Effect of Electrical Muscle Stimulation and Resistance Exercise Intervention on Physical and Brain Function in Middle-Aged and Older Women
Abstract
1. Introduction
2. Materials and Methods
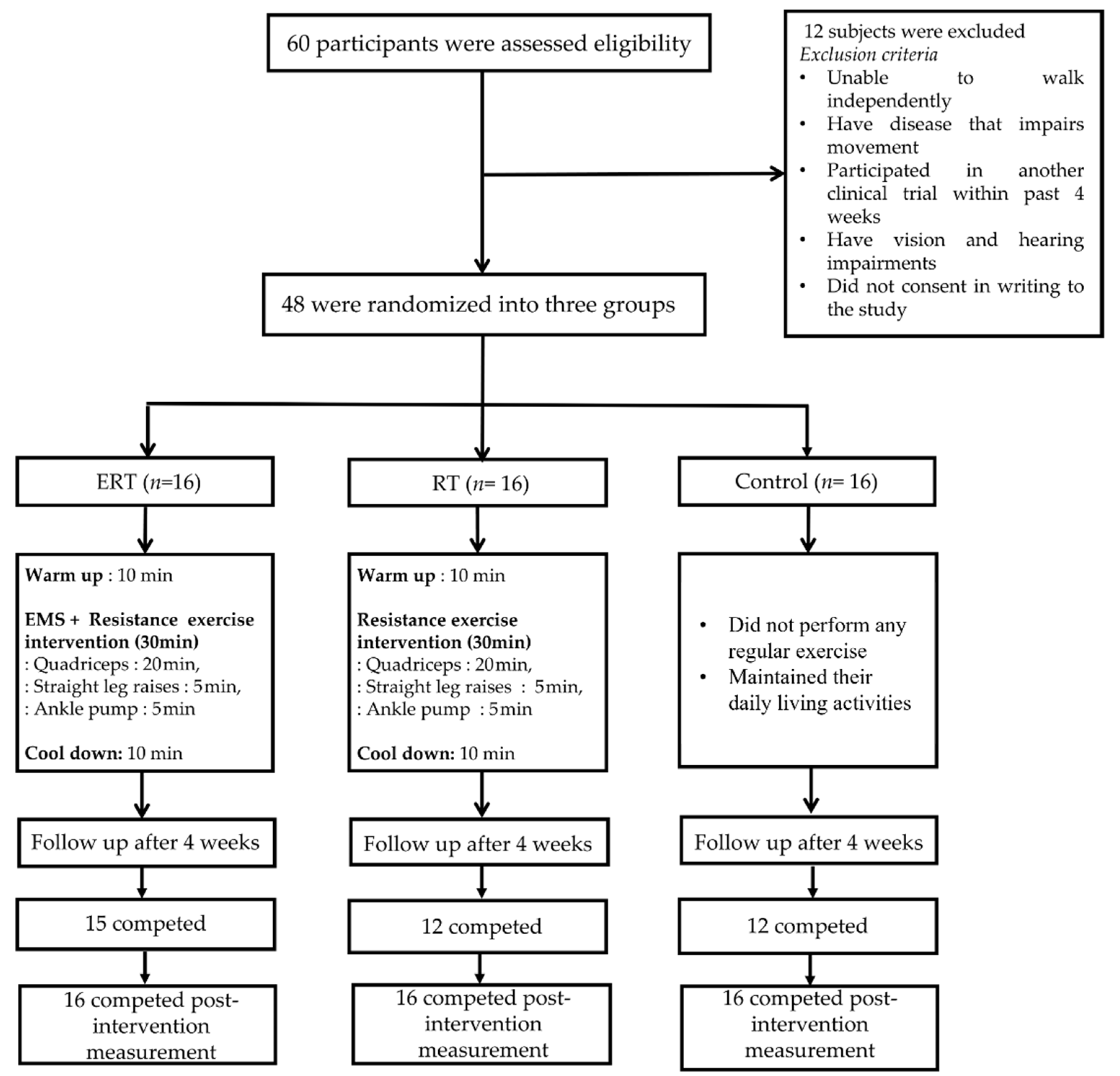
2.1. Study Design and Sample
2.2. Intervention
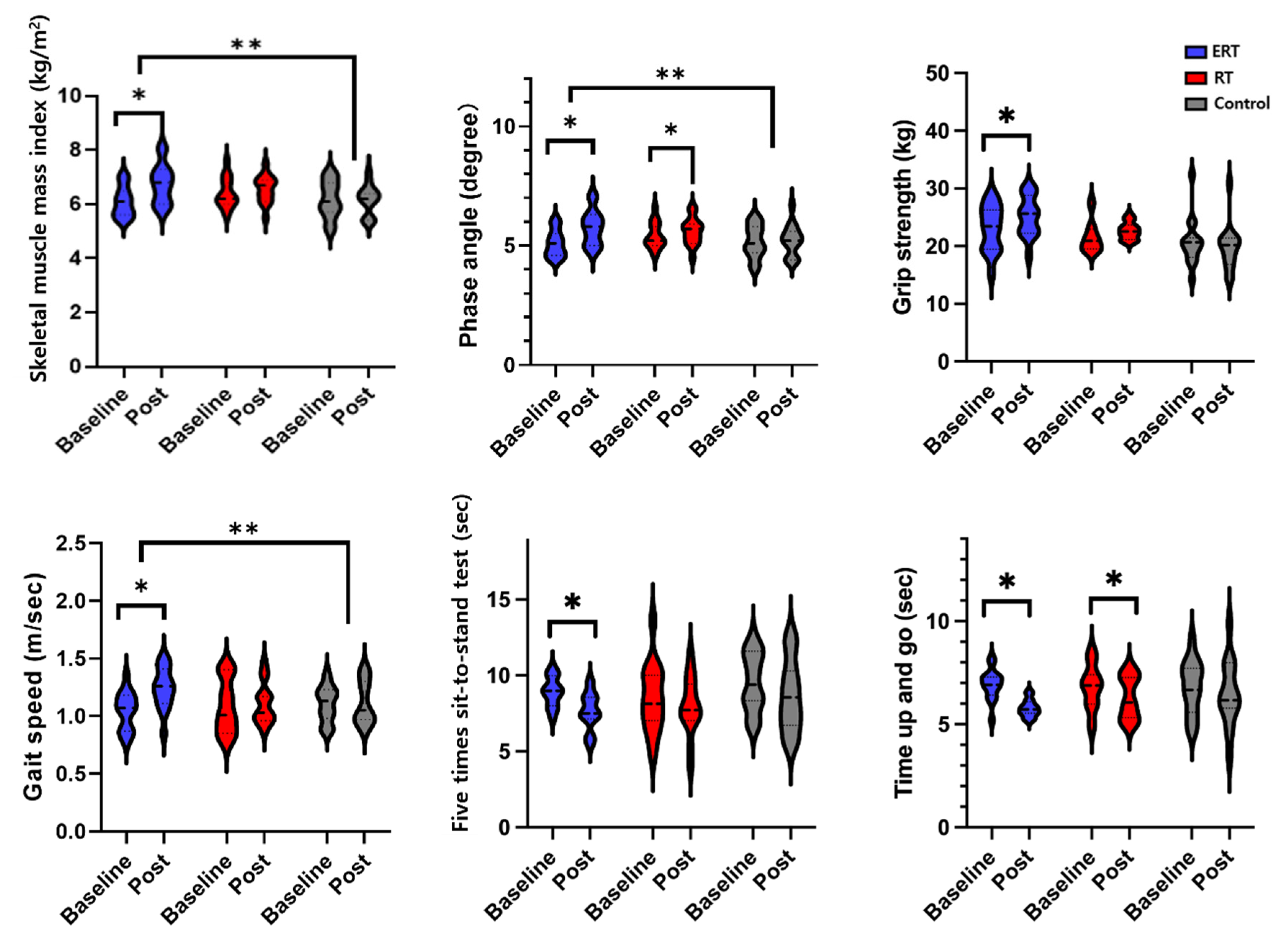
2.3. Physical Function
2.4. EEG Recording and Preprocessing
2.5. Statistical Analyses
3. Results
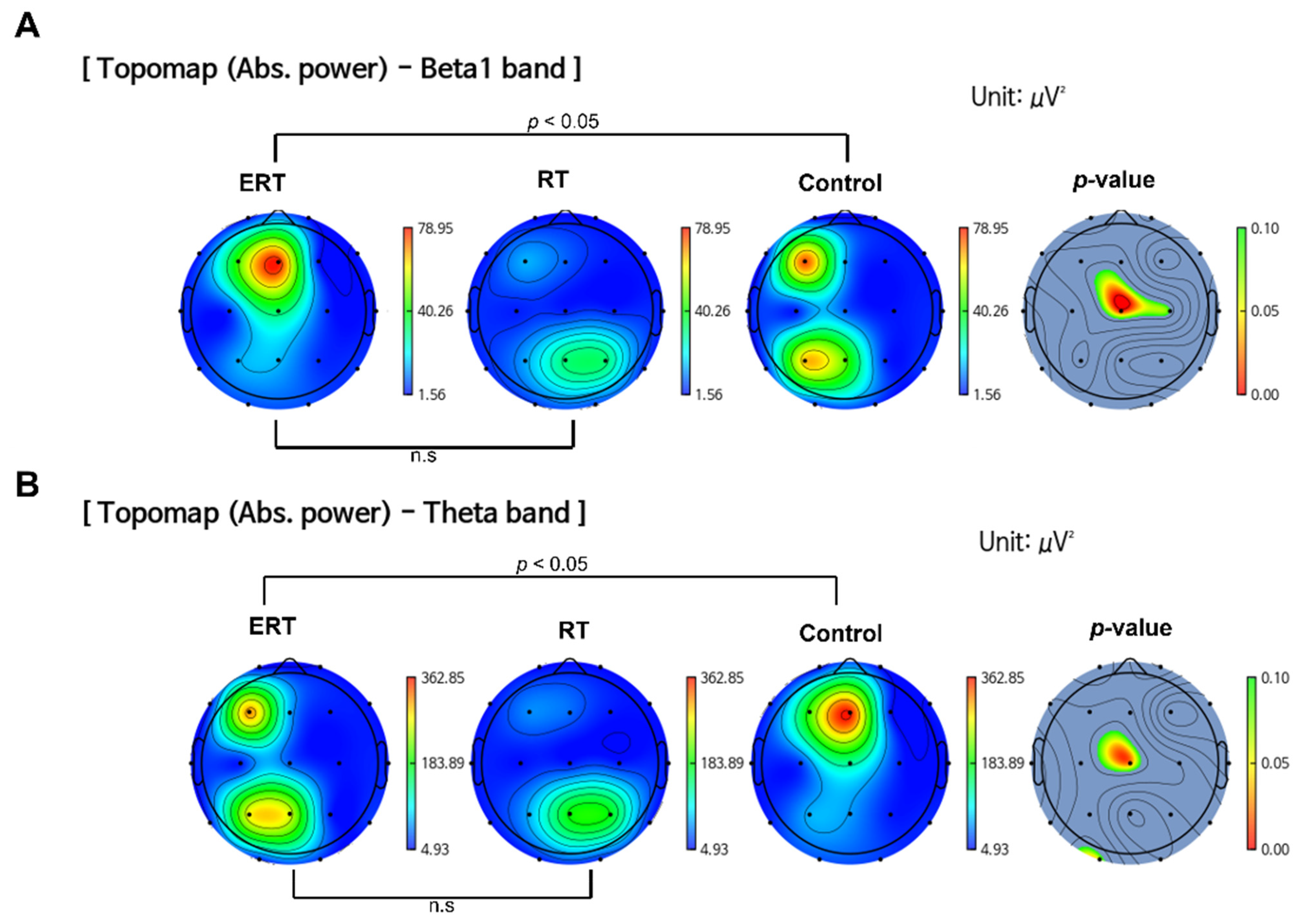
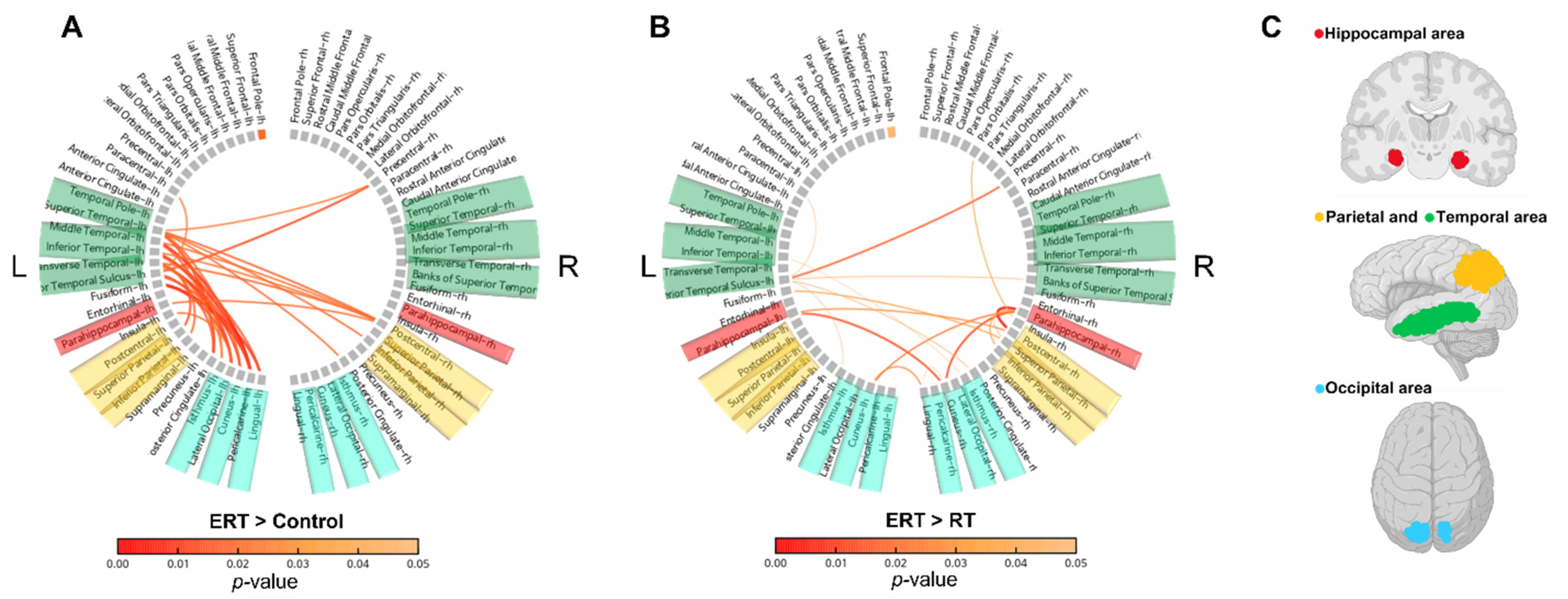
3.1. EEG
3.1.1. Resting State Absolute Band Power
3.1.2. Resting-State Functional Connectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B.; Health, A.; Study, B.C. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Muñoz-Cánoves, P. Regenerative decline of stem cells in sarcopenia. Mol. Asp. Med. 2016, 50, 109–117. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, I.; Cipolli, G.C.; Yassuda, M.S. Sarcopenia and cognitive impairment: Possible physiopathological causation or just a spurious association? Clin. Nutr. 2020, 39, 1622. [Google Scholar] [CrossRef] [PubMed]
- Beeri, M.S.; Leugrans, S.E.; Delbono, O.; Bennett, D.A.; Buchman, A.S. Sarcopenia is associated with incident Alzheimer’s dementia, m ild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 2021, 69, 1826–1835. [Google Scholar] [CrossRef]
- Wallace, L.M.; Theou, O.; Godin, J.; Andrew, M.K.; Bennett, D.A.; Rockwood, K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: A cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019, 18, 177–184. [Google Scholar] [CrossRef]
- Yu, L.; Boyle, P.A.; Leurgans, S.E.; Wilson, R.S.; Bennett, D.A.; Buchman, A.S. Incident mobility disability, mild cognitive impairment, and mortality in community-dwelling older adults. Neuroepidemiology 2019, 53, 55–62. [Google Scholar] [CrossRef]
- Kim, H.; Hirano, H.; Edahiro, A.; Ohara, Y.; Watanabe, Y.; Kojima, N.; Kim, M.; Hosoi, E.; Yoshida, Y.; Yoshida, H. Sarcopenia: Prevalence and associated factors based on different suggested definitions in community-dwelling older adults. Geriatr. Gerontol. Int. 2016, 16, 110–122. [Google Scholar] [CrossRef]
- Sayer, A.A.; Robinson, S.M.; Patel, H.P.; Shavlakadze, T.; Cooper, C.; Grounds, M.D. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing 2013, 42, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.L.; Jagust, W.J.; Dulberg, C.; Becker, J.T.; DeKosky, S.T.; Fitzpatrick, A.; Breitner, J.; Lyketsos, C.; Jones, B.; Kawas, C. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 2. Arch Neurol-Chic. 2003, 60, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Larson, E.B.; Bowen, J.D.; van Belle, G. Performance-based physical function and future dementia in older people. Arch. Intern. Med. 2006, 166, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Broadhouse, K.M.; Singh, M.F.; Suo, C.; Gates, N.; Wen, W.; Brodaty, H.; Jain, N.; Wilson, G.C.; Meiklejohn, J.; Singh, N. Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI. NeuroImage Clin. 2020, 25, 102182. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.; Cruz-Jentoft, A.; Arai, H.; Kritchevsky, S.; Guralnik, J.; Bauer, J.; Pahor, M.; Clark, B.; Cesari, M. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-j.; Latham, N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009, 2009, CD002759. [Google Scholar] [CrossRef]
- Grgic, J.; Garofolini, A.; Orazem, J.; Sabol, F.; Schoenfeld, B.J.; Pedisic, Z. Effects of resistance training on muscle size and strength in very elderly adults: A systematic review and meta-analysis of randomized controlled trials. Sport. Med. 2020, 50, 1983–1999. [Google Scholar] [CrossRef]
- Straight, C.R.; Lindheimer, J.B.; Brady, A.O.; Dishman, R.K.; Evans, E.M. Effects of resistance training on lower-extremity muscle power in middle-aged and older adults: A systematic review and meta-analysis of randomized controlled trials. Sport. Med. 2016, 46, 353–364. [Google Scholar] [CrossRef]
- Pahor, M.; Guralnik, J.M.; Ambrosius, W.T.; Blair, S.; Bonds, D.E.; Church, T.S.; Espeland, M.A.; Fielding, R.A.; Gill, T.M.; Groessl, E.J. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. Jama 2014, 311, 2387–2396. [Google Scholar] [CrossRef]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; Skelton, D.A. Resistance exercise as a treatment for sarcopenia: Prescription and delivery. Age Ageing 2022, 51, afac003. [Google Scholar] [CrossRef] [PubMed]
- Karatzanos, E.; Gerovasili, V.; Zervakis, D.; Tripodaki, E.-S.; Apostolou, K.; Vasileiadis, I.; Papadopoulos, E.; Mitsiou, G.; Tsimpouki, D.; Routsi, C. Electrical muscle stimulation: An effective form of exercise and early mobilization to preserve muscle strength in critically ill patients. Crit. Care Res. Pract. 2012, 2012, 432752. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, E.; Felicetti, G.; Maini, M.; Fracchia, C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: Effect of electrical stimulation. Chest 2003, 124, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Vivodtzev, I.; Pépin, J.-L.; Vottero, G.; Mayer, V.; Porsin, B.; Lévy, P.; Wuyam, B. Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest 2006, 129, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Nuhr, M.J.; Pette, D.; Berger, R.; Quittan, M.; Crevenna, R.; Huelsman, M.; Wiesinger, G.F.; Moser, P.; Fialka-Moser, V.; Pacher, R. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur. Heart J. 2004, 25, 136–143. [Google Scholar] [CrossRef]
- Banerjee, P.; Caulfield, B.; Crowe, L.; Clark, A. Prolonged electrical muscle stimulation exercise improves strength and aerobic capacity in healthy sedentary adults. J. Appl. Physiol. 2005, 99, 2307–2311. [Google Scholar] [CrossRef]
- Kern, H.; Barberi, L.; Löfler, S.; Sbardella, S.; Burggraf, S.; Fruhmann, H.; Carraro, U.; Mosole, S.; Sarabon, N.; Vogelauer, M. Electrical stimulation counteracts muscle decline in seniors. Front. Aging Neurosci. 2014, 6, 189. [Google Scholar] [CrossRef]
- Rahmati, M.; Gondin, J.; Malakoutinia, F. Effects of Neuromuscular Electrical Stimulation on Quadriceps Muscle Strength and Mass in Healthy Young and Older Adults: A Scoping Review. Phys. Ther. 2021, 101, pzab144. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Watanabe, K.; Kawade, S.; Takahashi, T.; Kimura, H.; Maruyama, H.; Hyngstrom, A. The effect of a portable electrical muscle stimulation device at home on muscle strength and activation patterns in locomotive syndrome patients: A randomized control trial. J. Electromyogr. Kinesiol. 2019, 45, 46–52. [Google Scholar] [CrossRef]
- Hasegawa, S.; Kobayashi, M.; Arai, R.; Tamaki, A.; Nakamura, T.; Moritani, T. Effect of early implementation of electrical muscle stimulation to prevent muscle atrophy and weakness in patients after anterior cruciate ligament reconstruction. J. Electromyogr. Kinesiol. 2011, 21, 622–630. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Caulfield, B.; Crowe, L.; Clark, A.L. Prolonged electrical muscle stimulation exercise improves strength, peak VO2, and exercise capacity in patients with stable chronic heart failure. J. Card. Fail. 2009, 15, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L.; Srinivasan, R.; Westdorp, A.F.; Wijesinghe, R.S.; Tucker, D.M.; Silberstein, R.B.; Cadusch, P.J. EEG coherency: I: Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 499–515. [Google Scholar] [CrossRef]
- Nolte, G.; Bai, O.; Wheaton, L.; Mari, Z.; Vorbach, S.; Hallett, M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 2004, 115, 2292–2307. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Dufor, O.; Merlet, I.; Berrou, C.; Wendling, F. EEG source connectivity analysis: From dense array recordings to brain networks. PLoS ONE 2014, 9, e105041. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Melov, S.; Tarnopolsky, M.A.; Beckman, K.; Felkey, K.; Hubbard, A. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2007, 2, e465. [Google Scholar] [CrossRef]
- Henwood, T.R.; Taaffe, D.R. Improved physical performance in older adults undertaking a short-term programme of high-velocity resistance training. Gerontology 2005, 51, 108–115. [Google Scholar] [CrossRef]
- Baker, B.S.; Weitzel, K.J.; Royse, L.A.; Miller, K.; Guess, T.M.; Ball, S.D.; Duren, D.L. Efficacy of an 8-week resistance training program in Older adults: A randomized controlled trial. J. Aging Phys. Act. 2020, 29, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sayers, S.P.; Gibson, K. A comparison of high-speed power training and traditional slow-speed resistance training in older men and women. J. Strength Cond. Res. 2010, 24, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Skelton, D.A.; Young, A.; Greig, C.A.; Malbut, K.E. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J. Am. Geriatr. Soc. 1995, 43, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Gondin, J.; Place, N.; Stevens-Lapsley, J.; Vivodtzev, I.; Minetto, M.A. Clinical use of neuromuscular electrical stimulation for neuromuscular rehabilitation: What are we overlooking? Arch. Phys. Med. Rehabil. 2018, 99, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.; Cyrino, E.; Antunes, M.; Santos, D.; Sardinha, L. Changes in phase angle and body composition induced by resistance training in older women. Eur. J. Clin. Nutr. 2016, 70, 1408–1413. [Google Scholar] [CrossRef]
- Matias, C.N.; Campa, F.; Nunes, C.L.; Francisco, R.; Jesus, F.; Cardoso, M.; Valamatos, M.J.; Homens, P.M.; Sardinha, L.B.; Martins, P. Phase Angle Is a Marker of Muscle Quantity and Strength in Overweight/Obese Former Athletes. Int. J. Environ. Res. Public Health 2021, 18, 6649. [Google Scholar] [CrossRef]
- Chan, J.; Lu, Y.-C.; Yao, M.M.-S.; Kosik, R.O. Correlation between hand grip strength and regional muscle mass in older Asian adults: An observational study. BMC Geriatr. 2022, 22, 206. [Google Scholar] [CrossRef]
- Peterson, M.D.; Rhea, M.R.; Sen, A.; Gordon, P.M. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res. Rev. 2010, 9, 226–237. [Google Scholar] [CrossRef]
- Agergaard, J.; Bülow, J.; Jensen, J.K.; Reitelseder, S.; Drummond, M.J.; Schjerling, P.; Scheike, T.; Serena, A.; Holm, L. Light-load resistance exercise increases muscle protein synthesis and hypertrophy signaling in elderly men. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E326–E338. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; Verdijk, L.B.; Snijders, T.; Hansen, D.; Vranckx, P.; Burd, N.A.; Dendale, P.; Van Loon, L.J. Neuromuscular electrical stimulation increases muscle protein synthesis in elderly type 2 diabetic men. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E614–E623. [Google Scholar] [CrossRef]
- Vega, S.R.; Knicker, A.; Hollmann, W.; Bloch, W.; Strüder, H. Effect of resistance exercise on serum levels of growth factors in humans. Horm. Metab. Res. 2010, 42, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101. [Google Scholar] [CrossRef]
- Magee, J.C.; Grienberger, C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is irisin a human exercise gene? Nature 2012, 488, E9–E10. [Google Scholar] [CrossRef]
- Moreau, D.; Dubots, P.; Boggio, V.; Guilland, J.C.; Cometti, G. Effects of electromyostimulation and strength training on muscle soreness, muscle damage and sympathetic activation. J. Sport. Sci. 1995, 13, 95–100. [Google Scholar] [CrossRef]
- Wong, R.A.; Jette, D.U. Changes in sympathetic tone associated with different forms of transcutaneous electrical nerve stimulation in healthy subjects. Phys. Ther. 1984, 64, 478–482. [Google Scholar] [CrossRef]
- Kondo, Y.; To, M.; Saruta, J.; Hayashi, T.; Sugiyama, H.; Tsukinoki, K. Role of TrkB expression in rat adrenal gland during acute immobilization stress. J. Neurochem. 2013, 124, 224–232. [Google Scholar] [CrossRef]
- Schiffer, T.; Schulte, S.; Sperlich, B.; Achtzehn, S.; Fricke, H.; Strüder, H.K. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci. Lett. 2011, 488, 234–237. [Google Scholar] [CrossRef]
- Ferris, L.T.; Williams, J.S.; Shen, C.-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sport. Exerc. 2007, 39, 728. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Minetto, M.A.; Farina, D.; Bottinelli, R. Electrical Stimulation for Neuromuscular Testing and Training: State-of-the Art and Unresolved Issues; Springer: Berlin/Heidelberg, Germany, 2011; Volume 111, pp. 2391–2397. [Google Scholar]
- Philp, A.; Macdonald, A.L.; Watt, P.W. Lactate—A signal coordinating cell and systemic function. J. Exp. Biol. 2005, 208, 4561–4575. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Pyun, J.-M.; Yeo, S.; Kang, D.W.; Jeong, H.T.; Kang, S.W.; Kim, S.; Youn, Y.C. Differences between memory encoding and retrieval failure in mild cognitive impairment: Results from quantitative electroencephalography and magnetic resonance volumetry. Alzheimer’s Res. Ther. 2021, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Musaeus, C.S.; Nielsen, M.S.; Østerbye, N.N.; Høgh, P. Decreased parietal beta power as a sign of disease progression in patients with mild cognitive impairment. J. Alzheimer’s Dis. 2018, 65, 475–487. [Google Scholar] [CrossRef]
- Babiloni, C.; Binetti, G.; Cassetta, E.; Dal Forno, G.; Del Percio, C.; Ferreri, F.; Ferri, R.; Frisoni, G.; Hirata, K.; Lanuzza, B. Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: A multicenter study. Clin. Neurophysiol. 2006, 117, 252–268. [Google Scholar] [CrossRef]
- Babiloni, C.; Frisoni, G.B.; Pievani, M.; Vecchio, F.; Lizio, R.; Buttiglione, M.; Geroldi, C.; Fracassi, C.; Eusebi, F.; Ferri, R. Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. Neuroimage 2009, 44, 123–135. [Google Scholar] [CrossRef]
- Michels, L.; Muthuraman, M.; Anwar, A.R.; Kollias, S.; Leh, S.E.; Riese, F.; Unschuld, P.G.; Siniatchkin, M.; Gietl, A.F.; Hock, C. Changes of functional and directed resting-state connectivity are associated with neuronal oscillations, ApoE genotype and amyloid deposition in mild cognitive impairment. Front. Aging Neurosci. 2017, 9, 304. [Google Scholar] [CrossRef]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscientist 2012, 18, 82–97. [Google Scholar] [CrossRef]
- Quan, M.; Xun, P.; Chen, C.; Wen, J.; Wang, Y.; Wang, R.; Chen, P.; He, K. Walking pace and the risk of cognitive decline and dementia in elderly populations: A meta-analysis of prospective cohort studies. J. Gerontol. Ser. A 2017, 72, 266–270. [Google Scholar] [CrossRef]
| Variable | Total | ERT | RT | Control | p-Value |
|---|---|---|---|---|---|
| (n = 48) | (n = 16) | (n = 16) | (n = 16) | ||
| Age (years) | 69.1 ± 5.3 | 69.2 ± 4.0 | 69.4 ± 5.2 | 68.6 ± 7.1 | 0.96 |
| Height (cm) | 154.5 ± 4.4 | 154.8 ± 4.3 | 154.5 ± 4.5 | 154.2 ± 4.7 | 0.90 |
| Weight (kg) | 58.4 ± 8.1 | 59.5 ± 11.3 | 57.6 ± 5.6 | 57.8 ± 5.7 | 0.99 |
| BMI (kg/m2) | 24.4 ± 2.8 | 24.1 ± 3.6 | 24.7 ± 2.6 | 24.4 ± 1.9 | 0.68 |
| SMI (kg/m2) | 6.2 ± 0.9 | 6.1 ± 1.0 | 6.4 ± 1.0 | 6.1 ± 1.0 | 0.60 |
| Phase angle (°) | 5.3 ± 1.0 | 5.1 ± 1.1 | 5.6 ± 1.0 | 5.2 ± 1.0 | 0.38 |
| Grip strength (kg) | 22.7 ± 4.2 | 23.8 ± 4.1 | 22.8 ± 3.2 | 21.3 ± 5.1 | 0.24 |
| Gait speed (m/s) | 1.08 ± 0.18 | 1.04 ± 0.16 | 1.12 ± 0.23 | 1.08 ± 0.16 | 0.55 |
| FSST (s) | 8.9 ± 1.8 | 8.9 ± 0.9 | 8.2 ± 2.3 | 9.6 ± 1.8 | 0.16 |
| TUG (s) | 6.7 ± 1.1 | 6.9 ± 0.7 | 6.6 ± 1.1 | 6.7 ± 1.4 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, N.; Yang, J.-G.; Bae, S.; Kim, G.-M.; Park, H.-J.; Park, H. Effect of Electrical Muscle Stimulation and Resistance Exercise Intervention on Physical and Brain Function in Middle-Aged and Older Women. Int. J. Environ. Res. Public Health 2023, 20, 101. https://doi.org/10.3390/ijerph20010101
Thapa N, Yang J-G, Bae S, Kim G-M, Park H-J, Park H. Effect of Electrical Muscle Stimulation and Resistance Exercise Intervention on Physical and Brain Function in Middle-Aged and Older Women. International Journal of Environmental Research and Public Health. 2023; 20(1):101. https://doi.org/10.3390/ijerph20010101
Chicago/Turabian StyleThapa, Ngeemasara, Ja-Gyeong Yang, Seongryu Bae, Gwon-Min Kim, Hye-Jin Park, and Hyuntae Park. 2023. "Effect of Electrical Muscle Stimulation and Resistance Exercise Intervention on Physical and Brain Function in Middle-Aged and Older Women" International Journal of Environmental Research and Public Health 20, no. 1: 101. https://doi.org/10.3390/ijerph20010101
APA StyleThapa, N., Yang, J.-G., Bae, S., Kim, G.-M., Park, H.-J., & Park, H. (2023). Effect of Electrical Muscle Stimulation and Resistance Exercise Intervention on Physical and Brain Function in Middle-Aged and Older Women. International Journal of Environmental Research and Public Health, 20(1), 101. https://doi.org/10.3390/ijerph20010101







