The Association between Apparent Temperature and Hospital Admissions for Cardiovascular Disease in Limpopo Province, South Africa
Abstract
1. Introduction
2. Materials and Methods
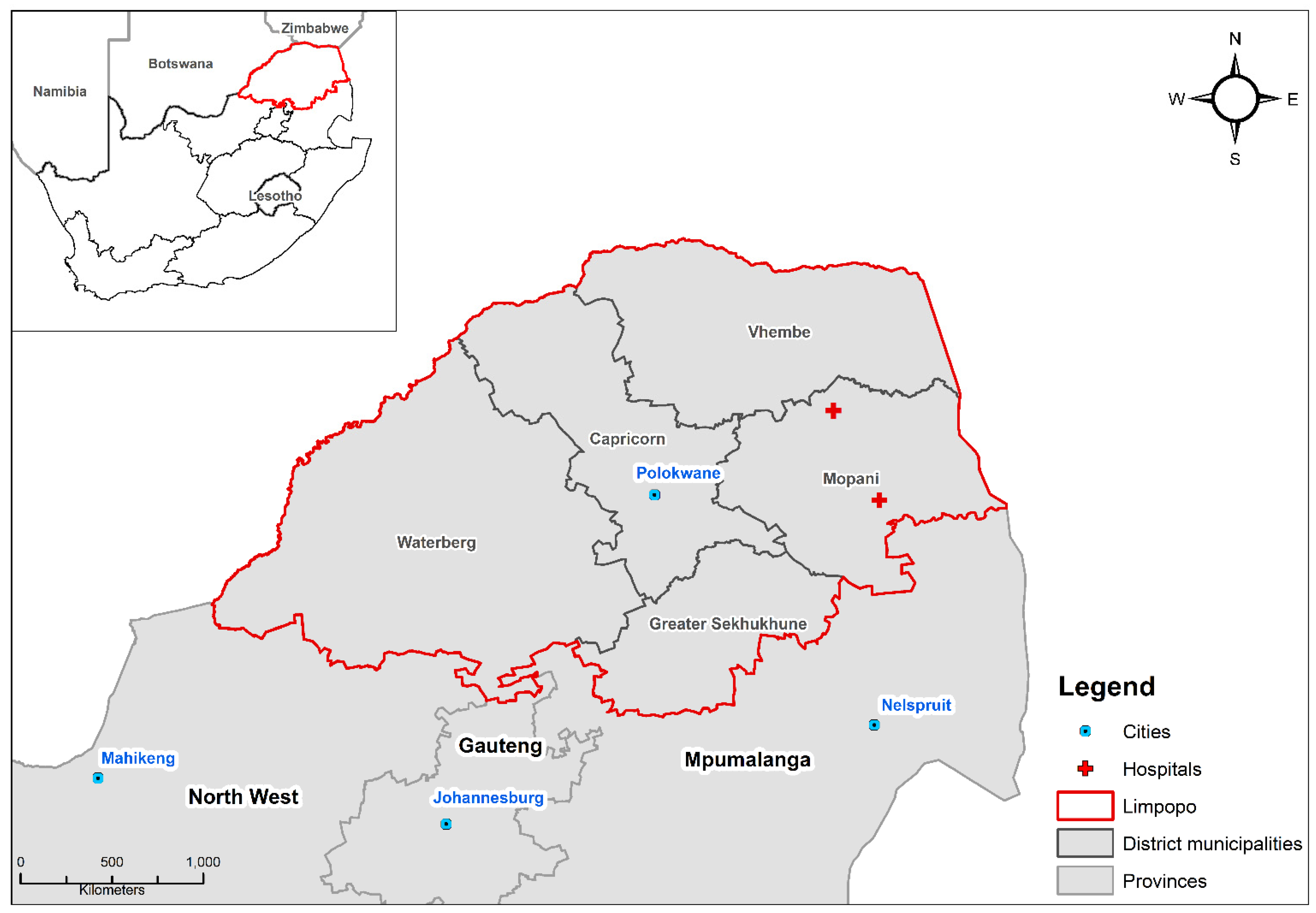
2.1. Study Setting
2.2. Data Sources
2.2.1. Health Data
2.2.2. Weather Data
2.3. Statistical Analyses
2.3.1. Statistical Model
2.3.2. Relative Risk and Optimal Temperature
2.3.3. Attributable Fraction
2.3.4. Sensitivity Analysis
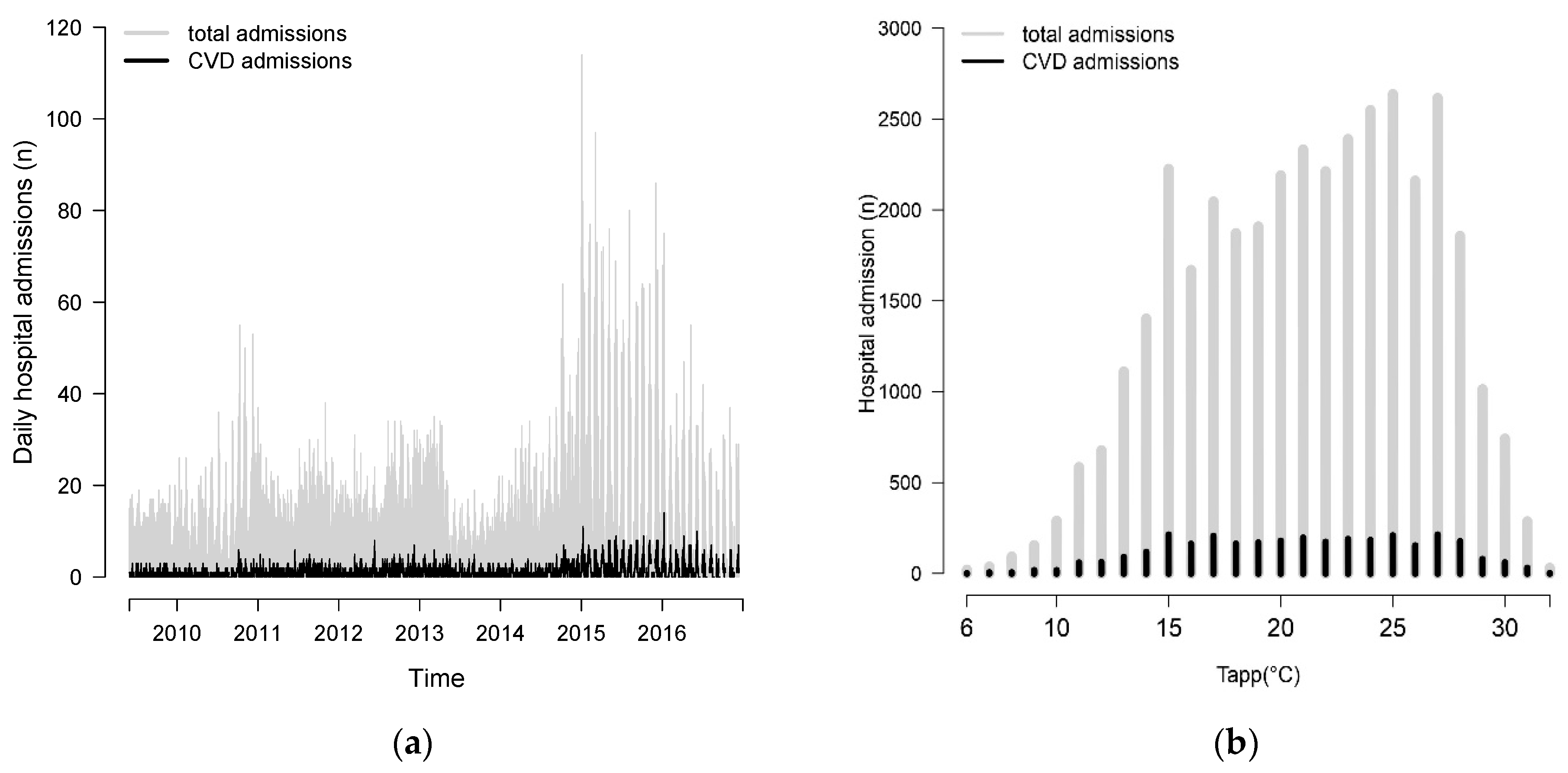
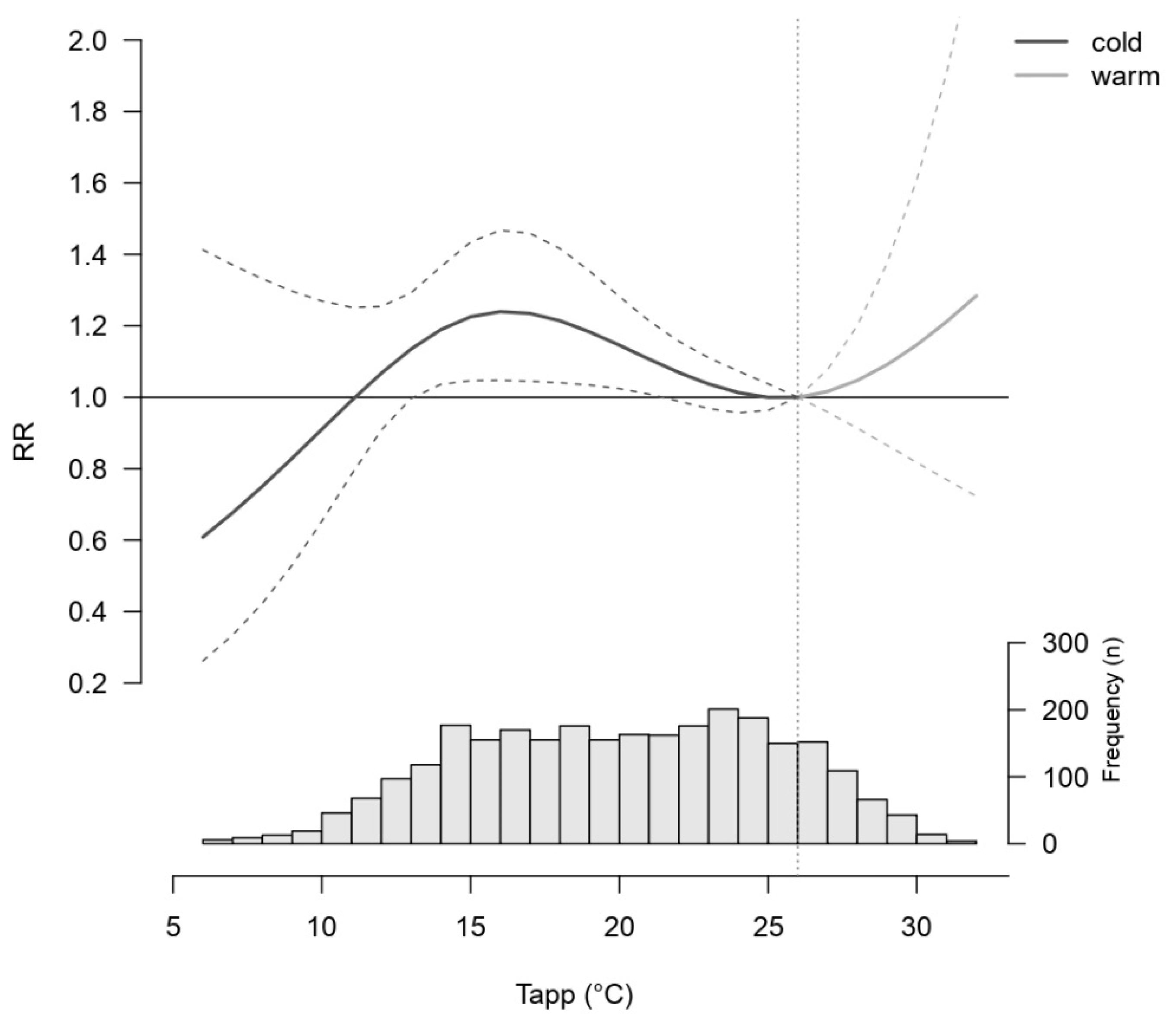
3. Results
Descriptive Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Cardiovascular Diseases Factsheet. 2021. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 March 2022).
- Papageorgiou, N. Cardiovascular Diseases-Genetic Susceptibility, Environmental Factors and their Interaction; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- De Blois, J.; Kjellstrom, T.; Agewall, S.; Ezekowitz, J.A.; Armstrong, P.W.; Atar, D. The Effects of Climate Change on Cardiac Health. Cardiology 2015, 131, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Cissé, G.; McLeman, R.; Adams, H.; Aldunce, P.; Bowen, K.; Campbell-Lendrum, D.; Clayton, S.; Ebi, K.L.; Hess, C.H.; Liu, Q.; et al. Health, wellbeing, and the changing structure of communities. In Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 1041–1170. [Google Scholar] [CrossRef]
- Vicedo-Cabrera, A.M.; Scovronick, N.; Sera, F.; Royé, D.; Schneider, R.; Tobias, A.; Astrom, C.; Guo, Y.; Honda, Y.; Hondula, D.M.; et al. The burden of heat-related mortality attributable to recent human-induced climate change. Nat. Clim. Chang. 2021, 11, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, Y.; Ye, T.; Gasparrini, A.; Tong, S.; Overcenco, A.; Urban, A.; Schneider, A.; Entezari, A.; Vicedo-Cabrera, A.M.; et al. Global, regional, and national burden of mortality associated with non-optimal ambient temperatures from 2000 to 2019: A three-stage modelling study. Lancet Planet. Health 2021, 5, e415–e425. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklöc, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Zafeiratou, S.; Samoli, E.; Dimakopoulou, K.; Rodpoulou, S.; Abalitis, A.; Gasparrini, A.; Stafoggia, M.; De’ Donato, D.; Rao, S.; Monteiro, A. A systematic review on the association between total and cardiopulmonary mortality/morbidity or cardiovascular risk factors with long-term exposure to increased or decreased ambient temperature. Sci. Total. Environ. 2021, 772, 145383. [Google Scholar] [CrossRef]
- Tobias, A.; Hashizume, M.; Honda, Y.; Sera, F.; Ng, C.F.S.; Kim, Y.; Royce, D.; Chung, Y.; Dang, T.N.; Kim, H.; et al. Geographical variations of the minimum mortality temperature at a global scale: A multicountry study. Environ. Epidemiol. 2021, 5, e169. [Google Scholar] [CrossRef]
- Liu, C.; Yavar, Z.; Sun, Q. Cardiovascular response to thermoregulatory challenges. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1793–H1812. [Google Scholar] [CrossRef]
- Sokolnicki, L.A.; Strom, N.A.; Roberts, E.K.; Kingsley-Berg, S.A.; Basu, A.; Charloudian, N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J. Appl. Physiol. 2009, 106, 566–570. [Google Scholar] [CrossRef]
- Ikäheimo, T.M. Cardiovascular diseases, cold exposure and exercise. Temperature 2018, 5, 123–146. [Google Scholar] [CrossRef]
- Keatinge, W.; Coleshaw, S.R.; Cotter, F.; Murphy, M.; Chelliah, R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: Factors in mortality from coronary and cerebral thrombosis in winter. BMJ 1984, 289, 1405–1408. [Google Scholar] [CrossRef]
- Shattock, M.J.; Tipton, M.J. ‘Autonomic conflict’: A different way to die during cold water immersion? J. Physiol. 2012, 590, 3219–3230. [Google Scholar] [CrossRef] [PubMed]
- Madaniyazi, L.; Armstrong, B.; Chung, Y.; Ng, C.F.S.; Seposo, X.; Kim, Y.; Tobias, A.; Guo, Y.; Sera, F.; Honda, Y.; et al. Seasonal variation in mortality and the role of temperature: A multi-country multi-city study. Int. J. Epidemiol. 2022, 51, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wolff, R.; Yu, W.; Vaneckova, P.; Pan, X.; Tong, S. Ambient Temperature and Morbidity: A Review of Epidemiological Evidence. Environ. Health Perspect. 2012, 120, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Qin, Y.; Shi, L.; Wei, G.; Zhu, H. Impact of temperature on morbidity: New evidence from China. J. Environ. Econ. Manag. 2021, 109, 102495. [Google Scholar] [CrossRef]
- Turner, L.R.; Barnett, A.G.; Connell, D.; Tong, S. Ambient temperature and cardiorespiratory morbidity: A systematic review and meta-analysis. Epidemiology 2012, 23, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hu, W.; Megnersen, K.; Guo, Y.; Pan, X.; Connell, D.; Tong, S. Time course of temperature effects on cardiovascular mortality in Brisbane, Australia. Heart 2011, 97, 1089–1093. [Google Scholar] [CrossRef]
- Guo, Y.; Gasparrini, A.; Li, S.; Sera, F.; Vicedo-Cabrera, A.M.; de Sousa, M.; Coelho, Z.S.; Saldiva, P.H.N.; Lavigne, E.; Tawatsupa, B.; et al. Quantifying excess deaths related to heatwaves under climate change scenarios: A multicountry time series modelling study. PLoS Med. 2018, 15, e1002629. [Google Scholar] [CrossRef]
- Scovronick, N.; Sera, F.; Acquaotta, F.; Garzena, D.; Fratianni, S.; Wright, C.Y.; Gasparrini, A. The association between ambient temperature and mortality in South Africa: A time-series analysis. Environ. Res. 2018, 161, 229–235. [Google Scholar] [CrossRef]
- Phung, D.; Guo, Y.; Thai, P.; Rutherford, S.; Wang, X.; Nguyen, M.; Manh Do, C.; Nguyen, N.H.; Alam, N.; Chu, C. The effects of high temperature on cardiovascular admissions in the most populous tropical city in Vietnam. Environ. Pollut. 2016, 208, 33–39. [Google Scholar] [CrossRef]
- Yang, J.; Yin, P.; Zhou, M.; Ou, C.-Q.; Guo, Y.; Gasparrini, A.; Liu, Y.; Yue, Y.; Gu, S.; Sang, S.; et al. Cardiovascular mortality risk attributable to ambient temperature in China. Heart 2015, 101, 1966–1972. [Google Scholar] [CrossRef]
- Rogelj, J.; Shindell, D.; Jiang, K.; Fifita, S.; Forster, P.; Ginzburg, V.; Handa, C.; Kheshgi, H.; Kobayashi, S.; Kriegler, E.; et al. Mitigation Pathways Compatible with 1.5 °C in the Context of Sustainable Development. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Global Burden of Disease 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Dong, J.-Y.; Yang, R.-Q.; Li, N. Air Temperature Affects the Hospital Admission for Cardiovascular Diseases among Rural Residents in Dingxi City. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2022, 44, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Bowry, A.D.; Lewey, J.; Dugani, S.B.; Choudhry, N.K. The burden of cardiovascular disease in low- and middle-income countries: Epidemiology and management. Can. J. Cardiol. 2015, 31, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, A.; Michels, K.; Wolfman, C.; Anand, N.; Sturke, R. Strengthening research capacity in LMICs to address the global NCD burden. Glob. Health Action 2020, 13, 1846904. [Google Scholar] [CrossRef] [PubMed]
- Odame, E.A.; Li, Y.; Zheng, S.; Vaidyanathan, A.; Silver, K. Assessing Heat-Related Mortality Risks among Rural Populations: A Systematic Review and Meta-Analysis of Epidemiological Evidence. Int. J. Environ. Res. Public Health 2018, 15, 1597. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, J.; Zhang, Q.; Sun, S.; Lei, R.; Zhang, C.; Cheng, H.; Ding, L.; Ding, R.; Xiao, C.; et al. The short-term effect of PM2.5/O3 on daily mortality from 2013 to 2018 in Hefei, China. Environ. Geochem. Health 2021, 43, 153–169. [Google Scholar] [CrossRef]
- Lokotola, C.L.; Wright, C.Y.; Wichmann, J. Temperature as a modifier of the effects of air pollution on cardiovascular disease hospital admissions in Cape Town, South Africa. Environ. Sci. Pollut. Res. 2020, 27, 16677–16685. [Google Scholar] [CrossRef]
- Shirinde, J.; Wichmann, J. Temperature modifies the association between air pollution and respiratory disease mortality in Cape Town, South Africa. Int. J. Environ. Health Res. 2022, 1–10. [Google Scholar] [CrossRef]
- IHME. South Africa Country Profile. 2019. Available online: https://www.healthdata.org/south-africa (accessed on 10 June 2022).
- Lyon, B. Southern Africa Summer Drought and Heat Waves: Observations and Coupled Model Behavior. J. Clim. 2009, 22, 6033–6046. [Google Scholar] [CrossRef]
- Burger, R.; Christian, C. Access to health care in post-apartheid South Africa: Availability, affordability, acceptability. Health Econ. Policy Law 2018, 15, 43–55. [Google Scholar] [CrossRef]
- Ntuli, S.T.; Maimela, E.; Alberts, M.; Choma, S.; Dikotope, S. Prevalence and associated risk factors of hypertension amongst adults in a rural community of Limpopo Province, South Africa. Afr. J. Prim. Health Care Fam. Med. 2015, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Shirinde, J.; Wichmann, J.; Voyi, K. Association between wheeze and selected air pollution sources in an air pollution priority area in South Africa: A cross-sectional study. Environ. Health 2014, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, F.; Adegoke, J.; Bopape, M.-J.M.; Naidoo, M.; Garland, R.; Thatcher, M.; McGregor, J.; Katzfey, J.; Werner, M.; Ichoku, C. Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ. Res. Lett. 2015, 10, 085004. [Google Scholar] [CrossRef]
- Garland, R.M.; Matooane, M.; Engelbrecht, F.A.; Bopape, M.-J.M.; Landman, W.A.; Naidoo, M.; Van der Merwe, J.; Wright, C.Y. Regional Projections of Extreme Apparent Temperature Days in Africa and the Related Potential Risk to Human Health. Int. J. Environ. Res. Public Health 2015, 12, 12577–12604. [Google Scholar] [CrossRef]
- Department of Cooperative Governance & Traditional Affairs. Mopani District Municipality Profile and Analysis. 2021. Available online: https://www.cogta.gov.za/ddm/wp-content/uploads/2020/11/Mopani-October-2020.pdf (accessed on 15 July 2022).
- Mbokodo, I.; Bopape, M.-J.; Chikoore, H.; Engelbrecht, F.; Nethengwe, N. Heatwaves in the Future Warmer Climate of South Africa. Atmosphere 2020, 11, 712. [Google Scholar] [CrossRef]
- Kruger, A.C.; Sekele, S.S. Trends in extreme temperature indices in South Africa: 1962-2009. Int. J. Clim. 2013, 33, 661–676. [Google Scholar] [CrossRef]
- Ikeda, T.; Kapwata, T.; Behera, S.K.; Manakwa, N.; Hashizume, M.; Sweijd, N.; Mathee, A.; Wright, C.Y. Climatic Factors in Relation to Diarrhoea Hospital Admissions in Rural Limpopo, South Africa. Atmosphere 2019, 10, 522. [Google Scholar] [CrossRef]
- Pedder, H.; Kapwata, T.; Howard, G.; Naidoo, R.N.; Kunene, Z.; Morris, R.W.; Mathee, A.; Wright, C.Y. Lagged Association between Climate Variables and Hospital Admissions for Pneumonia in South Africa. Int. J. Environ. Res. Public Health 2021, 18, 6191. [Google Scholar] [CrossRef]
- Kapwata, T.; Wright, C.Y.; du Preez, D.J.; Kunene, Z.; Mathee, A.; Ikeda, T.; Landman, W.; Maharaj, R.; Sweijd, N.; Minakawa, N.; et al. Exploring rural hospital admissions for diarrhoeal disease, malaria, pneumonia, and asthma in relation to temperature, rainfall and air pollution using wavelet transform analysis. Sci. Total. Environ. 2021, 791, 148307. [Google Scholar] [CrossRef]
- Steadman, R. Norms of Apparent Temperature in Australia; Australian Meteorological Magazine, 1994; Volume 43, pp. 1–16. Available online: http://www.bom.gov.au/jshess/docs/1994/steadman.pdf (accessed on 2 December 2022).
- Cabrera, A.M.V.; Sera, F.; Gasparrini, A. Hands-on Tutorial on a Modeling Framework for Projections of Climate Change Impacts on Health. Epidemiology 2019, 30, 321–329. [Google Scholar] [CrossRef]
- Guo, Y.; Barnett, A.G.; Pan, X.; Yu, W.; Tong, S. The Impact of Temperature on Mortality in Tianjin, China: A Case-Crossover Design with a Distributed Lag Nonlinear Model. Environ. Health Perspect. 2011, 119, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Gasparrini, A.; Hajat, S.; Smeeth, L.; Armstrong, B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013, 42, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Kondo, M.; McGregor, G.; Kim, H.; Hijioka, Y.; Yoshikawa, M.; Oka, K.; Takano, S.; Hales, S.; Kovats, R.S. Heat-related mortality risk model for climate change impact projection. Environ. Health Prev. Med. 2014, 19, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Leone, M. Attributable risk from distributed lag models. BMC Med. Res. Methodol. 2014, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Hajat, S.; Armstrong, B.G.; Gouveia, N.; Wilkson, P. Mortality displacement of heat-related deaths: A comparison of Delhi, Sao Paulo, and London. Epidemiology 2005, 16, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Guo, Y.; Yu, W.; Tong, S. Assessment of Short- and Long-Term Mortality Displacement in Heat-Related Deaths in Brisbane, Australia, 1996–2004. Environ. Health Perspect. 2015, 123, 766–772. [Google Scholar] [CrossRef]
- Schwartz, J.; Samet, J.M.; Patz, J.A. Hospital admissions for heart disease: The effects of temperature and humidity. Epidemiology 2004, 15, 755–761. [Google Scholar] [CrossRef]
- Mohammadi, D.; Zadeh, M.Z.; Sakhvidi, M.J.Z. Short-term exposure to extreme temperature and risk of hospital admission due to cardiovascular diseases. Int. J. Environ. Health Res. 2021, 31, 344–354. [Google Scholar] [CrossRef]
- Stuckler, D.; Basu, S.; McKee, M. Health care capacity and allocations among South Africa’s provinces: Infrastructure-inequality traps after the end of apartheid. Am. J. Public Health 2011, 101, 165–172. [Google Scholar] [CrossRef]
- Giang, P.N.; Dung, D.V.; Giang, K.B.; Vinhc, H.V.; Rocklöv, J. The effect of temperature on cardiovascular disease hospital admissions among elderly people in Thai Nguyen Province, Vietnam. Glob. Health Action 2014, 7, 23649. [Google Scholar] [CrossRef]
- Vaneckova, P.; Bambrick, H. Cause-Specific Hospital Admissions on Hot Days in Sydney, Australia. PLoS ONE 2013, 8, e55459. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Andersen, A.; Ketzel, M.; Ellermann, T.; Loft, S. Apparent Temperature and Cause-Specific Emergency Hospital Admissions in Greater Copenhagen, Denmark. PLoS ONE 2011, 6, e22904. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qui, H.; Sun, S.; Lin, H. Emergency Cardiovascular Hospitalization Risk Attributable to Cold Temperatures in Hong Kong. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.; Davídkovová, H.; Kyselý, J. Heat- and cold-stress effects on cardiovascular mortality and morbidity among urban and rural populations in the Czech Republic. Int. J. Biometeorol. 2014, 58, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Keates, A.K.; Redfern, A.; McMurray, J.J.V. Seasonal variations in cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 654–664. [Google Scholar] [CrossRef]
- Rosengren, A.; Smyth, A.; Rangarajan, S.; Ramasundarahettige, C.; Bangdiwala, S.I.; AlHabib, K.F.; Avezum, A.; Boström, K.B.; Chifamba, J.; Gulec, S.; et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: The Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob. Health 2019, 7, e748–e760. [Google Scholar] [CrossRef]
- Limpopo Treasury. Limpopo Socio-Economic Review and Outlook 2018/19 2018. p. 55. Available online: http://www.limtreasury.gov.za/lim_admin_trea/pages/sites/treasury_lim/documents/budget_statement/Limpopo%20Socio-Economic%20Review%20and%20Outlook%202018-19.pdf (accessed on 2 December 2022).
- Schulte, F.; Roosli, M.; Ragettli, M.S. Heat-related cardiovascular morbidity and mortality in Switzerland: A clinical perspective. Swiss Med. Wkly. 2021, 151, w30013. [Google Scholar] [CrossRef]
- Naidoo, S. The South African national health insurance: A revolution in health-care delivery! J. Public Health 2012, 34, 149–150. [Google Scholar] [CrossRef]
- Achebak, H.; Devolder, D.; Ballester, J. Trends in temperature-related age-specific and sex-specific mortality from cardiovascular diseases in Spain: A national time-series analysis. Lancet Planet. Health 2019, 3, e297–e306. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause transition and cardiovascular disease risk: Implications for timing of early prevention: A scientific statement from the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef]
- Bittel, J.; Henane, R. Comparison of thermal exchanges in men and women under neutral and hot conditions. J. Physiol. 1975, 250, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Horvath, S.M. Cardiovascular reactions to cold exposures differ with age and gender. J. Appl. Physiol. 1985, 58, 187–192. [Google Scholar] [CrossRef] [PubMed]


| AF | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| Non-optimal Tapps | 9.54% | 3.47% | 15.73% |
| Cold Tapps | 8.5% | 3.16% | 13.72% |
| Warm Tapps | 1.16% | −1.43% | 3.51% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bühler, J.L.; Shrikhande, S.; Kapwata, T.; Cissé, G.; Liang, Y.; Pedder, H.; Kwiatkowski, M.; Kunene, Z.; Mathee, A.; Peer, N.; et al. The Association between Apparent Temperature and Hospital Admissions for Cardiovascular Disease in Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 116. https://doi.org/10.3390/ijerph20010116
Bühler JL, Shrikhande S, Kapwata T, Cissé G, Liang Y, Pedder H, Kwiatkowski M, Kunene Z, Mathee A, Peer N, et al. The Association between Apparent Temperature and Hospital Admissions for Cardiovascular Disease in Limpopo Province, South Africa. International Journal of Environmental Research and Public Health. 2023; 20(1):116. https://doi.org/10.3390/ijerph20010116
Chicago/Turabian StyleBühler, Jacqueline Lisa, Shreya Shrikhande, Thandi Kapwata, Guéladio Cissé, Yajun Liang, Hugo Pedder, Marek Kwiatkowski, Zamantimande Kunene, Angela Mathee, Nasheeta Peer, and et al. 2023. "The Association between Apparent Temperature and Hospital Admissions for Cardiovascular Disease in Limpopo Province, South Africa" International Journal of Environmental Research and Public Health 20, no. 1: 116. https://doi.org/10.3390/ijerph20010116
APA StyleBühler, J. L., Shrikhande, S., Kapwata, T., Cissé, G., Liang, Y., Pedder, H., Kwiatkowski, M., Kunene, Z., Mathee, A., Peer, N., & Wright, C. Y. (2023). The Association between Apparent Temperature and Hospital Admissions for Cardiovascular Disease in Limpopo Province, South Africa. International Journal of Environmental Research and Public Health, 20(1), 116. https://doi.org/10.3390/ijerph20010116












