Prevalence and Subtype Distribution of Blastocystis Isolated from School-Aged Children in the Thai-Myanmar Border, Ratchaburi Province, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Population and Sample Collection
2.3. Extraction of Genomic DNA from Fecal Samples
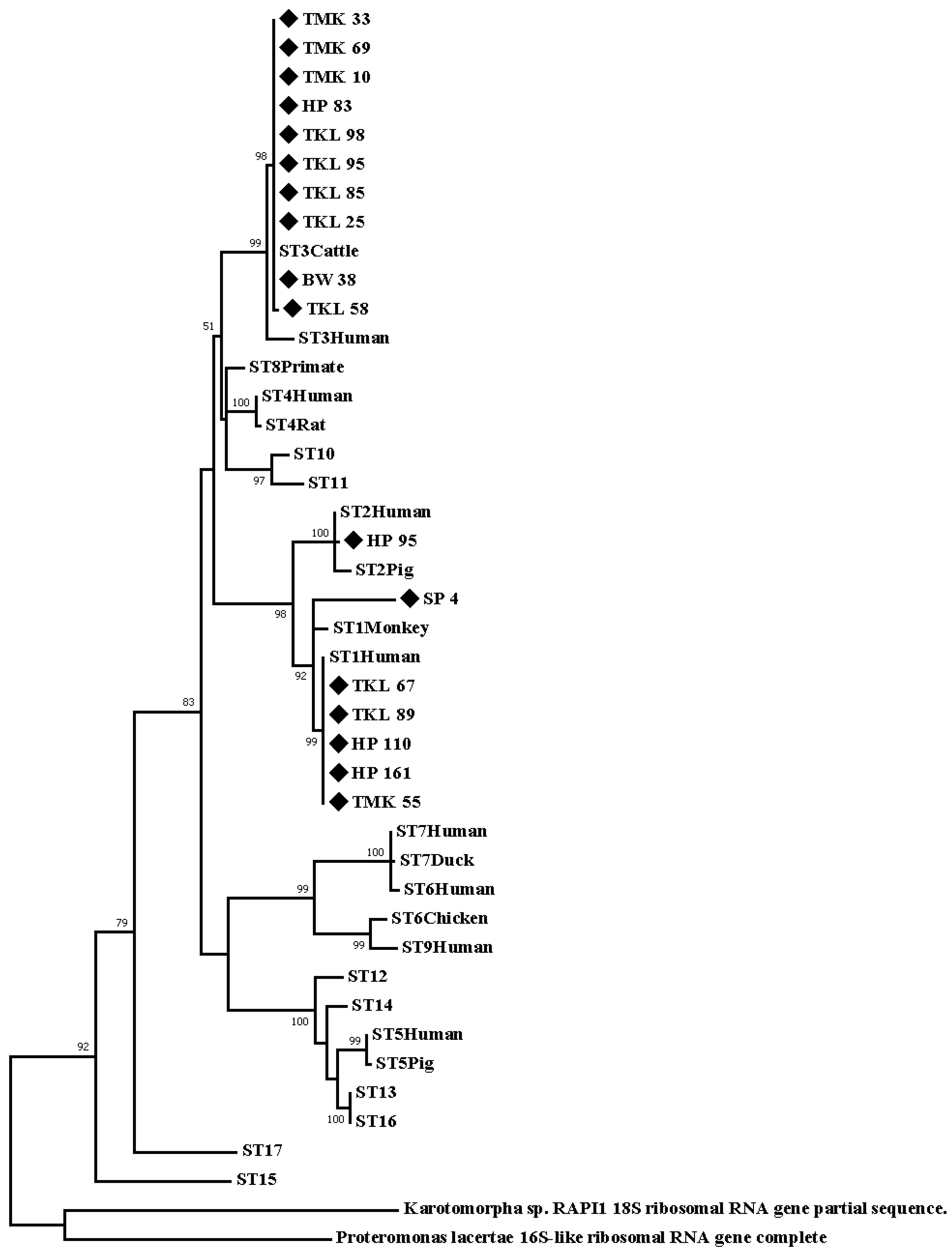
2.4. Sequencing and Phylogenic Analysis
2.5. Statistical Analysis
3. Results
3.1. Prevalence of Blastocystis Infection
3.2. Subtype Distribution of Blastocystis in School-Aged Children
3.3. Accession Number of Positive Samples and Phylogenic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korzeniewski, K.; Augustynowicz, A.; Smoleń, A.; Lass, A. Epidemiology of intestinal parasitic infections in school children in Ghazni Province, eastern Afghanistan. Pak. J. Med. Sci. 2015, 31, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Page, W.; Judd, J.A.; Bradbury, R.S. The Unique Life Cycle of Strongyloides stercoralis and Implications for Public Health Action. Trop. Med. Infect. Dis. 2018, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J. The drugs we have and the drugs we need against major helminth infections. Adv. Parasitol. 2010, 73, 197–230. [Google Scholar] [PubMed]
- Echagüe, G.; Sosa, L.; Díaz, V.; Ruiz, I.; Rivas, L.; Granado, D.; Funes, P.; Zenteno, J.; Pistilli, N.; Ramírez, M. Enteric parasitic disease in children under 5 years of age, indigenous and non-indigenous, from rural communities in Paraguay. Rev. Chil. Infectol. 2015, 32, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D.; Stensvold, C.R. Blastocystis: Getting to grips with our guileful guest. Trends Parasitol. 2013, 29, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R. Blastocystis: Genetic diversity and molecular methods for diagnosis and epidemiology. Trop. Parasitol. 2013, 3, 26–34. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Suresh, G.K.; Tan, K.S.; Thompson, R.A.; Traub, R.J.; Viscogliosi, E.; Yoshikawa, H.; Clark, C.G. Terminology for Blastocystis subtypes: A consensus. Trends Parasitol. 2007, 23, 93–96. [Google Scholar] [CrossRef]
- Valença-Barbosa, C.; Bomfim, T.C.B.; Teixeira, B.R.; Gentile, R.; da Costa Neto, S.F.; Magalhães, B.S.N.; de Almeida Balthazar, D.; da Silva, F.A.; Biot, R.; d’Avila Levy, C.M.; et al. Molecular epidemiology of Blastocystis isolated from animals in the state of Rio de Janeiro, Brazil. PLoS ONE 2019, 14, e0210740. [Google Scholar] [CrossRef]
- Moura, R.G.F.; Oliveira-Silva, M.B.; Pedrosa, A.L.; Nascentes, G.A.N.; Cabrine-Santos, M. Occurrence of Blastocystis spp. in domestic animals in Triângulo Mineiro area of Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 240–243. [Google Scholar] [CrossRef]
- Javanmard, E.; Niyyati, M.; Ghasemi, E.; Mirjalali, H.; Asadzadeh Aghdaei, H.; Zali, M.R. Impacts of human development index and climate conditions on prevalence of Blastocystis: A systematic review and meta-analysis. Acta Trop. 2018, 185, 193–203. [Google Scholar] [CrossRef]
- Greige, S.; El Safadi, D.; Khaled, S.; Gantois, N.; Baydoun, M.; Chemaly, M.; Benamrouz-Vanneste, S.; Chabé, M.; Osman, M.; Certad, G.; et al. First report on the prevalence and subtype distribution of Blastocystis sp. in dairy cattle in lebanon and assessment of zoonotic transmission. Acta Trop. 2019, 194, 23–29. [Google Scholar] [CrossRef]
- Saksirisampant, W.; Nuchprayoon, S.; Wiwanitkit, V.; Yenthakam, S.; Ampavasiri, A. Intestinal parasitic infestations among children in an orphanage in Pathum Thani province. J. Med. Assoc. Thail. 2003, 86, 263–270. [Google Scholar]
- Kyaw, P.P.; Paratthakonkun, C.; Yaicharoen, R.; Soonthornworasiri, N.; Prangthip, P.; Maneekan, P.; Zaw, A.P.; Thu, S.W.Y.M.; Arthan, D.; Nilkote, R. Prevalence of intestinal parasite and related factors among school children in Suan Phueng subdistrict, Ratchaburi, Thailand. In Proceedings of the International Conference on Applied Science and Health, Salaya, Thailand, 2 August 2018. [Google Scholar]
- Pipatsatitpong, D.; Rangsin, R.; Leelayoova, S.; Naaglor, T.; Mungthin, M. Incidence and risk factors of Blastocystis infection in an orphanage in Bangkok, Thailand. Parasit. Vectors 2012, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Thathaisong, U.; Siripattanapipong, S.; Mungthin, M.; Pipatsatitpong, D.; Tan-ariya, P.; Naaglor, T.; Leelayoova, S. Identification of Blastocystis subtype 1 variants in the Home for Girls, Bangkok, Thailand. Am. J. Trop. Med. Hyg. 2013, 88, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Tan, K.S.W.; Clark, C.G. Blastocystis. Trends Parasitol. 2020, 36, 315–316. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, S.; Ning, C.; Zhou, Y.; Teng, X.; Wu, X.; Chu, Y.; Yu, Y.; Chen, J.; Tian, L.; et al. Molecular Epidemiology and Risk Factors of Blastocystis sp. Infections Among General Populations in Yunnan Province, Southwestern China. Risk Manag. Healthc. Policy 2020, 13, 1791–1801. [Google Scholar] [CrossRef]
- Roberts, T.; Barratt, J.; Harkness, J.; Ellis, J.; Stark, D. Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of Blastocystis sp. In clinical stool samples. Am. J. Trop. Med. Hyg. 2011, 84, 308–312. [Google Scholar] [CrossRef]
- Kaewjai, C.; Prommano, O.; Tonsomboon, A. Detection of Blastocystis hominis using Simple direct smear compared with Jones’ medium cultivation in Thatu Subdistrict, Chiang Khan District, Loei Province. Thammasat Med. J. 2020, 20, 8–14. [Google Scholar]
- Srichaipon, N.; Nuchprayoon, S.; Charuchaibovorn, S.; Sukkapan, P.; Sanprasert, V. A Simple Genotyping Method for Rapid Differentiation of Blastocystis Subtypes and Subtype Distribution of Blastocystis spp. in Thailand. Pathogens 2019, 8, 38. [Google Scholar] [CrossRef]
- Aldahhasi, W.; Toulah, F.; Wakid, M. Evaluation of common microscopic techniques for detection of Blastocystis hominis. J. Egypt. Soc. Parasitol. 2020, 50, 33–40. [Google Scholar] [CrossRef]
- Poirier, P.; Wawrzyniak, I.; Albert, A.; El Alaoui, H.; Delbac, F.; Livrelli, V. Development and evatuation of real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: Prospective study of patients with hematological malignancies. J. Clin. Microbiol. 2011, 49, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, S.M.; Tawari, B.; Clark, C.G. DNA barcoding of Blastocystis. Protist 2006, 157, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kusolsuk, T.; Maipanich, W.; Nuamtanong, S.; Pubampen, S.; Sa-nguankiat, S.; Rojekittikhun, W.; Lekkla, A.; Tunyong, W.; Chettanadee, S.; Komalamisra, C. Parasitic and enteric bacterial infections among food handlers in tourist-area restaurants and educational-institution cafeterias, Sai-Yok District, Kanchanaburi Province, Thailand. J. Trop. Med. Parasitol. 2011, 34, 49. [Google Scholar]
- Hirata, T.; Nakamura, H.; Kinjo, N.; Hokama, A.; Kinjo, F.; Yamane, N.; Fujita, J. Prevalence of Blastocystis hominis and Strongyloides stercoralis infection in Okinawa, Japan. Parasitol. Res. 2007, 101, 1717–1719. [Google Scholar] [CrossRef]
- Suresh, K.; Smith, H. Comparison of methods for detecting for Blastocystis hominis. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 509–511. [Google Scholar] [CrossRef]
- Masucci, L.; Graffeo, R.; Bani, S.; Bugli, F.; Boccia, S.; Nicolotti, N.; Fiori, B.; Fadda, G.; Spanu, T. Intestinal parasites isolated in a large teaching hospital, Italy, 1 May 2006 to 31 December 2008. Eurosurveillance 2011, 16, 19891. [Google Scholar] [CrossRef]
- González-Moreno, O.; Domingo, L.; Teixidor, J.; Gracenea, M. Prevalence and associated factors of intestinal parasitisation: A cross-sectional study among outpatients with gastrointestinal symptoms in Catalonia, Spain. Parasitol. Res. 2011, 108, 87–93. [Google Scholar] [CrossRef]
- El Safadi, D.; Gaayeb, L.; Meloni, D.; Cian, A.; Poirier, P.; Wawrzyniak, I.; Delbac, F.; Dabboussi, F.; Delhaes, L.; Seck, M.; et al. Children of senegal river basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect. Dis. 2014, 14, 1471–2334. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Stensvold, C.R.; Rajilić-Stojanović, M.; Heilig, H.G.; De Vos, W.M.; O’Toole, P.W.; Cotter, P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330. [Google Scholar] [CrossRef]
- Popruk, S.; Udonsom, R.; Koompapong, K.; Mahittikorn, A.; Kusolsuk, T.; Ruangsittichai, J.; Palasuwan, A. Subtype distribution of Blastocystis in Thai-Myanmar border, Thailand. Korean J. Parasitol. 2015, 53, 13–19. [Google Scholar] [CrossRef]
- Gong, B.; Liu, X.; Wu, Y.; Xu, N.; Xu, M.; Yang, F.; Tong, L.; Zhou, K.; Cao, J.; Liu, A.; et al. Prevalence and subtype distribution of Blastocystis in ethnic minority groups on both sides of the China-Myanmar border, and assessment of risk factors. Parasite 2019, 26, 46. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.; Arendrup, M.; Jespersgaard, C.; Molbak, K.; Nielsen, H. Detecting Blastocystis using parasitologic and DNA-based methods: A comparative study. Diagn. Microbiol. Infect. Dis. 2007, 59, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Clark, C.G. Pre-empting Pandora’s Box: Blastocystis Subtypes Revisited. Trends Parasitol. 2020, 36, 229–232. [Google Scholar] [CrossRef]
- Yowang, A.; Tsaousis, A.D.; Chumphonsuk, T.; Thongsin, N.; Kullawong, N.; Popluechai, S.; Gentekaki, E. High diversity of Blastocystis subtypes isolated from asymptomatic adults living in Chiang Rai, Thailand. Infect. Genet. Evol. 2018, 65, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Popruk, N.; Prasongwattana, S.; Mahittikorn, A.; Palasuwan, A.; Popruk, S.; Palasuwan, D. Prevalence and subtype distribution of Blastocystis infection in patients with diabetes mellitus in Thailand. Int. J. Environ. Res. Public Health 2020, 17, 8877. [Google Scholar] [CrossRef] [PubMed]
- Sahimin, N.; Meor Termizi, F.H.; Rajamanikam, A.; Mohd Nazri, N.A.; Govind, S.K.; Mohd Zain, S.N. Prevalence and subtypes of Blastocystis among migrant workers from different working sectors in Peninsular Malaysia. Parasitol. Res. 2020, 119, 3555–3558. [Google Scholar] [CrossRef]
- Malatyalı, E.; Ertabaklar, H.; Ertuğ, S. Subtype Distribution of Blastocystis spp. with DNA Barcoding and Evaluation of Diagnostic Methods. Mikrobiyol. Bul. 2019, 53, 308–318. [Google Scholar] [CrossRef]
- Ascuña-Durand, K.; Salazar-Sánchez, R.S.; Castillo-Neyra, R.; Ballón-Echegaray, J. Relative Frequency of Blastocystis Subtypes 1, 2, and 3 in Urban and Periurban Human Populations of Arequipa, Peru. Trop. Med. Infect. Dis. 2020, 5, 178. [Google Scholar] [CrossRef]
- Gabrielli, S.; Furzi, F.; Sulekova, L.F.; Taliani, G.; Mattiucci, S. Occurrence of Blastocystis-subtypes in patients from Italy revealed association of ST3 with a healthy gut microbiota. Parasite Epidemiol. Control 2020, 9, e00134. [Google Scholar] [CrossRef]
- Oliveira-Arbex, A.P.; David, É.B.; Guimarães, S. Blastocystis genetic diversity among children of low-income daycare center in Southeastern Brazil. Infect. Genet. Evol. 2018, 57, 59–63. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Sánchez, L.V.; Bautista, D.C.; Corredor, A.F.; Flórez, A.C.; Stensvold, C.R. Blastocystis subtypes detected in humans and animals from Colombia. Infect. Genet. Evol. 2014, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Alfellani, M.A.; Stensvolde, C.R.; Vidal-Lipeidra, A.; Onuoha, E.S.H.; Fagbenro-Beyioku, A.F.; Clark, C.G. Variable grographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013, 126, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhang, Y.; Zhang, Q.; Li, J.; Zhang, L.; Wang, R. Genetic diversity of Blastocystis in kindergarten children in southern Xinjiang, China. Parasit. Vectors 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Riabi, T.R.; Mirjalali, H.; Haghighi, A.; Nejad, M.R.; Pourhoseingholi, M.A.; Poirier, P.; Delbace, F.; Wawrzyniake, I.; Reza, M.; Genetic, Z. Diversity analysis of Blastocystis subtypes from both symptomatic and asymptomatic subjects using a barcoding region from the 18S rRNA gene. Infect. Genet. Evol. 2018, 61, 119–126. [Google Scholar] [CrossRef] [PubMed]

| School | Participants/Total No. (Kindergarten & Grades 1–3) (%) | Number of Positive Samples (%) |
|---|---|---|
| TKL School | 98/239 (41.0) | 7/98 (7.14) |
| HP School | 170/228 (74.56) | 4/170 (2.35) |
| TMK School | 74/283 (26.15) | 4/74 (5.40) |
| BW School | 47/168 (27.98) | 1/47 (2.13) |
| SP School | 96/213 (45.07) | 1/96 (1.04) |
| RJ School | 23/85 (27.06) | 0/23 (0) |
| Total | 508/1216 (41.78) | 17/508 (3.35%) |
| Samples | Genbank Accession Number | Sex | Age | Blastocystis Subtype | Allele | Sequence Similarity (%) | Similar Genbank Reference Sequences (Source) |
|---|---|---|---|---|---|---|---|
| TKL25 | OM149811 | F | 6 | 3 | 34 | 99.64% | MT039586 (Human) |
| TKL58 | OM149812 | M | 7 | 3 | 36 | 99.64% | MT645665 (Human) |
| TKL67 | OM149813 | M | 8 | 3 | 34 | 99.64% | MT645669 (Human) |
| TKL85 | OM149814 | F | 8 | 3 | 34 | 99.44% | MT039597 (Human) |
| TKL89 | OM149815 | F | 11 | 1 | 4 | 99.63% | MT645664 (Human) |
| TKL95 | OM149816 | F | 8 | 3 | 34 | 98.92% | KY675330 (Human) |
| TKL98 | OM149817 | F | 9 | 3 | 34 | 99.63% | MT039597 (Human) |
| HP83 | OM149818 | M | 6 | 3 | 34 | 99.45 | MG011642 (Human) |
| HP85 | OM149819 | F | 6 | 2 | 9 | 99.11 | MT039594 (Human) |
| HP110 | OM149820 | M | 7 | 1 | 4 | 99.28 | MT042818 (Human) |
| HP161 | OM149822 | F | 6 | 1 | 4 | 100.00 | MK719642 (Human) |
| TMK10 | OM149823 | M | 5 | 3 | 34 | 99.28 | MK100354 (Human) |
| TMK33 | OM149824 | M | 7 | 3 | 34 | 99.82 | MT039556 (Human) |
| TMK55 | OM149825 | M | 9 | 1 | 4 | 99.64 | MG011607 (Human) |
| TMK69 | OM149826 | M | 5 | 3 | 34 | 100.00 | MG011642 (Human) |
| BW38 | OM149827 | M | 6 | 3 | 34 | 100.00 | MT039567 (Human) |
| SP4 | OM149828 | M | 9 | 1 | 4 | 99.80 | MH021854 (Rat) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu, A.; Sutthikornchai, C.; Mahittikorn, A.; Koompapong, K.; Chiabchalard, R.; Arthan, D.; Soonthornworasiri, N.; Popruk, S. Prevalence and Subtype Distribution of Blastocystis Isolated from School-Aged Children in the Thai-Myanmar Border, Ratchaburi Province, Thailand. Int. J. Environ. Res. Public Health 2023, 20, 204. https://doi.org/10.3390/ijerph20010204
Abu A, Sutthikornchai C, Mahittikorn A, Koompapong K, Chiabchalard R, Arthan D, Soonthornworasiri N, Popruk S. Prevalence and Subtype Distribution of Blastocystis Isolated from School-Aged Children in the Thai-Myanmar Border, Ratchaburi Province, Thailand. International Journal of Environmental Research and Public Health. 2023; 20(1):204. https://doi.org/10.3390/ijerph20010204
Chicago/Turabian StyleAbu, Amanee, Chantira Sutthikornchai, Aongart Mahittikorn, Khuanchai Koompapong, Rachatawan Chiabchalard, Dumrongkiet Arthan, Ngamphol Soonthornworasiri, and Supaluk Popruk. 2023. "Prevalence and Subtype Distribution of Blastocystis Isolated from School-Aged Children in the Thai-Myanmar Border, Ratchaburi Province, Thailand" International Journal of Environmental Research and Public Health 20, no. 1: 204. https://doi.org/10.3390/ijerph20010204
APA StyleAbu, A., Sutthikornchai, C., Mahittikorn, A., Koompapong, K., Chiabchalard, R., Arthan, D., Soonthornworasiri, N., & Popruk, S. (2023). Prevalence and Subtype Distribution of Blastocystis Isolated from School-Aged Children in the Thai-Myanmar Border, Ratchaburi Province, Thailand. International Journal of Environmental Research and Public Health, 20(1), 204. https://doi.org/10.3390/ijerph20010204






