Types and Distribution of Organic Amines in Organic Nitrogen Deposition in Strategic Water Sources
Abstract
:1. Introduction
2. Materials and Methods
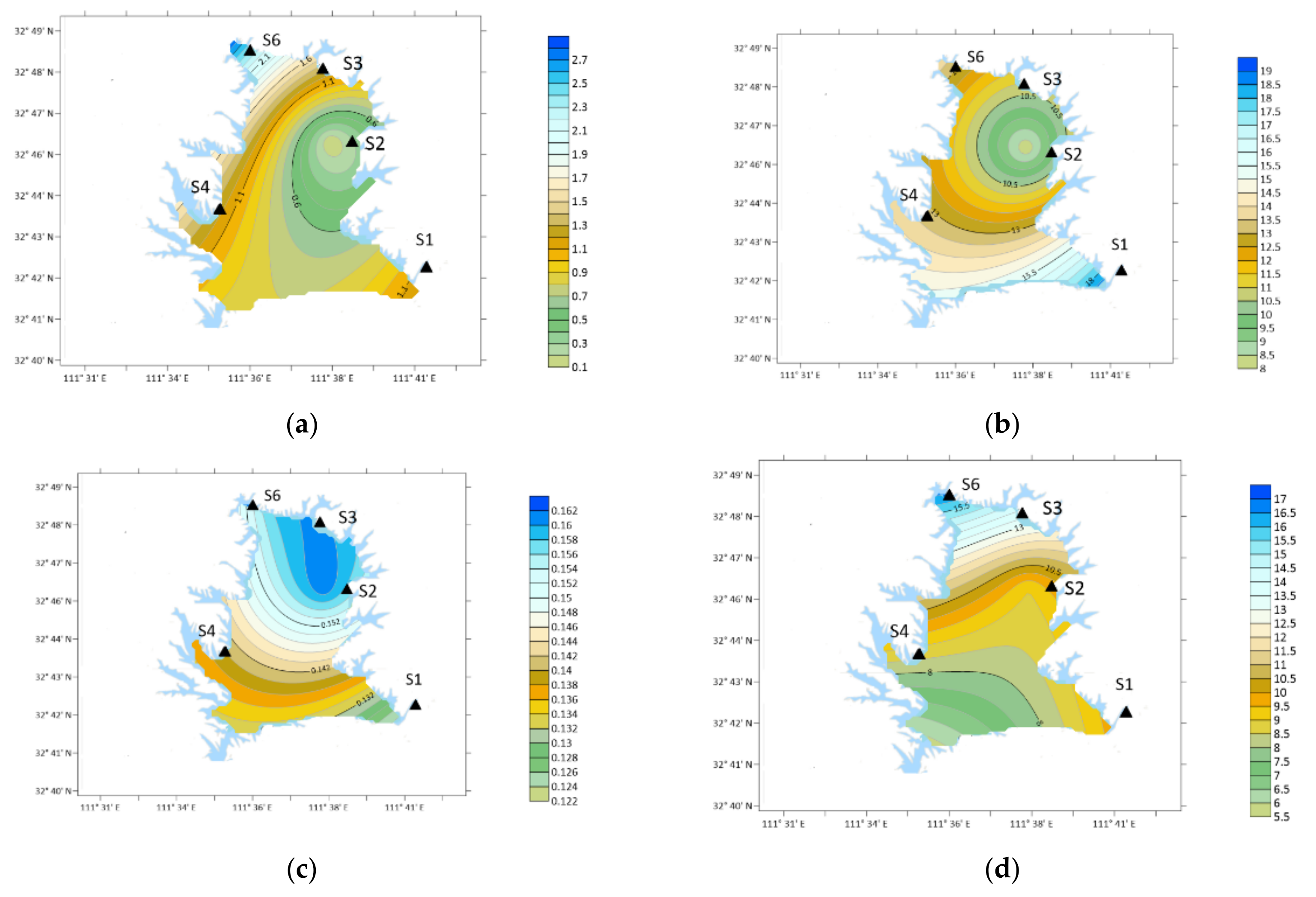
2.1. Sampling Point Locations and Functional Areas
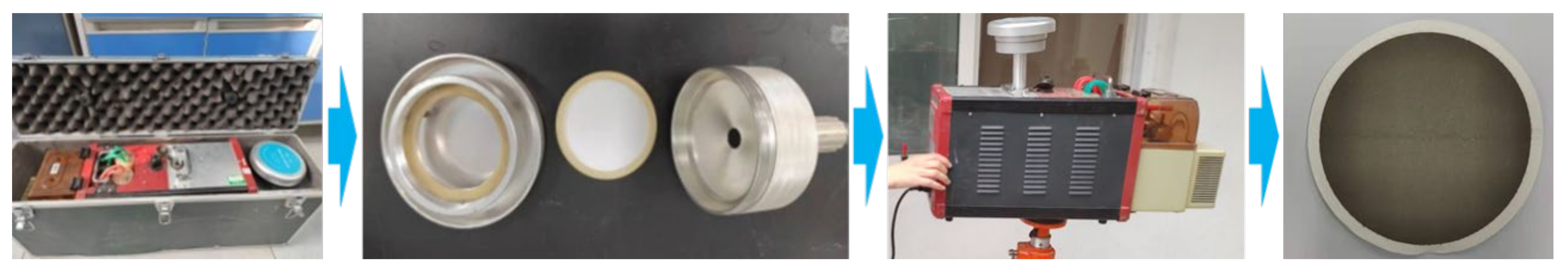
2.2. Sample Collection
2.3. Sample Pretreatment
2.4. Preparation of Standard Sample Solution
2.5. Chromatographic Conditions
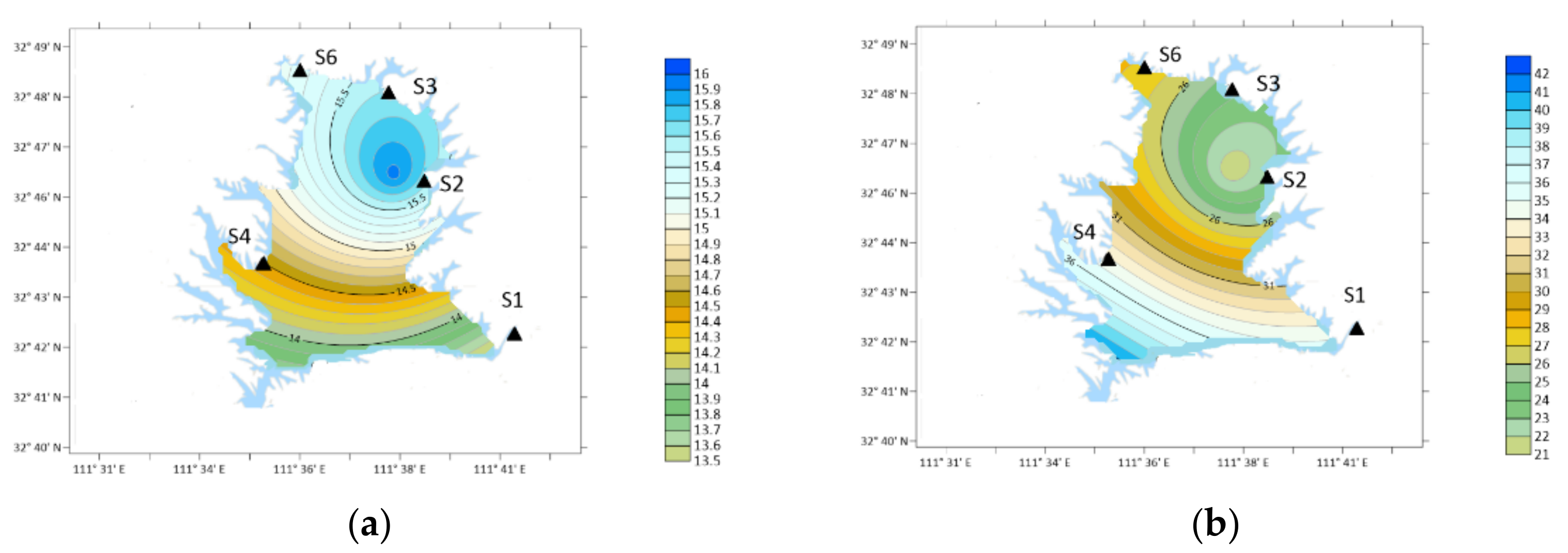
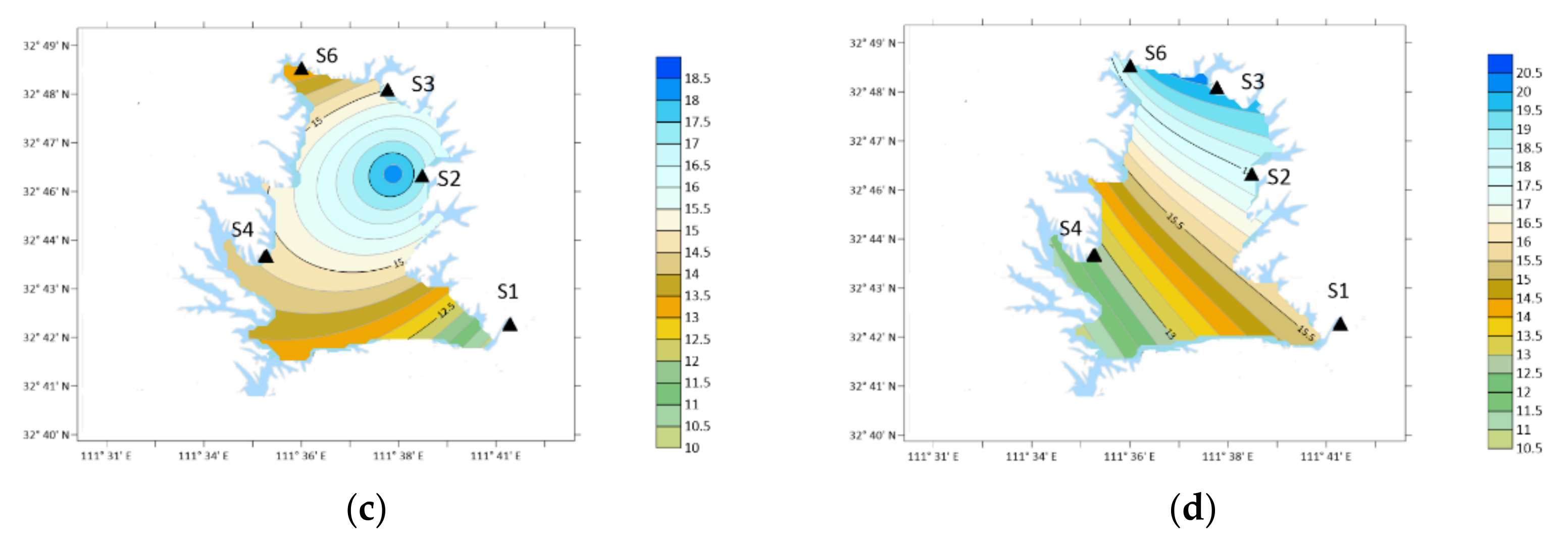
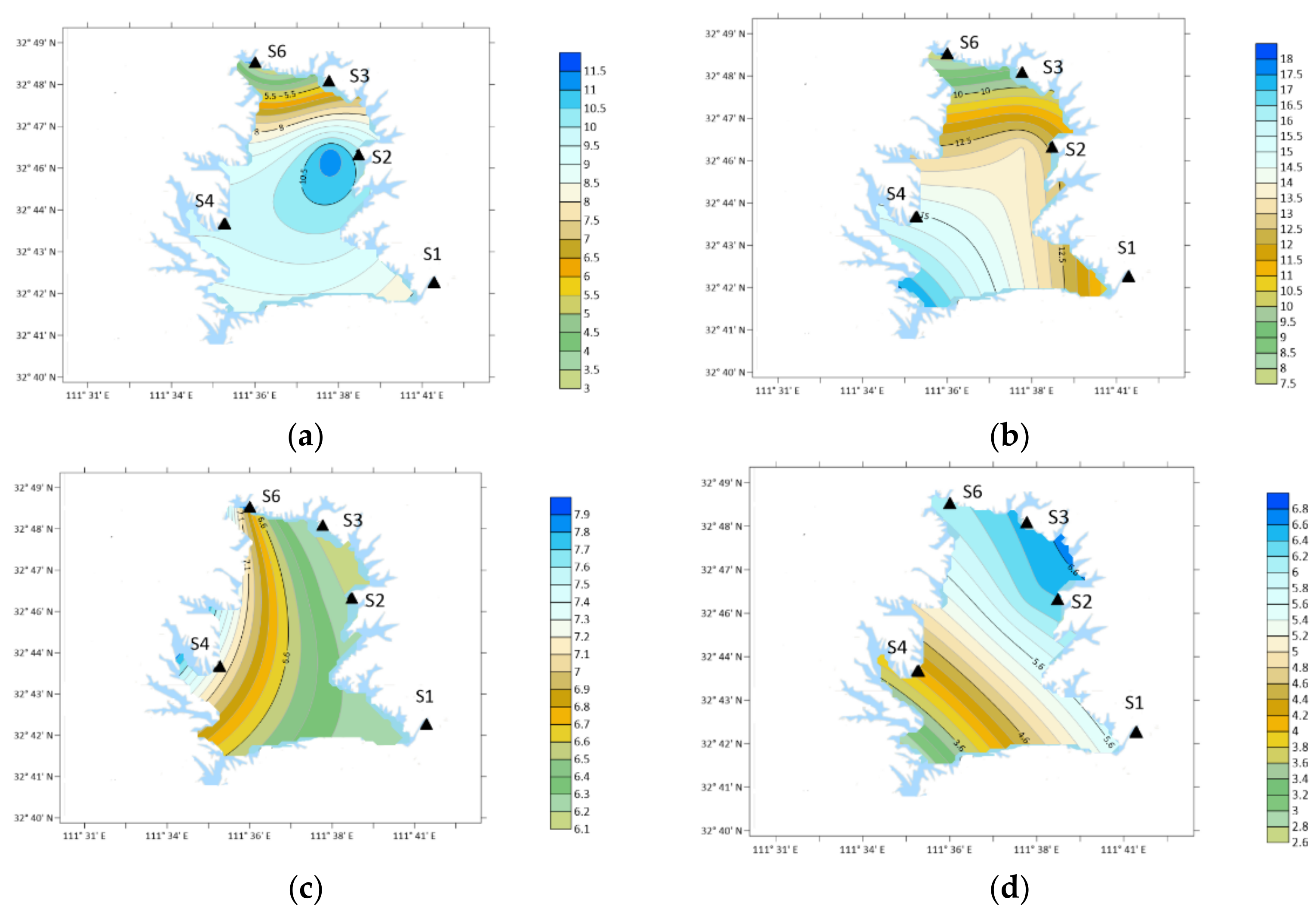
3. Results
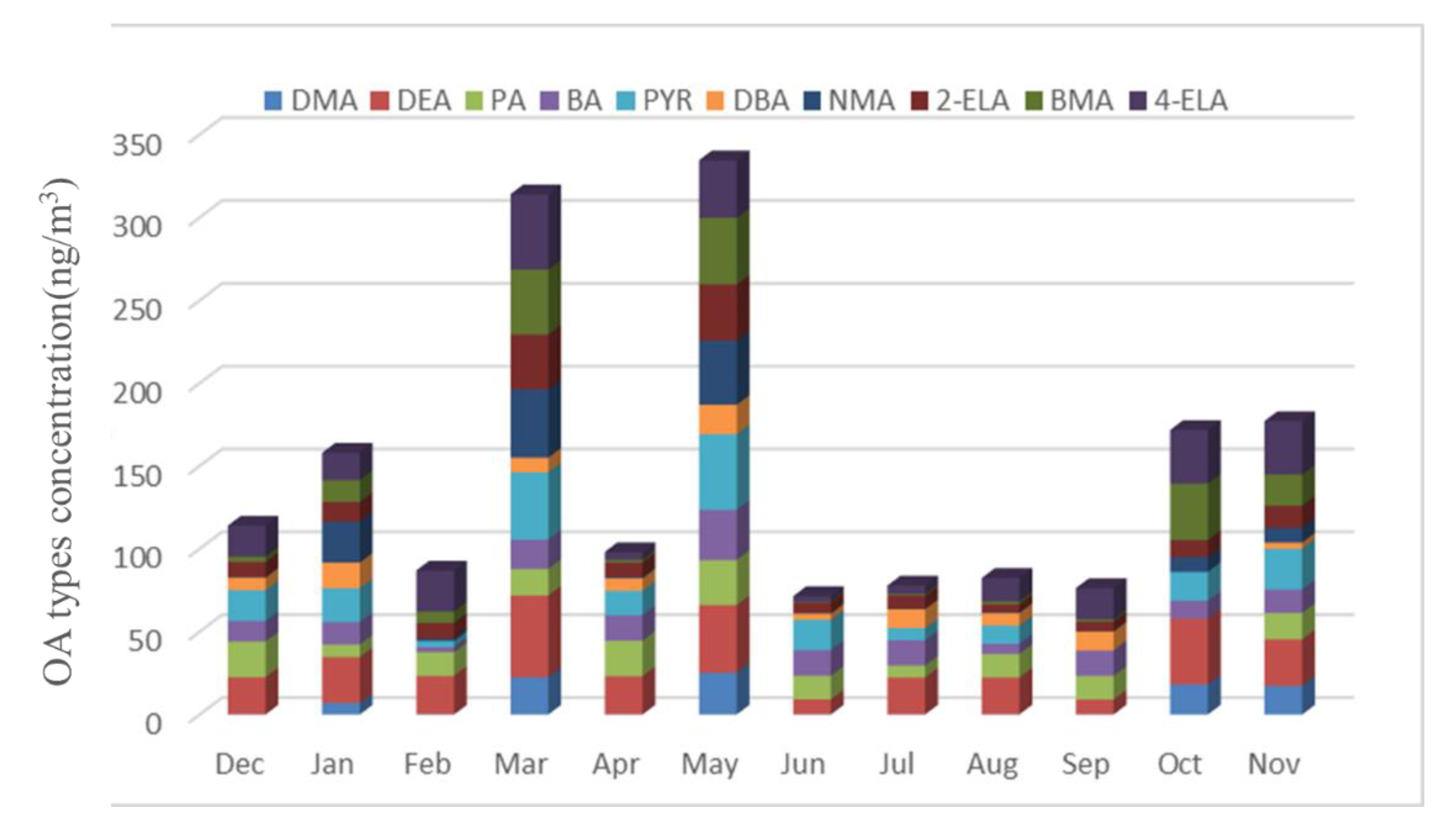
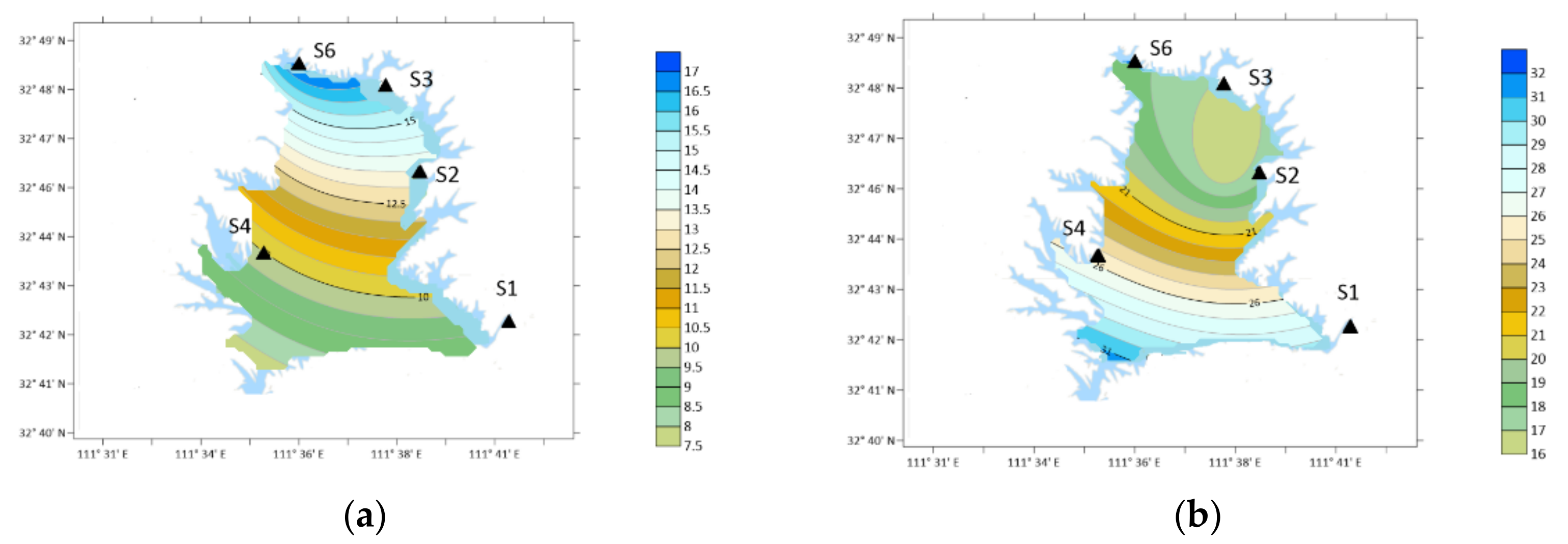
3.1. OA Types and Concentration
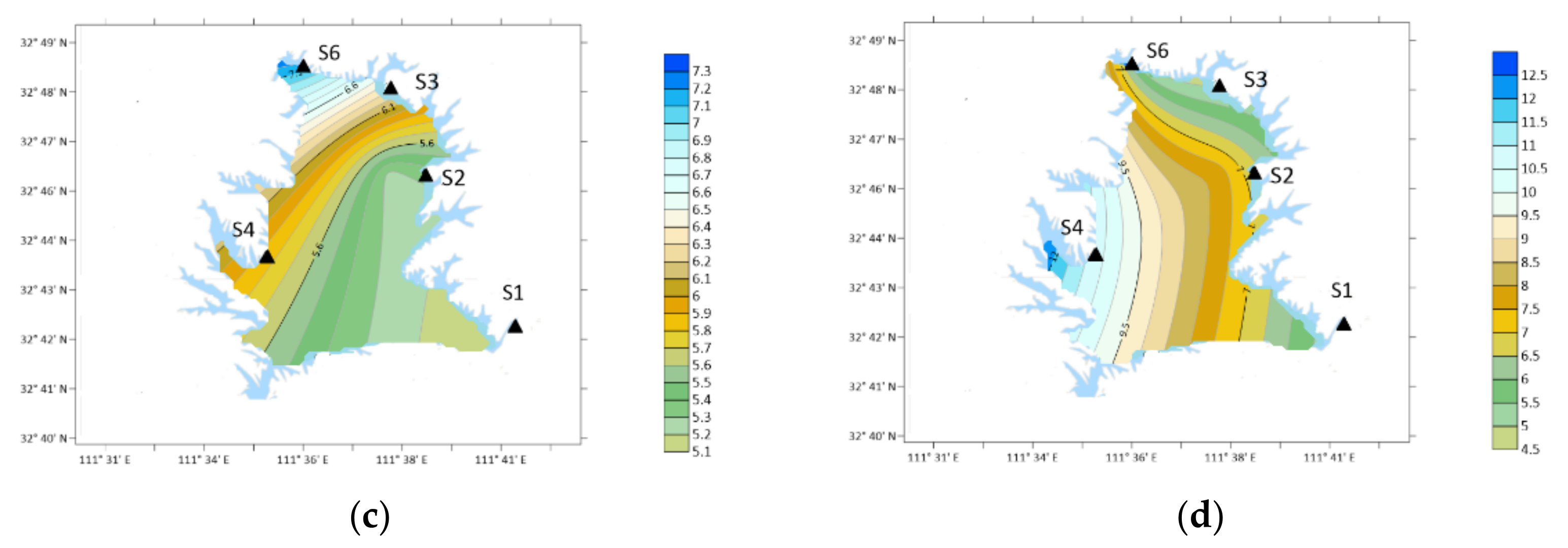
3.2. Characteristics of DMA
3.3. Characteristics of DEA
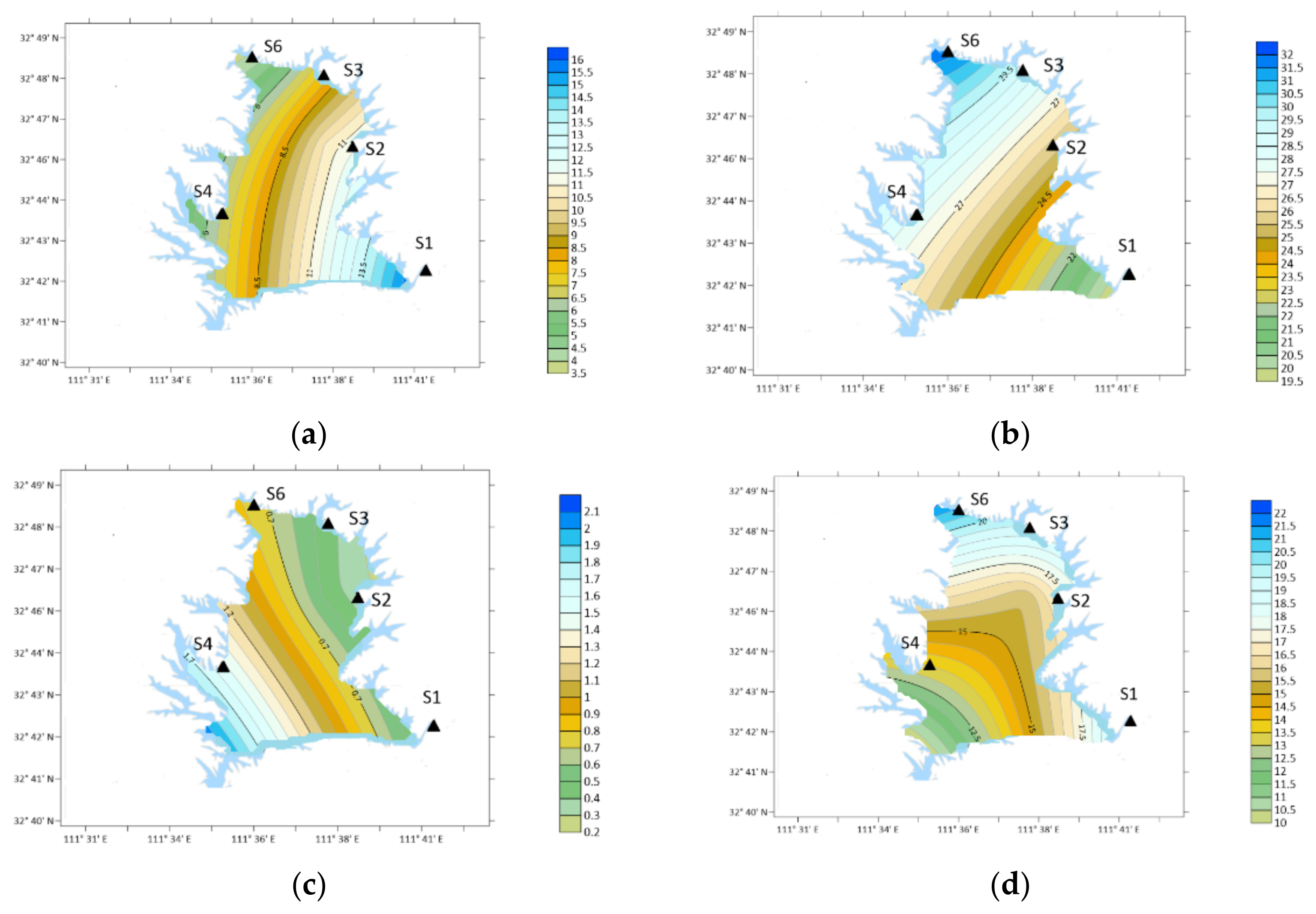
3.4. Characteristics of PA
3.5. Characteristics of BA
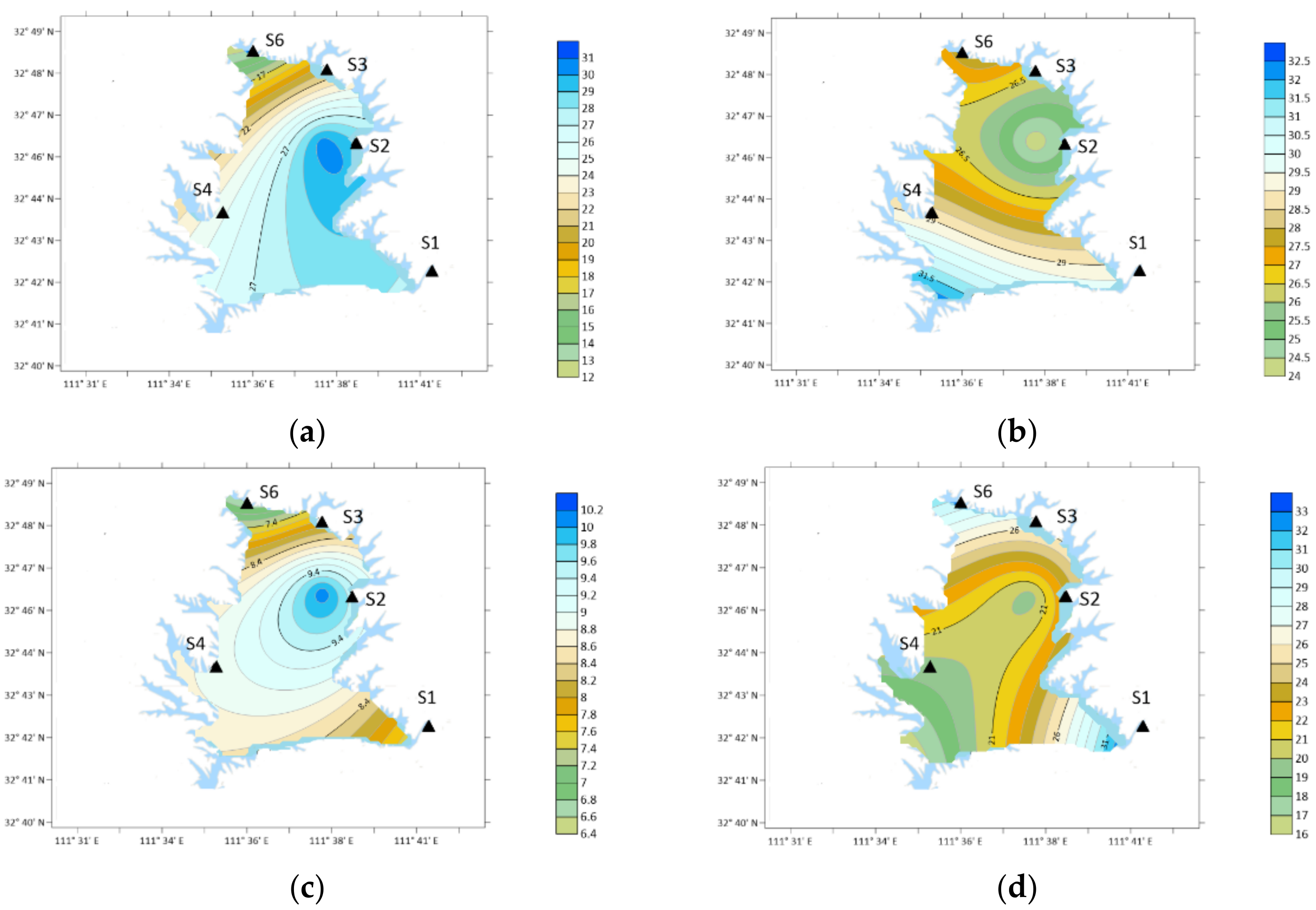
3.6. Characteristics of PYR
3.7. Characteristics of DBA
3.8. Characteristics of NMA
3.9. Characteristics of 2-ELA
3.10. Characteristics of BMA
3.11. Characteristics of 4-ELA
4. Discussion
5. Conclusions
- (1)
- Ten types of OA were detected in the atmospheric particle samples collected in five field monitoring stations around Xichuan Reservoir Area, namely DMA, DEA, PA, BA, PYR, DBA, NMA, 2-ELA, BMA and 4-ELA. The annual average concentrations were 7.64, 26.35, 14.51, 14.10, 18.55, 7.92, 10.56, 12.84, 13.46 and 21.00 ng/m3, respectively.
- (2)
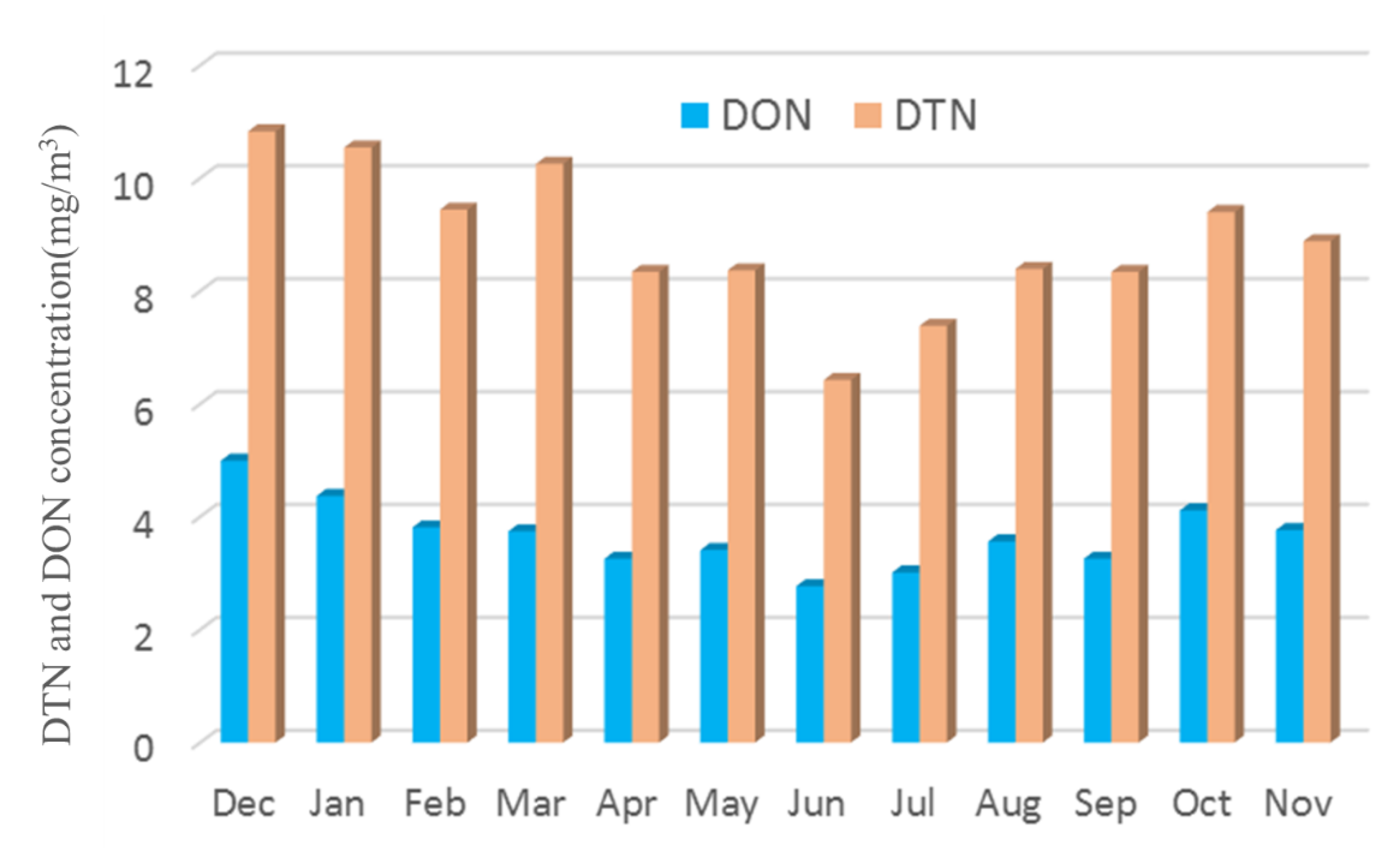
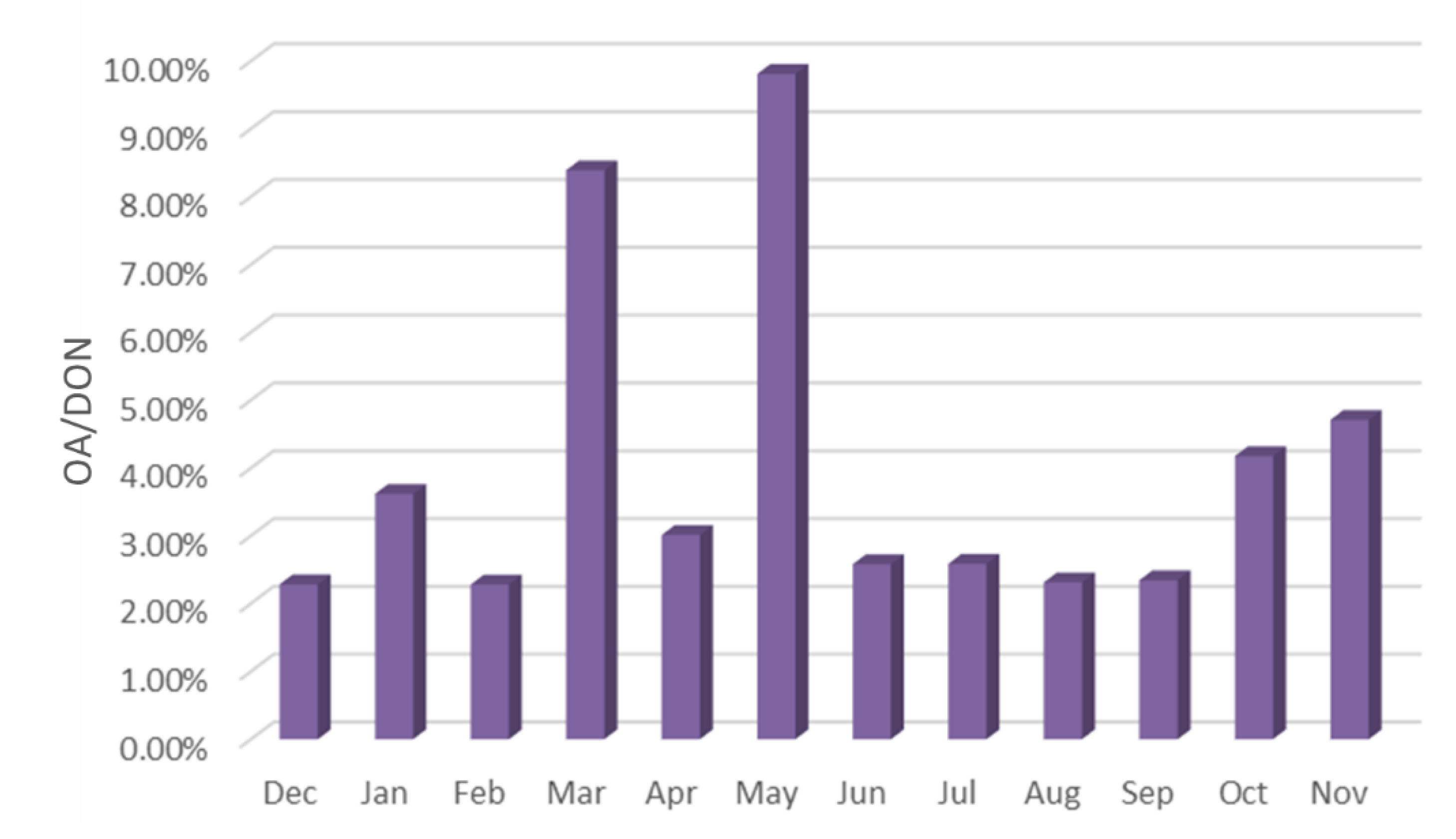
- The proportion of OA in organic nitrogen was different, the total nitrogen concentration was 6.42–10.82 mg/m3, and the DON concentration was 2.77–4.99 mg/m3. The total concentration of ten kinds of OA accounted for 2.28–9.81% of DON in the current month, of which the content of DEA was the highest, reaching 0.71%, the content of 4-ELA, PYR, PA and BA were 0.4–0.56%, and the content of DMA, DBA and NMA was 0.2–0.36%.
- (3)
- The sources of OA in the reservoir area have significant seasonal differences. The content of OA was the highest in spring, followed by autumn, and lower in summer and winter. The rainfall in spring and autumn is small, windy weather is frequent, and the source of road dust is relatively high. Because spring and autumn are the time of frequent agricultural activities, the concentration of OA is significantly higher than that in winter and summer. This shows that agricultural activities have a significant impact on the emission of OA. The rainfall in summer is large, and the particles in the air are washed by rain, resulting in the lowest concentration of OA in the sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhao, T.; Jiao, H.; Wu, L.; Xiao, C.; Guo, X.; Jin, C. Atmospheric Organic Nitrogen Deposition in Strategic Water Sources of China after COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2022, 19, 2734. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.J.; Wright, J.S.; Hall, J.W.; Gong, P.; Ni, S.; Qiao, S.; Huang, G.; et al. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Lu, X.; Tian, D.; Mao, Q.; Jing, X.; Wang, C.; Xia, N. Impacts of nitrogen deposition on forest ecosystems in China. In Atmospheric Reactive Nitrogen in China; Springer: Singapore, 2020; pp. 185–213. [Google Scholar] [CrossRef]
- Shen, H.; Dong, S.; Li, S.; Xiao, J.; Han, Y.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Li, Y.; et al. Effects of simulated N deposition on photosynthesis and productivity of key plants from different functional groups of alpine meadow on Qinghai-Tibetan plateau. Environ. Pollut. 2019, 251, 731–737. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Zhang, F.; Xu, W.; Liu, X.; Wang, Z.; Xie, Y. Soil Nitrous Oxide Emissions by Atmospheric Nitrogen Deposition over Global Agricultural Systems. Environ. Sci. Technol. 2021, 55, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Köchy, M.; Wilson, S. Nitrogen deposition and forest expansion in the northern Great Plains. J. Ecol. 2001, 89, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Huang, X.; Huang, C.; Yang, Y.; Yang, H.; Zhang, J.; Huang, T. High atmospheric wet nitrogen deposition and major sources in two cities of Yangtze River Delta: Combustion-related NH3 and non-fossil fuel NOx. Sci. Total Environ. 2022, 806, 150502. [Google Scholar] [CrossRef]
- Chen, F.; Xu, B.; Jiao, H.; Chen, X.; Shi, Y.; Wang, J.; Li, Z. Triaxial mechanical properties and microstructure visualization of BFRC. Constr. Build. Mater. 2021, 278, 122275. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Liu, X.; Xu, W.; Wen, Z. Atmospheric nitrogen deposition around the Dongting Lake, China. Atmos. Environ. 2019, 207, 197–204. [Google Scholar] [CrossRef]
- He, Y.; Zheng, Y.; Li, W.; Zhang, Z.; Ma, Y.; Zhao, T.; Ren, Y. Characteristics of eukaryotic phytoplankton community structure in early spring and its relationship with environmental factors in Danjiangkou Reservoir. Acta Sci. Circumst. 2021, 41, 2192–2200. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Ren, J.; Zhang, X.; Jiang, L. Nitrogen input increases Deyeuxia angustifolia litter decomposition and enzyme activities in a marshland ecosystem in Sanjiang plain. northeast China. Wetlands 2019, 39, 549–557. [Google Scholar] [CrossRef]
- Ackerman, D.; Millet, D.; Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Global Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Jickells, T.; Baker, A.R.; Cornell, C.; Nemitz, E. The cycling of organic nitrogen through the atmosphere. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130115. [Google Scholar] [CrossRef]
- Jiao, H.; Chen, W.; Wu, A.; Yu, Y.; Ruan, Z.; Honaker, R.; Chen, X.; Yu, J. Flocculated unclassified tailings settling efficiency improvement by particle collision optimization in the feedwell. Int. J. Miner. Metall. Mater. 2022, 44, 553. [Google Scholar] [CrossRef]
- Ge, X.; Wexler, A.S.; Clegg, S.L. Atmospheric amines—Part I. A review. Atmos. Environ. 2011, 45, 524–546. [Google Scholar] [CrossRef]
- Nie, E.; Zheng, G.; Gao, D.; Chen, T.; Yang, J.; Wang, Y. Emission characteristics of VOCs and potential ozone formation from a full-scale sewage sludge composting plant. Sci. Total Environ. 2019, 659, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Wu, Y.; Wang, H.; Chen, X.; Li, Z.; Wang, Y.; Zhang, B.; Liu, J. Micro-scale mechanism of sealed water seepage and thickening from tailings bed in rake shearing thickener. Miner. Eng. 2021, 173, 107043. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, Y.; Li, H.; Zeng, H.; Chen, W. The preconcentration, detection methods and degradation mechanisms of N-nitrosamine in soil: A review. Environ. Chem. 2021, 40, 3082–3092. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, T.; Jiao, H.; Wang, Y.; Li, H. Potential effect of porosity evolution of cemented paste backfill on selective solidification of heavy metal ions. Int. J. Environ. Res. Public Health 2020, 17, 814. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Wang, X.; Yang, F.; Bai, K.; Wu, C.; Liu, S. Particulate Amines in the Background Atmosphere of the Yangtze River Delta, China: Concentration, Size Distribution, and Sources. Adv. Atmos. Sci. 2021, 38, 1128–1140. [Google Scholar] [CrossRef]
- He, Y.; Su, X.; Ren, Y.; Wang, X.; Ouyang, Z. Spatiotemporal differentiation of urban water resource utilization efficiency of eco-geographic regions in China. Acta Ecol. Sin. 2020, 40, 20. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, A.K.; Kumar, A.; Tripathi, R.; Shahid, M.; Bhattacharyya, P.; Raja, R.; Panda, B.B. Carbon and nitrogen mineralization kinetics in soil of rice–rice system under long term application of chemical fertilizers and farmyard manure. Eur. J. Soil Biol. 2013, 58, 113–121. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Chen, J.; Qin, D.; Ji, Y.; Li, J.; An, T. Mechanism of atmospheric organic amines reacted with ozone and implications for the formation of secondary organic aerosols. Sci. Total Environ. 2020, 737, 139830. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xu, Y. Effects of relative humidity on ozone and secondary organic aerosol formation from the photooxidation of benzene and ethylbenzene. Aerosol Sci. Tech. 2014, 48, 1–12. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Tang, G.; Wu, D. Wet and dry deposition of atmospheric nitrogen at ten sites in Northern China. Atmos. Chem. Phys. 2012, 12, 6515–6535. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Shen, Z.; Cao, J.; Li, X.; Zhao, J.; Liu, P.; Wang, Y.; Zhou, J. Seasonal Variations of Water-Soluble Inorganic Ions in Atmospheric Particles Over Xi’an. Environ. Chem. 2009, 28, 261–266. [Google Scholar] [CrossRef]
- Schade, G.W.; Crutzen, P.J. Emission of aliphatic amines from animal husbandry and their reactions: Potential source of N2O and HCN. J. Atmos. Chem. 1995, 22, 319–346. [Google Scholar] [CrossRef]
- Zhang, Q. The South-to-North Water Transfer Project of China: Environmental Implications and Monitoring Strategy. J. Am. Water Resour. Assoc. 2009, 45, 1238–1247. [Google Scholar] [CrossRef]
- Tai, C.; Zhang, K.; Zhou, T.; Zhao, T.; Wang, Q.; He, X. Distribution Characteristics of Polycyclic Aromatic Hydrocarbons in Runoff from the Middle Line Source Area of South-to-North Water Diversion Project. Environ. Sci. 2011, 32, 1932–1939. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China Home Page. Available online: https://www.mee.gov.cn/hjzl/shj/dbsszyb/ (accessed on 14 March 2022).
- Liu, F.; Bi, X.; Zhang, G.; Lian, X.; Fu, Y.; Yang, Y.; Lin, Q.; Jiang, F.; Wang, X.; Sheng, G. Gas-to-particle partitioning of atmospheric amines observed at a mountain site in southern China. Atmos. Environ. 2018, 195, 1–11. [Google Scholar] [CrossRef]
- Shen, W.; Ren, L.; Zhao, Y.; Zhou, L.; Dai, L.; Ge, X.; Kong, S.; Yan, Q.; Xu, H.; Jiang, Y.; et al. Corrigendum to “C1-C2 alkyl aminiums in urban aerosols: Insights from ambient and fuel combustion emission measurements in the Yangtze River Delta region of China”. Environ. Pollut. 2020, 257, 113741. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.F.; Ho, S.S.H.; Huang, R.J.; Chuang, H.C.; Cao, J.J.; Han, Y.; Lui, K.H.; Ning, Z.; Chuang, K.J.; Cheng, T.J.; et al. Chemical composition and bioreactivity of PM2.5 during 2013 haze events in China. Atmos. Environ. 2016, 126, 162–170. [Google Scholar] [CrossRef]
- Yu, P.; Hu, Q.; Li, K.; Zhu, Y.; Liu, X.; Gao, H.; Yao, X. Characteristics of dimethylaminium and trimethylaminium in atmospheric particles ranging from supermicron to nanometer sizes over eutrophic marginal seas of China and oligotrophic open oceans. Sci. Total Environ. 2016, 572, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.F.; Ho, S.S.H.; Huang, R.J.; Liu, S.X.; Cao, J.J.; Zhang, T.; Chuang, H.C.; Chan, C.S.; Hu, D.; Tian, L. Characteristics of water-soluble organic nitrogen in fine particulate matter in the continental area of China. Atmos. Environ. 2015, 106, 252–261. [Google Scholar] [CrossRef]
- Akyüz, M. Simultaneous determination of aliphatic and aromatic amines in ambient air and airborne particulate matters by gas chromatography-mass spectrometry. Atmos. Environ. 2008, 42, 3809–3819. [Google Scholar] [CrossRef]
- Calderón, S.; Poor, N.; Campbell, S. Estimation of the particle and gas scavenging contributions to wet deposition of organic nitrogen. Atmos. Environ. 2007, 41, 4281–4290. [Google Scholar] [CrossRef]
- Yang, H.; Xu, J.; Wu, W.S.; Wan, C.; Yu, J. Chemical Characterization of Water-Soluble Organic Aerosols at Jeju Island Collected During ACE-Asia. Environ. Chem. 2004, 1, 13–17. [Google Scholar] [CrossRef]


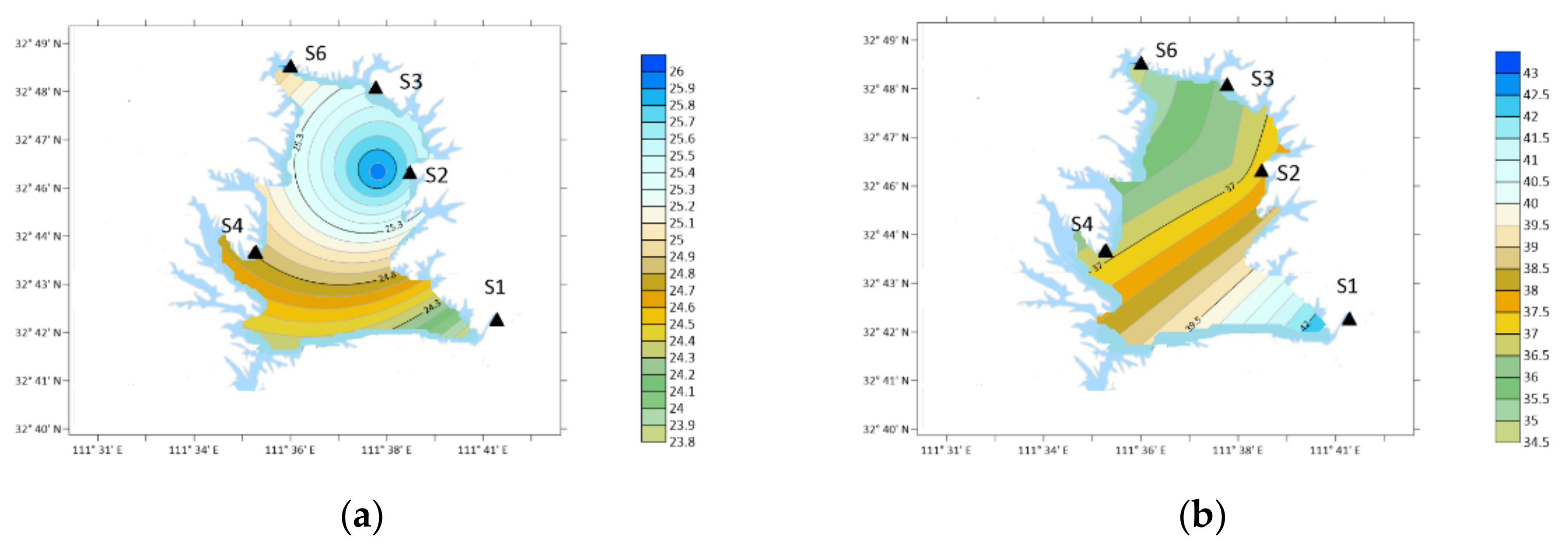
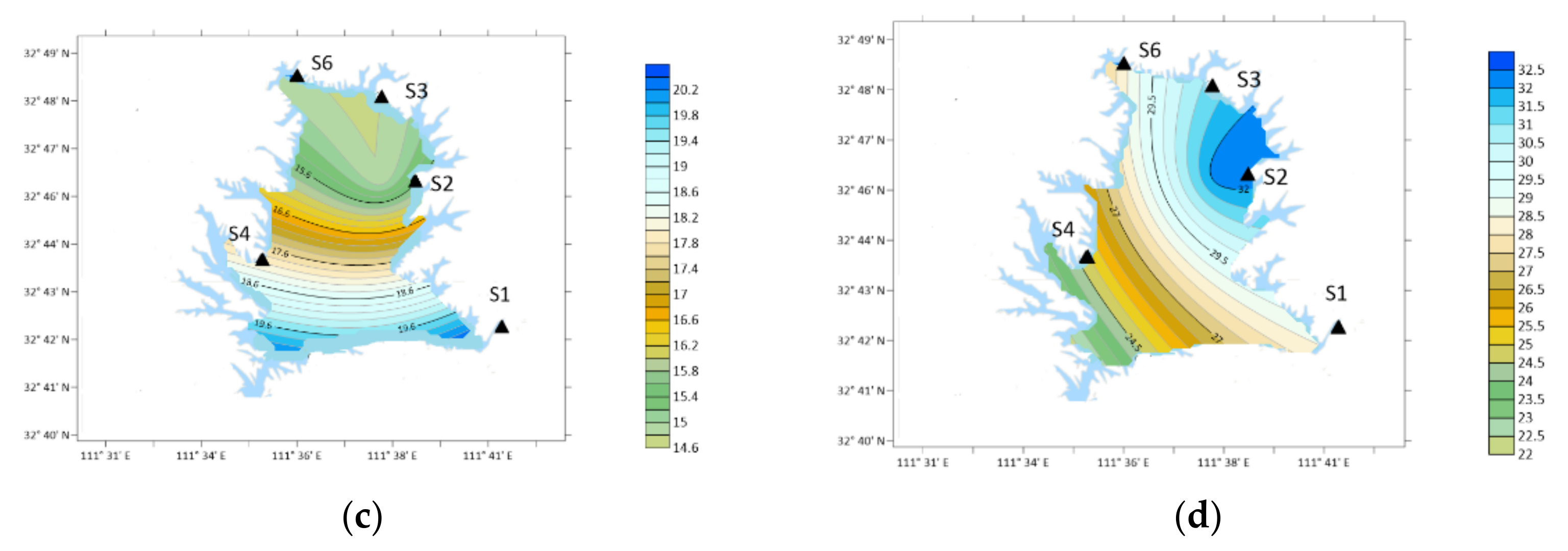
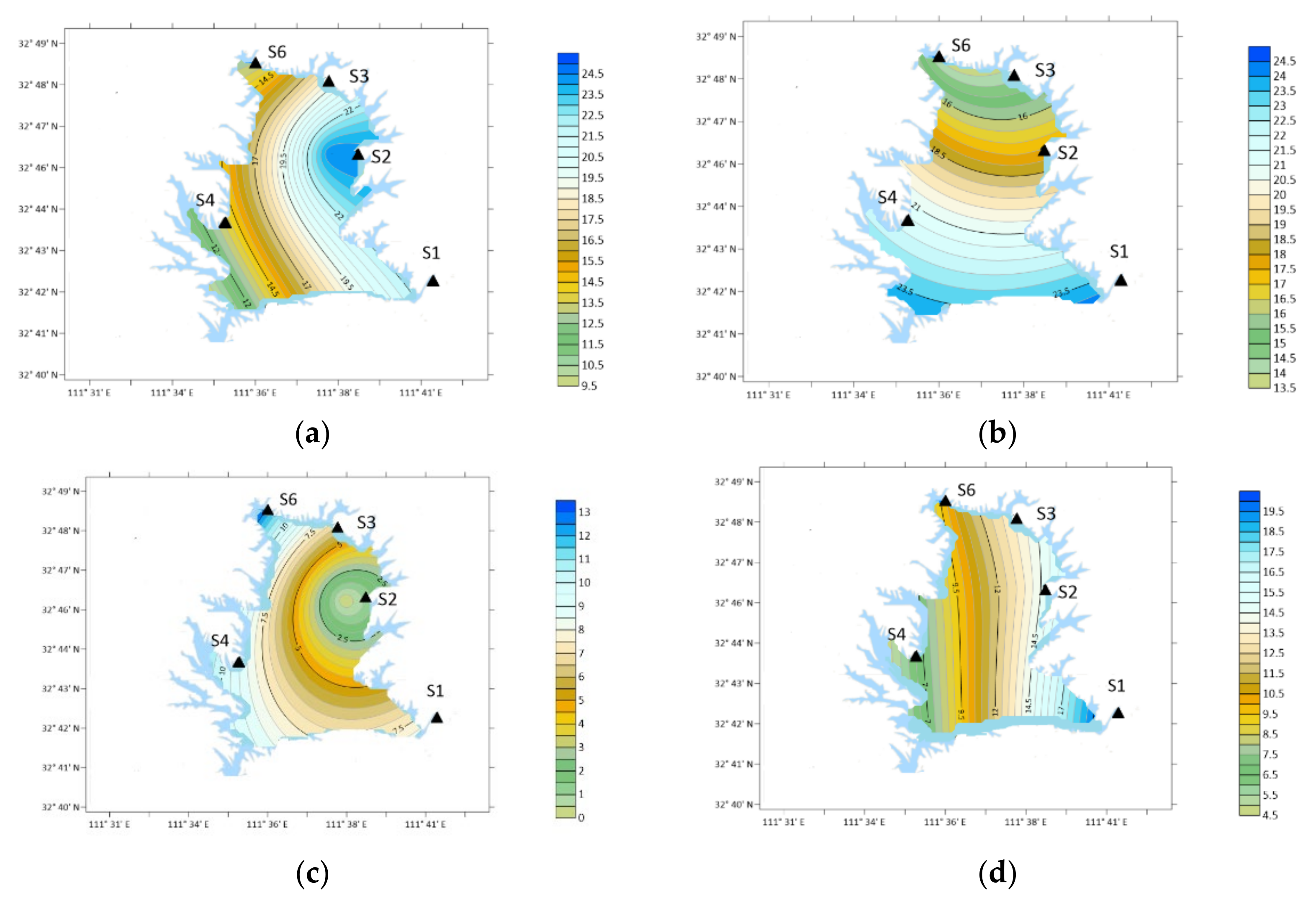
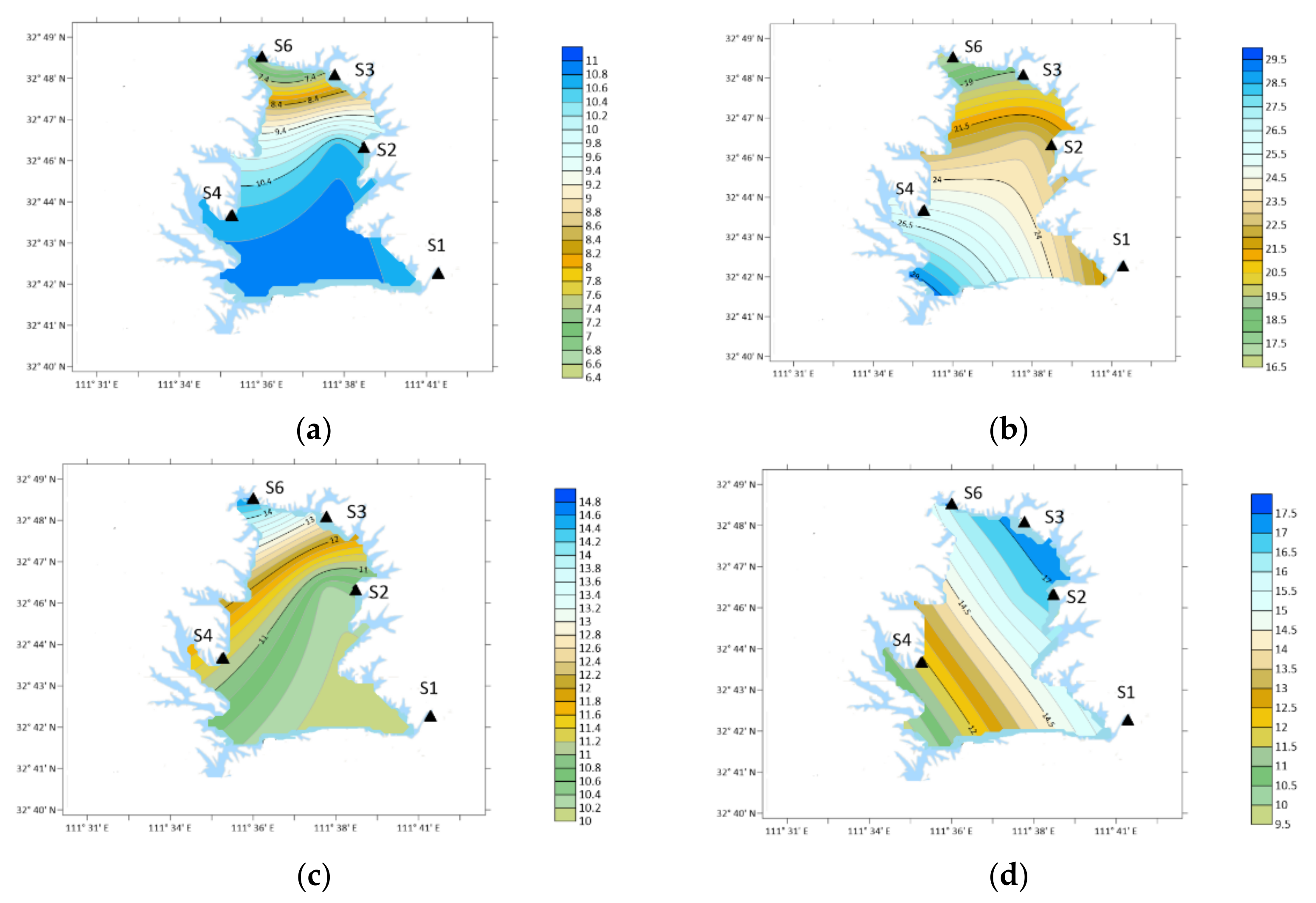

| Place | Sampling Type | Sampling Date (Year~Month) | Concentration/ng·m−3 | Analytical Method | ||||
|---|---|---|---|---|---|---|---|---|
| MA | EA | DMA | TMA | AN | ||||
| Nanjing | Industrial park | 12 2017~01 2018 | 9.6 ± 5 | 2.6 ± 1.4 | 12.6 ± 4.8 | 5.5 ± 2 | 2.5 ± 1.1 | IC |
| 03 2018~04 2018 | 12.3 ± 3.9 | 5.8 ± 1.9 | 15.3 ± 5.6 | 7.6 ± 1.6 | 5.1 ± 1.7 | |||
| 07 2018~08 2018 | 16.9 ± 5.6 | 10.8 ± 4.6 | 26.6 ± 14 | 12.3 ± 3.9 | 10.6 ± 4.2 | |||
| 10 2018~11 2018 | 14.2 ± 9 | 6.9 ± 4 | 22.2 ± 13.6 | 9.5 ± 4.6 | 6.3 ± 4.3 | |||
| Guangzhou | Mountain area | 10 2016 | 8.79 ± 7.80 | - | 2.37 ± 3.15 | - | - | GC—MS [32] |
| 05 2017~06 2017 | 11.9 ± 9.77 | - | 5.03 ± 2.23 | - | - | |||
| Nanjing, Yangzhou | Urban area | 11 2015~04 2016 | 5.7 ± 3.2 | 20.3 ± 16.6 | 7.9 ± 5.4 | - | - | HPLC [33] |
| Beijing | Urban area | 01 2013~02 2013 | 31 ± 10.8 | 14.8 ± 6.5 | 4.3 ± 1.7 | - | 5.7 ± 2.2 | HPLC [34] |
| Xi’an | Urban area | 01 2013~02 2013 | 24.7 ± 9.7 | 12.6 ± 5.6 | 3.8 ± 1.6 | - | 5.1 ± 2.2 | |
| Xiamen | Urban area | 01 2013~02 2013 | 10.2 ± 2.0 | 5.3 ± 1.4 | 1.7 ± 0.4 | - | 2.5 ± 0.8 | |
| HongKong, China | Urban area | 01 2013~02 2013 | 12.1 ± 2.4 | 4.5 ± 1.1 | 1.5 ± 0.5 | - | 2.0 ± 0.6 | |
| Eastern China | Ocean | 08 2015 | - | - | 34.2 ± 5.4 | 55.8 ± 7.8 | - | IC [35] |
| 04 2015 | - | - | 9.9 ± 5.4 | 16.8 ± 9.8 | - | |||
| Xi’an | Urban area | 03 2009~05 2009 | 16.9 ± 5.5 | 9.7 ± 3.9 | - | - | - | HPLC [36] |
| 06 2009~08 2009 | 6.2 ± 2 | 3.8 ± 1.4 | - | - | - | |||
| 09 2008~11 2008 | 14.7 ± 9.9 | 8.4 ± 4.2 | - | - | - | |||
| 12 2008~02 2009 | 22.3 ± 10.4 | 11.5 ± 4.1 | - | - | - | |||
| Zonguldak Province, Turkey | Ocean | 10 2006~04 2007 | 4.48 ± 1.75 | 4.37 ± 2.27 | 4.58 ± 2.28 | - | 5.52 ± 2.05 | HPLC [37] |
| 05 2007~09 2007 | 2.33 ± 1.30 | 2.19 ± 1.13 | 2.79 ± 1.55 | - | 3.63 ± 2.36 | |||
| Tampa Province, USA | Ocean | 06 2005~08 2005 | - | - | 31 ± 28 | - | - | IC [38] |
| Jeju Island, Korea | Ocean | 03 2001~04 2001 | 13.5 | 3.1 | - | - | - | HPLC [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhao, T.; Jiao, H.; Wu, L.; Xiao, C.; Guo, X. Types and Distribution of Organic Amines in Organic Nitrogen Deposition in Strategic Water Sources. Int. J. Environ. Res. Public Health 2022, 19, 4151. https://doi.org/10.3390/ijerph19074151
Yang Y, Zhao T, Jiao H, Wu L, Xiao C, Guo X. Types and Distribution of Organic Amines in Organic Nitrogen Deposition in Strategic Water Sources. International Journal of Environmental Research and Public Health. 2022; 19(7):4151. https://doi.org/10.3390/ijerph19074151
Chicago/Turabian StyleYang, Yixuan, Tongqian Zhao, Huazhe Jiao, Li Wu, Chunyan Xiao, and Xiaoming Guo. 2022. "Types and Distribution of Organic Amines in Organic Nitrogen Deposition in Strategic Water Sources" International Journal of Environmental Research and Public Health 19, no. 7: 4151. https://doi.org/10.3390/ijerph19074151
APA StyleYang, Y., Zhao, T., Jiao, H., Wu, L., Xiao, C., & Guo, X. (2022). Types and Distribution of Organic Amines in Organic Nitrogen Deposition in Strategic Water Sources. International Journal of Environmental Research and Public Health, 19(7), 4151. https://doi.org/10.3390/ijerph19074151







