Medicine Shortages: Gaps Between Countries and Global Perspectives. Front. Pharmacol. 2019, 10, 763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videau, M.; Chemali, L.; Stucki, C.; Saavedra-Mitjans, M.; Largana, S.; Guerin, A.; Bonnabry, P.; Delhauteur, B.; VAN Hees, T.; Lebel, D.; et al. Drug Shortages in Canada and Selected European Countries: A Cross-Sectional, Institution-Level Comparison. Can. J. Hosp. Pharm. 2019, 72, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Parliament, Council. Directive 2001/83/EC of the European Parliament and of the Council on the Community Code Relating to Medicinal Products for Human Use 2001/83/Ec. 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32001L0083&from=en (accessed on 14 March 2021).
- Miljković, N.; Gibbons, N.; Batista, A.; Fitzpatrick, R.W.; Underhill, J.; Horák, P. Results of EAHP’s 2018 Survey on Medicines Shortages. Eur. J. Hosp. Pharm. 2019, 26, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Susan, T.; Erin, B.; Corby-Edwards, A.K.; Glassgold, J.M.; Johnson, J.A.; Lister, S.A.; Sarata, A.K. The Food and Drug Administration Safety and Innovation Act (FDASIA, P.L. 112-144), Report, February 4 2013; Library of Congress: Washington, DC, USA, 2013; Available online: https://digital.library.unt.edu/ark:/67531/metadc808297/ (accessed on 24 June 2021).
- Tan, Y.X.; Moles, R.J.; Chaar, B.B. Medicine shortages in Australia: Causes, impact and management strategies in the community setting. Int. J. Clin. Pharm. 2016, 38, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.R.; Birt, A.; James, K.B.; Kokko, H.; Salverson, S.; Soflin, D.L.; Pharm, B.S. ASHP Guidelines on Managing Drug Product Shortages in Hospitals and Health Systems. Am. J. Health-Syst. Pharm. 2009, 66, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The drug shortage crisis in the United States: Causes, impact, and management strategies. Pharm. Ther. Peer-Rev. J. Formul. Manag. 2011, 36, 740–757. [Google Scholar]
- American Society of Health-System Pharmacists. Drug Shortages: Current Shortages. Database: ASHP. 2021. Available online: https://www.ashp.org/Drug-Shortages/Current-Shortages (accessed on 9 March 2021).
- Martinelli Consulting. Database: Martinelli. 2021. Available online: https://www.drugshortage.ch/ (accessed on 6 March 2021).
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2013. Oslo, 2012. 2021. Available online: https://www.whocc.no/filearchive/publications/1_2013guidelines.pdf (accessed on 23 May 2021).
- Reed, B.N.; Fox, E.R.; Konig, M.; Jackevicius, C.A.; Masoudi, F.A.; Rabinstein, A.A.; Page, R.L. The impact of drug shortages on patients with cardiovascular disease: Causes, consequences, and a call to action. Am. Heart J. 2016, 175, 130–141. [Google Scholar] [CrossRef] [PubMed]
- The Economist Intelligence Unit (EIU) Report, Supported by the European Society for Medical Oncology (ESMO) Country Profile: Cancer Medicines Shortages. 2019, pp. 1–8. Available online: https://www.esmo.org/content/download/197324/3552968/1/ESMO-Country-profile-Belgium.pdf (accessed on 18 April 2021).
- Griffith, M.M.; Gross, A.E.; Sutton, S.H.; Bolon, M.K.; Esterly, J.S.; Patel, J.A.; Postelnick, M.J.; Zembower, T.R.; Scheetz, M.H. The Impact of Anti-infective Drug Shortages on Hospitals in the United States: Trends and Causes. Clin. Infect. Dis. 2012, 54, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danon, J.J.; Reekie, T.A.; Kassiou, M. Challenges and Opportunities in Central Nervous System Drug Discovery. Trends Chem. 2019, 1, 612–624. [Google Scholar] [CrossRef]
- Spanish Agency of Medicines and Health Products. Database: AEMPS. Available online: https://cima.aemps.es/cima/publico/home.html (accessed on 12 March 2021).
- Országos Gyógyszerészeti és Élelmezés-Egészségügyi Intézet. Database: OGYEI. Available online: https://ogyei.gov.hu/gyogyszeradatbazis (accessed on 9 March 2021).
- Federal Agency for Medicines and Health Products, Unavailable Medicines. Database: FAMHP. 2021. Available online: https://www.famhp.be/en/items-HOME/unavailability_of_medicinal_products (accessed on 4 March 2021).
- Therapeutic Goods Administration, Medicine Shortages Information Initiative. Database: TGA. 2021. Available online: https://apps.tga.gov.au/prod/MSI/search (accessed on 8 March 2021).
- World Population Dashboard, United Nations Population Fund. Database: UNFPA. 2021. Available online: https://www.unfpa.org/data/world-population-dashboard (accessed on 18 May 2021).
- Wet Betreffende de Verplichte Verzekering Voor Geneeskundige Verzorging en Uitkeringen Gecoördineerd Op. Available online: http://www.ejustice.just.fgov.be/cgi_loi/change_lg.pl?language=nl&la=N&cn=1994071438&table_name=wet (accessed on 5 March 2021).
- Bochenek, T.; Abilova, V.; Alkan, A.; Asanin, B.; Beriain, I.D.M.; Besovic, Z.; Bonanno, P.V.; Bucsics, A.; Davidescu, M.; De Weerdt, E.; et al. Systemic Measures and Legislative and Organizational Frameworks Aimed at Preventing or Mitigating Drug Shortages in 28 European and Western Asian Countries. Front. Pharmacol. 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española de Medicamentos y Productos Sanitarios AEMPS. How Are Medicines and Medical Devices Regulated in Spain. Available online: https://www.aemps.gob.es/publicaciones/publica/regulacion_med-PS/v2/docs/reg_med-PS-v2-light-ing.pdf?x80916. (accessed on 12 April 2021).
- Therapeutic Goods Act 1989 (TG Act). Available online: https://www.legislation.gov.au/Details/C2017C00226 (accessed on 2 May 2021).
- Cook, J. Therapeutic Goods (Reportable Medicines) Determination December 2018. 2021. Available online: https://www.legislation.gov.au/Details/F2018L01678 (accessed on 3 May 2021).
- Australian Government Department of Health. Management and Communication of Medicine Shortages in Australia, A New Protocol. Available online: https://www.tga.gov.au/sites/default/files/consultation-management-and-communication-of-medicine-shortages-in-australia-a-new-protocol.pdf (accessed on 12 April 2021).
- Cook, J. Therapeutic Goods (Medicines Watch List) Determination December 2018. Available online: https://www.legislation.gov.au/Details/F2018L01679 (accessed on 5 May 2021).
- Act XCV of 2005 on Medicinal Products for Human Use and on the Amendment of Other Regulations Related to Medicinal Products. 2020, pp. 1–76. Available online: https://net.jogtar.hu/getpdf?docid=a0500095.tv&targetdate=&printTitle=Act+XCV+of+2005&dbnum=62&getdoc=1#:~:text=1%20Promulgated%20on%2015%20July,and%20international%20regulations%20and%20recommendations (accessed on 9 April 2021).
- American Society of Health-System Pharmacists. Drug Shortages Statistics. Database: ASHP. 2021. Available online: https://www.ashp.org/Drug-Shortages/Shortage-Resources/Drug-Shortages-Statistics (accessed on 10 May 2021).
- Miljković, N.; Godman, B.; van Overbeeke, E.; Kovačević, M.; Tsiakitzis, K.; Apatsidou, A.; Nikopoulou, A.; Yubero, C.G.; Horcajada, L.P.; Stemer, G.; et al. Risks in Antibiotic Substitution Following Medicine Shortage: A Health-Care Failure Mode and Effect Analysis of Six European Hospitals. Front. Med. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List 2019. Database: WHO. 2021. Available online: https://apps.who.int/iris/handle/10665/325771 (accessed on 11 May 2021).
- Hogerzeil, H.V. The concept of essential medicines: Lessons for rich countries. BMJ 2004, 329, 1169–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MARS 1964. Loi sur les Médicaments. Available online: https://www.ejustice.just.fgov.be/cgi_loi/change_lg.pl?language=fr&la=F&cn=1964032530&table_name=loi (accessed on 12 March 2021).
- Belgisch Moniteur Staatsblad Belge. Available online: http://www.ejustice.just.fgov.be/cgi/welcome.pl (accessed on 26 March 2021).
- European Commission Health and Food Safety Directorate-General Summary of Responses to the Questionnaire on the Measures Implemented in the Member States Territories in the Context of Article 81 of Directive 2001/83/EC. 2017. Available online: https://ec.europa.eu/health/sites/default/files/files/committee/ev_20180525_summary_en.pdf (accessed on 12 April 2021).
- Service Public Federal Sante Publique Sdlcaee. 25 April 2014. Royal Decree Amending the Royal Decree of 14 December 2006 Relating to Medicinal Products for Human and Veterinary Use. 2021, pp. 1–10. Available online: https://www.ejustice.just.fgov.be/cgi_loi/change_lg.pl?language=fr&la=F&cn=2006121431&table_name=loi (accessed on 12 March 2021).
- SR 531.215.32. Verordnung Vom 12. August 2015 Über Die Meldestelle für Lebenswichtige Humanarzneimittel. 2013, pp. 1–24. Available online: https://www.fedlex.admin.ch/eli/cc/2015/544/de (accessed on 13 May 2021).
- Bart, T.N. Parallel Trade of Pharmaceuticals: A Review of Legal, Economic, and Political Aspects. Value Health 2008, 11, 996–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiss Agency for Therapeutic Products. Database Swissmedic. 2021. Available online: https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/marketsurveillance/out-of-stock.html (accessed on 12 April 2021).
- Royal Decree 1345/2007 of 11 October, Which Regulates the Procedure for Authorisation, Registration and Dispensation of Industrially Manufactured Medicaments for Human Use Conditions. 2021, pp. 1–91, Database. Available online: https://www.global-regulation.com/translation/spain/1445110/royal-decree-1345---2007-of-11-october%252c-which-regulates-the-procedure-for-authorisation%252c-registration-and-dispensation-of-industrially-manufacture.html (accessed on 12 April 2021).
- Agencia, D.C.; Sanitarios, A.P.; Decreto, R.; Espa, A.; Sanitarios, P.; Decreto, R. Circular no 3/2011. 2011, pp. 1–7. Available online: https://www.legisweb.com.br/legislacao/?id=392406 (accessed on 24 April 2021).
- Law 29/2006 of 26 July, on Guarantees and Rational Use of Medicines and Medical Devices. Available online: https://www.global-regulation.com/translation/spain/1446301/law-29-2006-of-26-july%252c-on-guarantees-and-rational-use-of-medicines-and-medical-devices.html (accessed on 26 April 2021).
- Jensen, V.; Kimzey, L.M.; Goldberger, M.J. FDA’s role in responding to drug shortages. Am. J. Health Pharm. 2002, 59, 1423–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US Food and Drug Administration. Database FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-publishes-list-essential-medicines-medical-countermeasures-critical-inputs-required-executive (accessed on 28 March 2021).
- National Medical Stockpile the National Medical Stockpile Is a Strategic Reserve of Drugs, Vaccines, Antidotes and Personal Protective Equipment for Use in National Health Emergencies. We Emergency and Able to Meet High Levels of Demand. 2021; 19–21 April. Database TGA. Available online: https://www.health.gov.au/initiatives-and-programs/national-medical-stockpile (accessed on 8 May 2021).
- Therapeutic Goods Administration, Exporting Therapeutic Goods. Available online: https://www.tga.gov.au/exporting-therapeutic-goods-0 (accessed on 25 May 2021).
- 44/2004 of the Minister for Health, Labour Affairs and Family on the Prescription and Dispensing of Medicinal Products for Human Use. 2020, pp. 1–30. Available online: https://net.jogtar.hu/jogszabaly?docid=a0400044.esc (accessed on 8 May 2021).
- Government Regulation 448/2017. On Authorisation of the Prescription and Individual Use of Medicinal Products for Human Use. Paragraph 5(1). 2020, pp. 1–8. Available online: https://net.jogtar.hu/jogszabaly?docid=A1700448.KOR (accessed on 11 March 2021).
- King of Spain. Royal Legislative Decree 1/2015, of July 24, Approving the Consolidated Text of the Law on Guarantees and Rational Use of Medicines and Medical Devices. 2021, pp. 1–163. Available online: https://www.global-regulation.com/translation/spain/615883/royal-legislative-decree-1-2015%252c-of-july-24%252c-approving-the-revised-text-of-the-law-on-guarantees-and-rational-use-of-medicines-and-medical-devices.html (accessed on 14 May 2021).
- Royal Decree 177/2014 of 21 March, the Reference Price System and Homogeneous Groups of Medicines in the National Health System Is Regulated, and Certain Information Systems on Financing and Price. 2021, pp. 1–17. Available online: https://www.global-regulation.com/translation/spain/1457557/royal-decree-177-2014-of-21-march%252c-the-reference-price-system-and-homogeneous-groups-of-medicines-in-the-national-health-system-is-regulated%252c-and-.html (accessed on 12 March 2021).
- Guidance on Detection and Notification of Shortages of Medicinal Products for Marketing Authorisation Holders (MAHs) in the Union (EEA). Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidance-detection-notification-shortages-medicinal-products-marketing-authorisation-holders-mahs_en.pdf (accessed on 15 March 2022).
- Good Practice Guidance for Communication to the Public on Medicines’ Availability Issues Recommendations for EU National Competent Authorities and EMA to Ensure Adequate Public Information. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/good-practice-guidance-communication-public-medicines-availability-issues_en.pdf (accessed on 15 March 2022).

| Country | Allocation of Critical Shortages |
|---|---|
| Switzerland | If there is no domestic alternative [13]. |
| Spain | If there is no domestic alternative [19]. |
| Hungary | If there is no domestic alternative [20]. |
| US | The “shortage risk index” was defined with the ratio of unavailable and available presentations. If this number is higher than 5, the shortage is considered critical. If the database states: “there are no presentations available” or “there is insufficient supply for usual ordering”, despite that the index will be lower than 5, the shortage had been considered critical. |
| Belgium | According to the FAMHP decision tree and in case it is necessary to import from abroad [21]. |
| Australia | According to the TGA definition, if the medicine is included on the Medicines Watch List (MWL) or if the shortage has the potential impact to have a life-threatening or serious impact on patients and there is not likely to be a sufficient supply of potential substitutes automatically considered as critical [22]. |
| Country | Manager of Database | Definition of Shortage | Source of Information | Scope of Report | Countermeasures |
|---|---|---|---|---|---|
| Belgium | Federal Agency for Medicines and Health Products (FAMHP) | Unable to deliver for an uninterrupted period of four days [24]. | MAH should notify the FAMHP within 7 days after the start of the unavailability when a drug will be unavailable for longer than 14 days [21]. | Concerns about a certain presentation. The entire range of medicine is not unavailable. | Emergency import Greater responsibility to full-line distributors MAH should give an exact reason and compensate costs |
| Switzerland | Martinelli Consulting GmbH | Supplies not satisfying demand and orders [13]. | The website is based on voluntary reports from companies and users [13]. | Drugs officially approved in Switzerland are listed in Martinelli database. | Emergency import Strategic stockpiling Define essentiality |
| Spain | Spanish Agency of Medicines and Medical Products (AEMPS) | The number of available units is below the level of national or local consumption needs [25]. | AEMPS database lists current or anticipated supply problems for different presentations. If a quick solution is expected, not included in the list [19]. | Concerns about a certain presentation. The entire range of medicine is not unavailable [26]. | Define essentiality Emergency import Maintain MA and production Delivery in 24 working hours Export ban |
| US | American Society of Health-System Pharmacists (ASHP) | Supply issue that affects how a pharmacy prepares, dispenses a drug, or influences patient care when prescribers must use an alternative agent [10]. | Voluntary reports from practitioners, patients, and others [12]. | ASHP lists every drug shortage reported through the online report form as soon as it is investigated and confirmed, usually within 24–72 h [12]. | Define essentiality Emergency import Reevaluates voluntary recalls Expedite changes Maintain MA and production Drugs into smaller units ASHP management practice |
| Australia | Therapeutic Goods Administration (TGA) | The supply of medicine will not (or may not) meet the demand for the medicine at the subsequent six months, including all patients who take (may need to take) [27]. | MAHs are required to report all registered medicines in 2–10 days upon severity [22]. | The medicines are set out in Therapeutic Goods Determination [28]. | Emergency import Export registration National Medical Stockpile [29] Medicine Watch List (MWL) [30]. |
| Hungary | National Institute of Pharmacy and Nutrition (OGYEI) | If the MAH is unable to maintain adequate and continuous supplies of specific medicinal products, or unwilling to supply the preparation temporarily or permanently [31]. | Before the final delivery to the wholesaler, but in a maximum of two months [31]. | Concerns about a certain presentation. The entire range of medicine is not unavailable. | Recommending alternatives Shortage declaration by CA Emergency import Export ban |
| Country | Date | No. of Shortages (a) | Considered as Critical (b) | Population in Millions (c) | Shortage per Million People (d = a/c) | Critical Shortage per Million People (e = b/c) | Percentage of Critical/All Shortages (f = e/d) |
|---|---|---|---|---|---|---|---|
| Belgium | 4 Mar 2021 | 227 | 41 | 11.6 | 19.57 | 3.53 | 18.1 |
| Spain | 6 Mar 2021 | 267 | 51 | 46.7 | 5.72 | 1.09 | 19.1 |
| Hungary | 9 Mar 2021 | 250 | 63 | 9.6 | 26.04 | 6.56 | 25.2 |
| Switzerland | 6 Mar 2021 | 173 | 38 | 8.7 | 19.89 | 4.37 | 22.0 |
| Average of examined European countries | - | 229.25 | 48.25 | - | 17.805 * | 3.89 | 21.1 |
| US | 9 Mar 2021 | 683 | 93 | 332.9 | 2.05 | 0.28 | 13.6 |
| Australia | 8 Mar 2021 | 244 | 25 | 25.8 | 9.46 | 0.97 | 10.2 |
| Critical_BI | Proportion | Std. Err. | Logit (95% Conf. Interval) | ||
|---|---|---|---|---|---|
| 0 | 0.8313449 | 0.0087199 | 0.8135458 | 0.8477629 | |
| 1 | 0.1686551 | 0.0087199 | 0.1522371 | 0.1864542 | |
| ATC | Number of All Shortages (N) | Observed (k) | Expected (k) | Expected (p) | Observed (p) |
| C | 639 | 55 | 107.7706089 | 0.16866 | 0.08607 |
| J | 369 | 105 | 62.2337319 | 0.16866 | 0.28455 |
| L | 176 | 39 | 29.6832976 | 0.16866 | 0.22159 |
| N | 660 | 112 | 111.312366 | 0.16866 | 0.16970 |
| Belgium | Switzerland | Spain | Hungary | Average of Examined European Countries | US | Australia | |
|---|---|---|---|---|---|---|---|
| No. of critical shortages/million people | 3.53 | 4.37 | 1.09 | 6.56 | 3.89 | 0.28 | 0.97 |
| No. of critical shortages on WHO Essential List/million people | 3.19 | 1.61 | 0.75 | 3.96 | 2.38 | 0.28 | 0.74 |
| WHO Essential/all critical shortages (%) | 90.36 | 36.82 | 68.76 | 60.34 | 64.07 | 45.06 | 75.92 |
| Compulsory Stockpiling | Measures for Essential Medicines—Legal Definitions | Notification Responsibilitiy—Legal Background | Measures Affecting Wholesalers | Export Bans | Emergency Imports | |
|---|---|---|---|---|---|---|
| BE | Full-line wholesalers required to have a range of products in stock for the needs of the given geographic territory/ies [36]. | Not defined. | Not providing the exact reasons is considered a clear legal violation [37]. | Full-line wholesalers are assigned besides regular ones with special responsibilities and privileges [36,38]. | Temporarily to medicinal products for which a shortage is notified [37]. | Based on a doctor’s request wholesalers may temporarily import medicine from the EU, if no substitutes are available in BE, in specific quantities requested by the doctor [39]. |
| CH | Delegated to companies at a defined level of stocks in Ordinance [40]. | Compounds for which there are no or only limited substitutes have been affected by a supply shortage over the previous three years [40]. | Determined in 531.215.32 Ordinance [40]. | Managing the strategic level of inventory stock delegated by the federal government in local law [40]. | Parallel export does not become significant due to high prices [41]. | Upon application for temporary import submission [42]. |
| ES | CA may demand commercialization to grant the suspension, or the revocation of the product [43]. | Essential if the pharmaceutical gap cannot fully cover or have a high economic impact. Critical if it has no available therapeutic alternatives and has a complex manufacturing process, and/or has only one supplier [38]. | Spanish Medicine and Products Devices Agency Circular No. 3/2011 [44]. | All wholesalers are required to deliver within 24 working hours [38]. | If lack of medicinal products causes a pharmaceutical gap [45]. | The Spanish Agency of Medicinal Products and Medical Devices can also approve the import of medicines labeled in other languages or with an expiry date shorter than 6 months [38]. |
| US | FDA supports MAHs to maintain production [46]. | If used to treat or prevent a serious disease or condition, and there is no other adequate available source [47]. | Safety and Innovation Act in 2012 [8]. | * Not applicable. | * Not applicable. | FDA may allow emergency importation [46]. |
| A | National Medical Stockpile maintains the strategic reserve of products [48]. | Included on Medicines Watch List, has a potential life-threatening or serious impact, or has no potential substitutes [22]. | Therapeutic Goods (Reportable Medicines) Determination 2018 [28]. | Wholesalers also have a duty to notify authorities about the expected duration of a discontinuation [29]. | Only for MAH or designated entity [49]. | Therapeutic Goods Act has been amended to assist import [27]. |
| HU | Products decreed by the minister should be available in the quantity defined therein [31]. | Not defined. | Act XCV of 2005 on Medicinal Products for Human Use [31]. | Authorized wholesale distributors shall be required to procure and supply the medicinal products as their authorization for wholesale distribution pertains [31]. | The active substances decreed by the minister for a period not exceeding one year [31]. | On the wholesaler’s request “contingent-approval” [50] or the physician statement “individual approval of OGYEI” [51]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turbucz, B.; Major, M.; Zelko, R.; Hanko, B. Proposal for Handling of Medicine Shortages Based on a Comparison of Retrospective Risk Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4102. https://doi.org/10.3390/ijerph19074102
Turbucz B, Major M, Zelko R, Hanko B. Proposal for Handling of Medicine Shortages Based on a Comparison of Retrospective Risk Analysis. International Journal of Environmental Research and Public Health. 2022; 19(7):4102. https://doi.org/10.3390/ijerph19074102
Chicago/Turabian StyleTurbucz, Bela, Martin Major, Romana Zelko, and Balazs Hanko. 2022. "Proposal for Handling of Medicine Shortages Based on a Comparison of Retrospective Risk Analysis" International Journal of Environmental Research and Public Health 19, no. 7: 4102. https://doi.org/10.3390/ijerph19074102






