The Effect of Yoga on Health-Related Fitness among Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Synthesis Methods
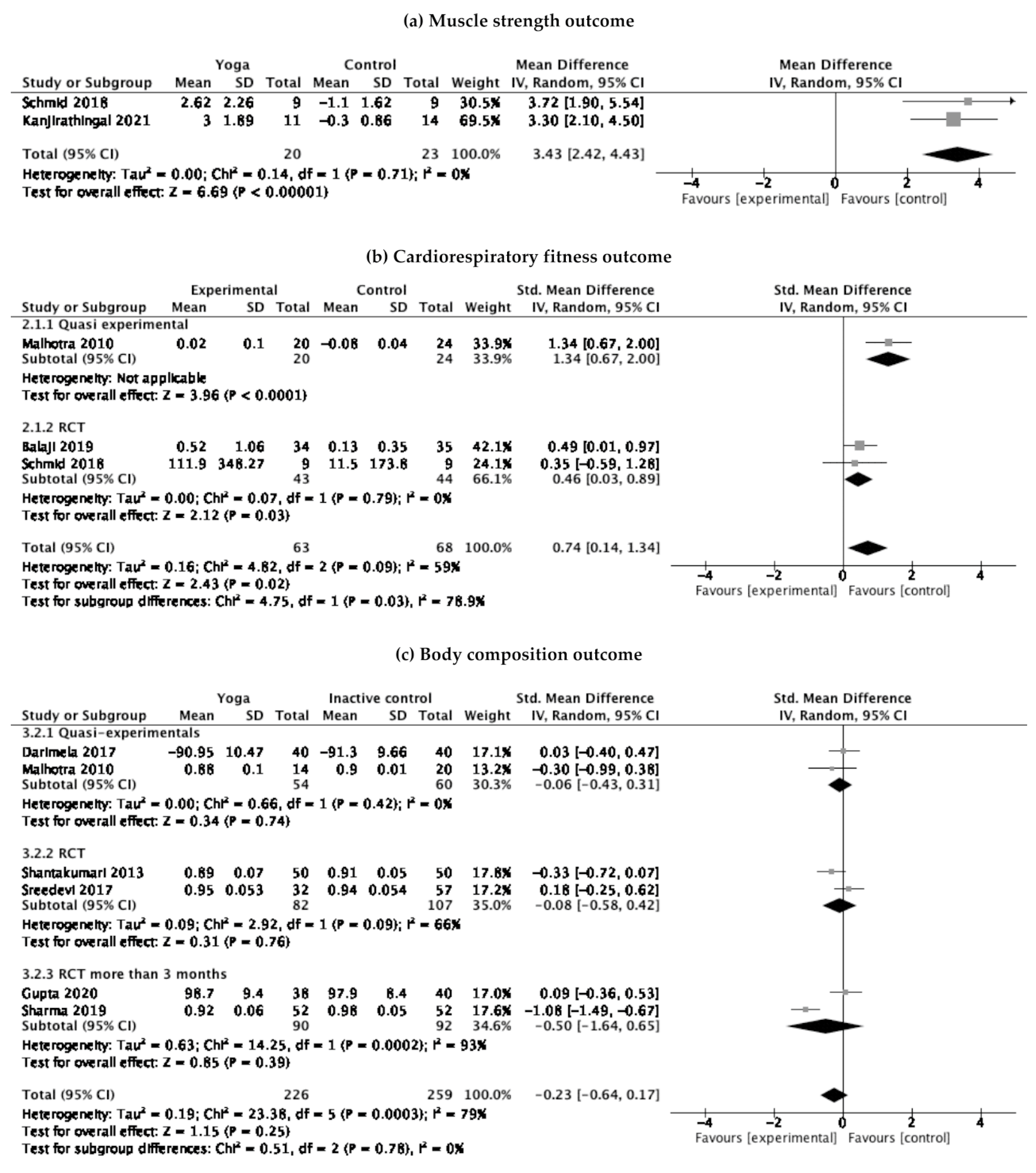
3. Results
3.1. Risk of Bias
3.2. Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganasegeran, K.; Hor, C.P.; Jamil, M.; Loh, H.C.; Noor, J.M.; Hamid, N.A.; Suppiah, P.D.; Abdul Manaf, M.R.; Ch’ng, A.; Looi, I. A Systematic Review of the Economic Burden of Type 2 Diabetes in Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 5723. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Png, M.E.; Yoong, J.; Phan, T.P.; Wee, H.L. Current and future economic burden of diabetes among working-age adults in Asia: Conservative estimates for Singapore from 2010-2050. BMC Public Health 2016, 16, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, T.S.; LaMonte, M.J.; Barlow, C.E.; Blair, S.N. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch. Intern. Med. 2005, 165, 2114–2120. [Google Scholar] [CrossRef]
- Gerrits, E.G.; Landman, G.W.; Nijenhuis-Rosien, L.; Bilo, H.J. Limited joint mobility syndrome in diabetes mellitus: A minireview. World J. Diabetes 2015, 6, 1108–1112. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Iliadis, F.; Angelopoulou, N.; Sailer, N.; Fotiadis, G.; Voliotis, K.; Vitta, I.; Liapis, C.D.; Alevizos, M. Cardiorespiratory capacity is associated with favourable cardiovascular risk profile in patients with Type 2 diabetes. J. Diabetes Its Complicat. 2009, 23, 160–166. [Google Scholar] [CrossRef]
- Sénéchal, M.; Ayers, C.R.; Szczepaniak, L.S.; Gore, M.O.; See, R.; Abdullah, S.M.; Berry, J.D.; McGuire, D.K.; McGavock, J.M. Is cardiorespiratory fitness a determinant of cardiomyopathy in the setting of type 2 diabetes? Diabetes Vasc. Dis. Res. 2014, 11, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; Senmaru, T.; et al. Sarcopenia Is Associated with a Risk of Mortality in People with Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 783363. [Google Scholar] [CrossRef]
- Ai, Y.; Xu, R.; Liu, L. The prevalence and risk factors of sarcopenia in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Heshka, S.; Ruggiero, A.; Bray, G.A.; Foreyt, J.; Kahn, S.E.; Lewis, C.E.; Saad, M.; Schwartz, A.V.; Look AHEAD Research Group. Altered body composition in type 2 diabetes mellitus. Int. J. Obes. 2008, 32, 780–787. [Google Scholar] [CrossRef] [Green Version]
- Jarvie, J.L.; Pandey, A.; Ayers, C.R.; McGavock, J.M.; Sénéchal, M.; Berry, J.D.; Patel, K.V.; McGuire, D.K. Aerobic Fitness and Adherence to Guideline-Recommended Minimum Physical Activity Among Ambulatory Patients with Type 2 Diabetes Mellitus. Diabetes Care 2019, 42, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K. Visceral fat and insulin resistance—causative or correlative? Br. J. Nutr. 2000, 83, S71–S77. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.; Bruunsgaard, H.; Weis, N.; Hendel, H.W.; Andreassen, B.U.; Eldrup, E.; Dela, F.; Pedersen, B.K. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech. Ageing Dev. 2003, 124, 495–502. [Google Scholar] [CrossRef]
- Saini, M.; Kulandaivelan, S.; Bansal, V.K.; Saini, V.; Sharma, S.; Kaur, J.; Sondh, A. Pulmonary Pathology Among Patients with Type 2 Diabetes Mellitus: An Updated Systematic Review and Meta-analysis. Curr. Diabetes Rev. 2020, 16, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Grassi, G.; Cuspidi, C. Cardiorespiratory fitness in patients with type 2 diabetes: A missing piece of the puzzle. Heart Fail. Rev. 2021, 26, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Cartagena, M.V.; Tort-Nasarre, G.; Arnaldo, E.R. Barriers and Facilitators for Physical Activity in Adults with Type 2 Diabetes Mellitus: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 5359. [Google Scholar] [CrossRef]
- Anderson, J.G.; Taylor, A.G. The metabolic syndrome and mind-body therapies: A systematic review. J. Nutr. Metab. 2011, 2011, 276419. [Google Scholar] [CrossRef] [Green Version]
- Larson-Meyer, D.E. A Systematic Review of the Energy Cost and Metabolic Intensity of Yoga. Med. Sci. Sports Exerc. 2016, 48, 1558–1569. [Google Scholar] [CrossRef]
- Innes, K.E.; Selfe, T.K. Yoga for Adults with Type 2 Diabetes: A Systematic Review of Controlled Trials. J. Diabetes Res. 2016, 2016, 6979370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaramakrishnan, D.; Fitzsimons, C.; Kelly, P.; Ludwig, K.; Mutrie, N.; Saunders, D.H.; Baker, G. The effects of yoga compared to active and inactive controls on physical function and health related quality of life in older adults-systematic review and meta-analysis of randomised controlled trials. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration. 2011. Available online: www.handbook.cochrane.org (accessed on 5 October 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clin. Res. Ed.) 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Duyx, B.; Swaen, G.; Urlings, M.; Bouter, L.M.; Zeegers, M.P. The strong focus on positive results in abstracts may cause bias in systematic reviews: A case study on abstract reporting bias. Syst. Rev. 2019, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Abbade, L.P.F.; Nwosu, I.; Jin, Y.; Leenus, A.; Maaz, M.; Wang, M.; Bhatt, M.; Zielinski, L.; Sanger, N.; et al. A scoping review of comparisons between abstracts and full reports in primary biomedical research. BMC Med Res. Methodol. 2017, 17, 181. [Google Scholar] [CrossRef]
- Sterne, J.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin. Res. Ed.) 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ (Clin. Res. Ed.) 2020, 368, l6890. [Google Scholar] [CrossRef] [Green Version]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P., Green, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008. [Google Scholar]
- Guidelines for the measurement of respiratory function. Recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir. Med. 1994, 88, 165–194. [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darimela, U.R.; Killi, A. Effect of physical therapy on blood glycemic parameters in women with type 2 diabetes mellitus. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 484–487. [Google Scholar] [CrossRef]
- Kanjirathingal, J.P.; Mullerpatan, R.P.; Nehete, G.; Raghuram, N. Effect of Yogasana Intervention on Standing Balance Performance among People with Diabetic Peripheral Neuropathy: A Pilot Study. Int. J. Yoga 2021, 14, 60–70. [Google Scholar] [CrossRef]
- Malhotra, V.; Singh, S.; Sharma, S.B.; Gupta, P.; Prasad, A.; Prasad, A.; Tandon, O.P.; Madhu Sv Jai Ganga, R. The Status of NIDDM Patients After Yoga Asanas: Assessment of Important Parameters. J. Clin. Diagn. Res. 2010, 4, 2652–2667. [Google Scholar]
- Balaji, R.; Ramanathan, M.; Bhavanani, A.B.; Ranganadin, P.; Balachandran, K. Effectiveness of Adjuvant Yoga Therapy in Diabetic Lung: A Randomized Control Trial. Int. J. Yoga 2019, 12, 96–102. [Google Scholar] [CrossRef]
- Gupta, U.; Gupta, Y.; Jose, D.; Mani, K.; Jyotsna, V.P.; Sharma, G.; Tandon, N. Effectiveness of yoga-based exercise program compared to usual care, in improving hba1c in individuals with type 2 diabetes: A randomized control trial. Int. J. Yoga 2020, 13, 233–238. [Google Scholar] [CrossRef]
- Schmid, A.A.; Atler, K.E.; Malcolm, M.P.; Grimm, L.A.; Klinedinst, T.C.; Marchant, D.R.; Marchant, T.P.; Portz, J.D. Yoga improves quality of life and fall risk-factors in a sample of people with chronic pain and Type 2 Diabetes. Complementary Ther. Clin. Pract. 2018, 31, 369–373. [Google Scholar] [CrossRef]
- Shantakumari, N.; Sequeira, S.; El Deeb, R. Effects of a yoga intervention on lipid profiles of diabetes patients with dyslipidemia. Indian Heart J. 2013, 65, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Bhardwaj, S.; Jangir, S.; Gupta, B. Influence of yoga on status of lipid indices in type 2 diabetes mellitus subjects. Int. J. Diabetes Dev. Ctries. 2020, 40, 410–415. [Google Scholar] [CrossRef]
- Skoro-Kondza, L.; Tai, S.S.; Gadelrab, R.; Drincevic, D.; Greenhalgh, T. Community based yoga classes for type 2 diabetes: An exploratory randomised controlled trial. BMC Health Serv. Res. 2009, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreedevi, A.; Gopalakrishnan, U.A.; Karimassery Ramaiyer, S.; Kamalamma, L. A Randomized controlled trial of the effect of yoga and peer support on glycaemic outcomes in women with type 2 diabetes mellitus: A feasibility study. BMC Complementary Altern. Med. 2017, 17, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanini, A.; Crisafulli, E.; D’Andria, M.; Gregorini, C.; Cherubino, F.; Zampogna, E.; Azzola, A.; Spanevello, A.; Schiavone, N.; Chetta, A. Minimum Clinically Important Difference in 30-s Sit-to-Stand Test After Pulmonary Rehabilitation in Subjects with COPD. Respir. Care 2019, 64, 1261–1269. [Google Scholar] [CrossRef]
- Kafaja, S.; Clements, P.J.; Wilhalme, H.; Furst, D.E.; Tseng, C.H.; Hyun, K.; Goldin, J.; Volkmann, E.R.; Roth, M.; Tashkin, D.P.; et al. Reliability and Minimal Clinically Important Differences (MCID) of Forced Vital Capacity: Post-Hoc Analyses from the Scleroderma Lung Studies (SLS-I and II) [abstract]. Arthritis Rheumatol. 2016, 68 (Suppl. 10). Available online: https://acrabstracts.org/abstract/reliability-and-minimal-clinically-important-differences-mcid-of-forced-vital-capacity-post-hoc-analyses-from-the-scleroderma-lung-studies-sls-i-and-ii/ (accessed on 5 October 2021).
- Lehecka, B.J.; Stoffregen, S.; May, A.; Thomas, J.; Mettling, A.; Hoover, J.; Hafenstine, R.; Hakansson, N.A. Gluteal Muscle Activation During Common Yoga Poses. Int. J. Sports Phys. Ther. 2021, 16, 662–670. [Google Scholar] [CrossRef]
- Oranchuk, D.J.; Storey, A.G.; Nelson, A.R.; Cronin, J.B. Isometric training and long-term adaptations: Effects of muscle length, intensity, and intent: A systematic review. Scand. J. Med. Sci. Sports 2019, 29, 484–503. [Google Scholar] [CrossRef]
- Puente-Maestu, L.; Stringer, W.W. Physical activity to improve health: Do not forget that the lungs benefit too. Eur. Respir. J. 2018, 51, 1702468. [Google Scholar] [CrossRef] [Green Version]
- Shin, S. Meta-Analysis of the Effect of Yoga Practice on Physical Fitness in the Elderly. Int. J. Environ. Res. Public Health 2021, 18, 11663. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, K.; Kim, C.; Kang, S.J.; Kim, H.J.; Yoon, S.; Shin, Y.A. Effects of exercise on the body composition and lipid profile of individuals with obesity: A systematic review and meta-analysis. J. Obes. Metab. Syndr. 2019, 28, 278–294. [Google Scholar] [CrossRef] [Green Version]
- Lauche, R.; Langhorst, J.; Lee, M.S.; Dobos, G.; Cramer, H. A systematic review and meta-analysis on the effects of yoga on weight-related outcomes. Prev. Med. 2016, 87, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Du Prel, J.B.; Hommel, G.; Röhrig, B.; Blettner, M. Confidence interval or p-value?: Part 4 of a series on evaluation of scientific publications. Dtsch. Arztebl. Int. 2009, 106, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Paez, A. Grey literature: An important resource in systematic reviews. J. Evid. Based Med. 2017, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]

| Study id Country Funding Source | Study Design | Participants (Number, Mean Age (SD), Gender Proportion, Presence of Diabetic Complication) | Intervention Characteristics (Type, Frequency, Session Duration, Length of Intervention) | Control Group(s) | Outcome Measures |
|---|---|---|---|---|---|
| Darimela (2017), India, Funding not stated [36] | Quasi- experimental | n = 160, age range 36–48, 100% female, complication not described | Hatha yoga, up to 60–70 min per session, frequency not described, 6 months | 1. Active control: exercise 2. Active control: walking exercise 3. Inactive control | Body composition: hip circumference |
| Kanjirathingal (2021), India, MGM School of Physiotherapy, MGM Institute of Health Sciences, Navi Mumbai, India [37] | Quasi- experimental | n = 35, mean age (SD): yoga group = 55.5 (7), mean age (SD): balance exercise group = 58.7 (5.6), mean age (SD): control group = 57.7 (6), 51.4% female, diabetic peripheral neuropathic pain | Hatha yoga, 3 times a week, 1 h per session, 12 weeks | 1. Active control: usual care + balance exercise 2. Inactive control: usual care + wait-list control | Muscle strength: chair stand test, step-up test; balance: star excursion balance test, single limb stance test; fall-related outcome: modified fall efficacy scale |
| Malhotra (2010), India, Funding NA [38] | Quasi- experimental | n = 62, age range = 30–60, gender not available, complication not described | Hatha yoga, every day, 30–40 min per session, 40 days | 1. Inactive control: usual care + mild exercise advice | Cardiorespiratory fitness: lung function test (slow vital capacity, forced expiratory volume for 1 second, peak expiratory flow rate, maximal voluntary ventilation, forced vital capacity) body composition: waist-to-hip ratio |
| Balaji (2019) India, Sri Balaji Vidyapeeth funds the CYTER and all of its activities in yoga therapy, education, and research [39] | RCT | n = 72, mean age (SD) = 49.6 (5.88), 31.9% female, diabetic lung function | Hatha yoga, thrice a week, 60 min per session, 4 months | 1. Inactive control: Usual care + advice on diet | Cardiorespiratory fitness: Lung function test (Forced expiratory volume for 1 second, forced vital capacity, forced expiratory volume for 1 second/forced vital capacity ratio) |
| Gupta (2020), India Centre for Integrative Medicine and Research, All India Institute of Medical Science [40] | RCT | n = 81, mean age (SD) = 50.6 (8.5), 44.4% female, 42% were on blood pressure medication, 44.4% were on lipid-lowering medication | Integrated yoga; 45 min/session; 3 classes/week for the first 2 weeks, 2 classes/week for the next 2 weeks, and 1 class/month for the last 3 months; 4 months | Inactive control: dietary and walking advice | Body composition: waist circumference |
| Schmid (2018) USA, Colorado State University Prevention Research Center [41] | RCT | n = 18, mean age (SD) = 54.95 (9.94), 66.67% female, diabetic peripheral neuropathic pain | Hatha yoga, twice a week, duration not available, 8 weeks | 1. Inactive control: usual care + wellness education | Cardiorespiratory fitness: 6-min walk test; muscle strength: upper extremity strength (chair stand test), lower extremity strength (arm curl test); balance: The Fullerton Advanced Balance Scale Quality of life: Rand 36-Item Health Survey |
| Shantakumari (2013), India, Funding NA [42] | RCT | n = 100, mean age = 45, 48% female, dyslipidemia | 1 h/session, daily, 3 months | Inactive control | Body composition: waist-to-hip ratio |
| Sharma (2020), India, Rajasthan University of Health Sciences [43] | RCT | n = 104, age range = 30–65, 45.19% female, dyslipidemia | 40 min/session, 5 days/week, 6 months | Inactive control | Body composition: waist-to-hip ratio |
| Skoro-Kondza (2009), UK, Novo Nordisk Research Foundation [44] | RCT | n = 59, mean age (SD) = 60 (10), 61.02% female, type 2 diabetes mellitus without complication | 90 min/session, 2 days/week, 12 weeks | Inactive control: lifestyle leaflet and advice + waiting list yoga | Body composition: waist-to-hip ratio; quality of life: audit of diabetes-dependent QoL |
| Sreedevi (2017) India, Fogarty International Centre, National Institutes of Health [45] | RCT | n = 124, mean age (SD)= 51.9 (7.3); 100% female, dyslipidemia | 60 min/session, 2 days/week, 3 months | 1. Inactive control: standard advice on diet and exercise 2. Inactive control: peer-support on management of diabetes, diet, and exercise | Body composition: waist-to-hip ratio |
| Study id, Design | Cardiorespiratory Fitness | Muscle Strength | Body Composition | Balance | Fall-Related Outcome | Quality of Life | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| vs. Inactive Control | vs. Active Control | vs. Inactive Control | vs. Active Control | vs. Inactive Control | vs. Active Control | vs. Inactive Control | vs. Active Control | vs. Inactive Control | ||
| Darimela (2017), Quasi-experimental [36] |  |  | ||||||||
| Kanjirathingal (2021), Quasi-experimental [37] |  |  |  |  |  |  | ||||
| Malhotra (2010), Quasi-experimental [38] |  |  | ||||||||
| Balaji (2019), RCT [39] |  | |||||||||
| Gupta (2020), RCT [40] |  | |||||||||
| Schmid (2018), RCT [41] |  |  |  | |||||||
| Shantakumari (2013), RCT [42] |  | |||||||||
| Sharma (2020), RCT [43] |  | |||||||||
| Skoro-Kondza (2009), RCT [44] |  |  | ||||||||
| Sreedevi (2017), RCT [45] |  | |||||||||
 statistically significant favoring yoga;
statistically significant favoring yoga;  no statistically significant difference;
no statistically significant difference;  statistically significant favoring comparator.
statistically significant favoring comparator.| ROB 2.0 | Randomization Process | Deviation from Intended Intervention | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Results | Overall Bias | ||
|---|---|---|---|---|---|---|---|---|
| Balaji 2019 [39] | Some concerns | Low | Low | Low | Low | Some concerns | ||
| Gupta 2020 [40] | Low | Low | Low | Low | Low | Low | ||
| Schmid 2018 [41] | Some concerns | Low | Low | Low | Low | Some concerns | ||
| Shantakumari (2013) [42] | Some concerns | Low | Low | High | Low | High | ||
| Sharma (2020) [43] | High | Low | Low | High | Low | High | ||
| Skoro-Kondza (2009) [44] | Low | Low | Low | Some concerns | Some concerns | Some concerns | ||
| Sreedevi (2017) [45] | Some concerns | Low | Some concerns | Low | Low | Some concerns | ||
| ROBINS-I | Confounding | Selection of participants | Classification of intervention | Deviation from intended intervention | Missing data | Measurement of outcome | Selection of reported results | Overall bias |
| Darimela (2017) [36] | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate risk of bias |
| Kanjirathingal (2021) [37] | Moderate | Low | Low | Low | Low | Low | Low | Moderate risk of bias |
| Malhotra (2010) [38] | Low | Low | Low | Low | Serious | Low | Low | Serious risk of bias |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wibowo, R.A.; Nurámalia, R.; Nurrahma, H.A.; Oktariani, E.; Setiawan, J.; Icanervilia, A.V.; Agustiningsih, D. The Effect of Yoga on Health-Related Fitness among Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4199. https://doi.org/10.3390/ijerph19074199
Wibowo RA, Nurámalia R, Nurrahma HA, Oktariani E, Setiawan J, Icanervilia AV, Agustiningsih D. The Effect of Yoga on Health-Related Fitness among Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(7):4199. https://doi.org/10.3390/ijerph19074199
Chicago/Turabian StyleWibowo, Rakhmat Ari, Riskah Nurámalia, Herlin Ajeng Nurrahma, Eva Oktariani, Jajar Setiawan, Ajeng Viska Icanervilia, and Denny Agustiningsih. 2022. "The Effect of Yoga on Health-Related Fitness among Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 7: 4199. https://doi.org/10.3390/ijerph19074199
APA StyleWibowo, R. A., Nurámalia, R., Nurrahma, H. A., Oktariani, E., Setiawan, J., Icanervilia, A. V., & Agustiningsih, D. (2022). The Effect of Yoga on Health-Related Fitness among Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(7), 4199. https://doi.org/10.3390/ijerph19074199






