Average and Interindividual Effects to a Comprehensive Cardiovascular Rehabilitation Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Concurrent Exercise Training
2.4. Nutritional Counseling
2.5. Psychological Support
2.6. Lifestyle Education
2.7. Measurements
2.7.1. Cardiopulmonary Exercise Test
2.7.2. Anthropometric and Body Composition Parameters
2.7.3. Quality of Life, Anxiety, and Depression
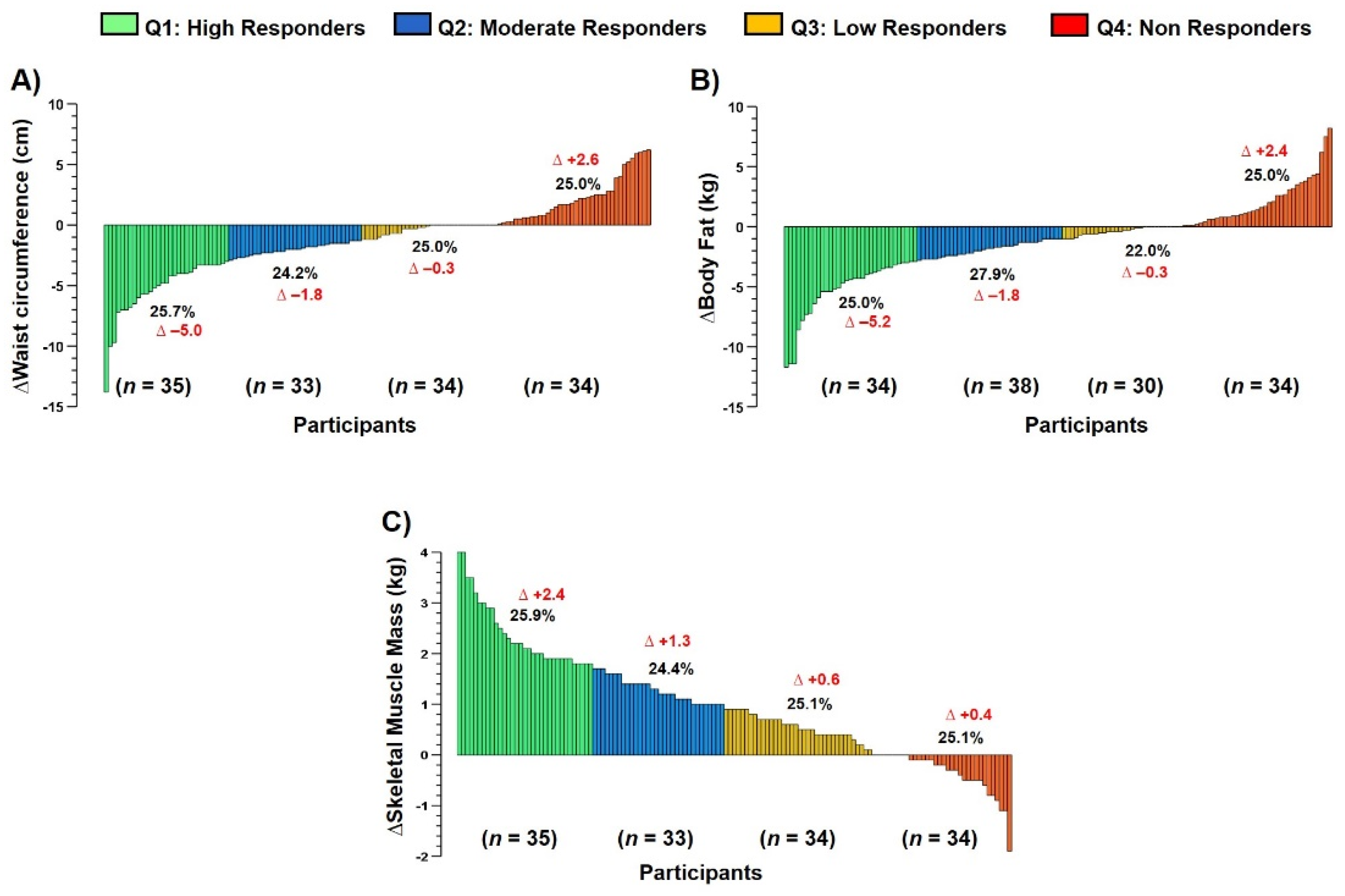
2.7.4. Interindividual Variability to Exercise Training
2.8. Statistical Analysis
3. Results
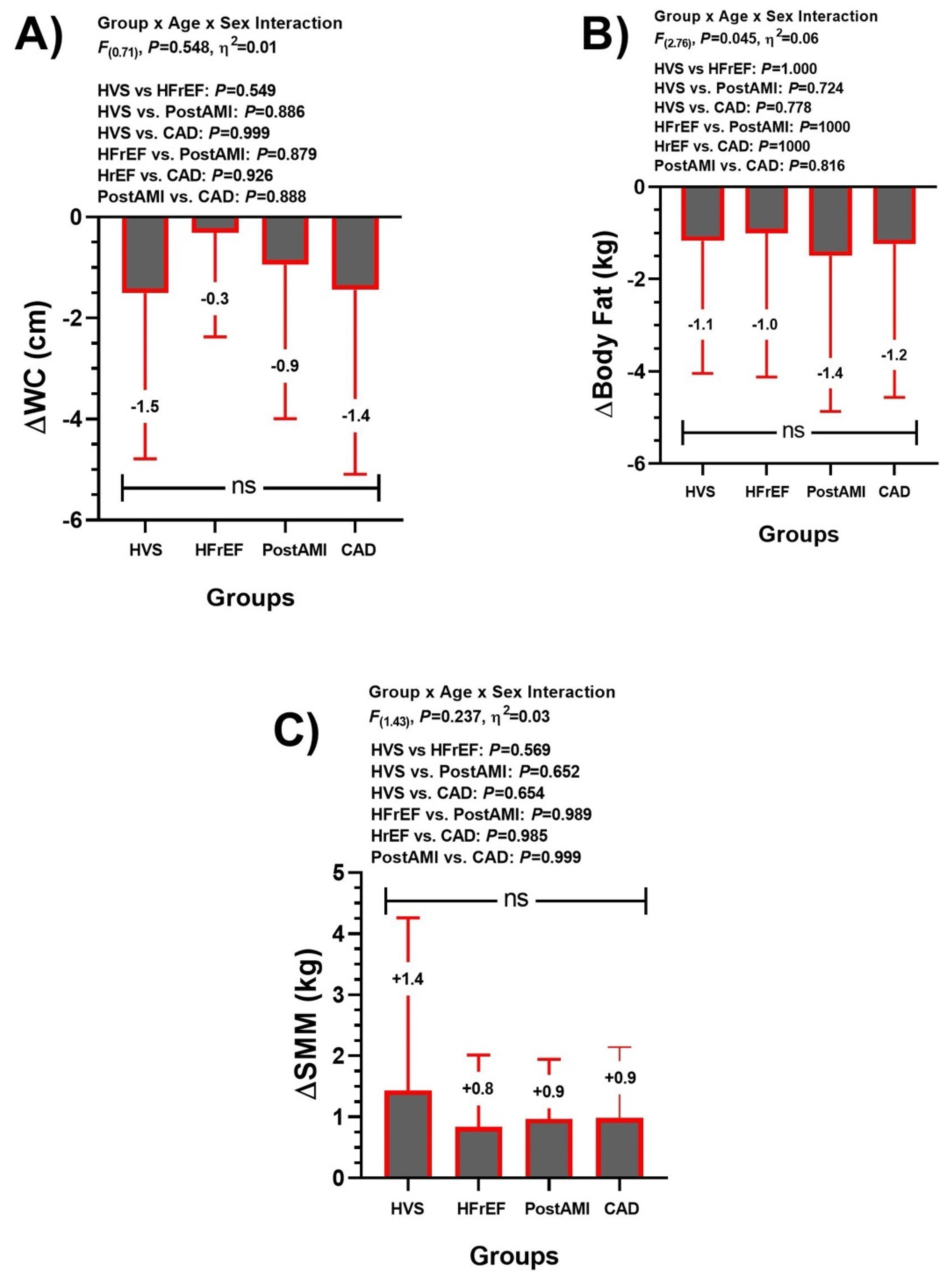
3.1. Anthropometric/Body Composition Group Comparison (Main Outcomes)
3.2. Cardiorespiratory Performance Group Comparison (Main Outcomes)
3.3. Training-Induced Changes at Anthropometric/Body Composition (Secondary Outcomes)
3.4. Training-Induced Changes at Cardiorespiratory Fitness (Secondary Outcomes)
3.5. Training-Induced Changes in Quality of Life and Emotional Health Parameters (Secondary Outcomes)
3.6. Interindividual Variability to Anthropometric/Body Composition Parameters (Main Outcomes)
3.7. Interindividual Variability to Cardiorespiratory Parameters among Groups (Main Outcomes)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kabboul, N.N.; Tomlinson, G.; Francis, T.A.; Grace, S.L.; Chaves, G.; Rac, V.; Daou-Kabboul, T.; Bielecki, J.M.; Alter, D.A.; Krahn, M. Comparative Effectiveness of the Core Components of Cardiac Rehabilitation on Mortality and Morbidity: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2018, 7, 514. [Google Scholar] [CrossRef] [Green Version]
- Balady, G.J.; Ades, P.A.; Bittner, V.A.; Franklin, B.A.; Gordon, N.F.; Thomas, R.J.; Tomaselli, G.F.; Yancy, C.W. Referral, Enrollment, and Delivery of Cardiac Rehabilitation/Secondary Prevention Programs at Clinical Centers and beyond: A Presidential Advisory from the American Heart Association. Circulation 2011, 124, 2951–2960. [Google Scholar] [CrossRef] [Green Version]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease: The Task Force on Sports Cardiology and Exercise in Patients with Cardiovascular Disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Price, K.J.; Gordon, B.A.; Bird, S.R.; Benson, A.C. A Review of Guidelines for Cardiac Rehabilitation Exercise Programmes: Is There an International Consensus? Eur. J. Prev. Cardiol. 2016, 23, 1715–1733. [Google Scholar] [CrossRef]
- Abreu, A.; Frederix, I.; Dendale, P.; Janssen, A.; Doherty, P.; Piepoli, M.F.; Voeller, H.; Davos, C.H. Standardization and Quality Improvement of Secondary Prevention through Cardiovascular Rehabilitation Programmes in Europe. A Position Statement of the Secondary Prevention and Rehabilitation Section of The. Eur. J. Prev. Cardiol. 2021, 28, 496–509. [Google Scholar] [CrossRef]
- Candelaria, D.; Zecchin, R.; Ferry, C.; Ladak, L.; Randall, S.; Gallagher, R. Shorter Wait Times to Cardiac Rehabilitation Associated with Greater Exercise Capacity Improvements: A Multisite Study. J. Cardiopulm. Rehabil. Prev. 2021, 41, 243–248. [Google Scholar] [CrossRef]
- Andjic, M.; Spiroski, D.; Vidakovic, T.; Lazovic, M.; Babic, D.; Ristic, A.; Mazic, S.; Zdravkovic, M.; Otasevic, P. Effect of Short-Term Exercise Training in Patients Following Acute Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. Eur. J. Phys. Rehabil. Med. 2016, 52, 364–369. [Google Scholar]
- Benzer, W.; Platter, M.; Oldridge, N.B.; Schwann, H.; Machreich, K.; Kullich, W.; Mayr, K.; Philippi, A.; Gassner, A.; Dörler, J. Short-Term Patient-Reported Outcomes after Different Exercise-Based Cardiac Rehabilitation Programmes. Eur. J. Prev. Cardiol. 2007, 14, 441–447. [Google Scholar] [CrossRef]
- Zoch-Lesniak, B.; Dobberke, J.; Schlitt, A.; Bongarth, C.; Glatz, J.; Spörl-Dönch, S.; Koran, I.; Völler, H.; Salzwedel, A. Performance Measures for Short-Term Cardiac Rehabilitation in Patients of Working Age: Results of the Prospective Observational Multicenter Registry OutCaRe. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100043. [Google Scholar] [CrossRef]
- Reich, B.; Benzer, W.; Harpf, H.; Hofmann, P.; Mayr, K.; Ocenasek, H.; Podolsky, A.; Pokan, R.; Porodko, M.; Puelacher, C. Efficacy of Extended, Comprehensive Outpatient Cardiac Rehabilitation on Cardiovascular Risk Factors: A Nationwide Registry. Eur. J. Prev. Cardiol. 2020, 27, 1026–1033. [Google Scholar] [CrossRef]
- da Silva, A.K.F.; da Barbosa, M.P.C.d.R.; Bernardo, A.F.B.; Vanderlei, F.M.; Pacagnelli, F.L.; Vanderlei, L.C.M. Cardiac Risk Stratification in Cardiac Rehabilitation Programs: A Review of Protocols. Braz. J. Cardiovasc. Surg. 2014, 29, 255–265. [Google Scholar] [CrossRef] [Green Version]
- American Association of Cardiovascular & Pulmonary Rehabilitation. Guidelines for Pulmonary Rehabilitation Programs; Human Kinetics: Champaign, IL, USA, 2011; ISBN 0736096531. [Google Scholar]
- Bouchard, C.; Blair, S.N.; Church, T.S.; Earnest, C.P.; Hagberg, J.M.; Häkkinen, K.; Jenkins, N.T.; Karavirta, L.; Kraus, W.E.; Leon, A.S. Adverse Metabolic Response to Regular Exercise: Is It a Rare or Common Occurrence? PLoS ONE 2012, 7, e37887. [Google Scholar] [CrossRef]
- Álvarez, C.; Ramírez-Campillo, R.; Ramírez-Vélez, R.; Izquierdo, M. Effects and Prevalence of Nonresponders after 12 Weeks of High-Intensity Interval or Resistance Training in Women with Insulin Resistance: A Randomized Trial. J. Appl. Physiol. 2017, 122, 985–996. [Google Scholar] [CrossRef]
- Álvarez, C.; Ramírez-Campillo, R.; Lucia, A.; Ramírez-Vélez, R.; Izquierdo, M. Concurrent Exercise Training on Hyperglycemia and Comorbidities Associated: Non-responders Using Clinical Cutoff Points. Scand. J. Med. Sci. Sports 2019, 29, 952–967. [Google Scholar] [CrossRef]
- Álvarez, C.; Guede-Rojas, F.; Ramírez-Campillo, R.; Andrade, D.C.; Vásquez-Gómez, J.; Rodríguez-Rodríguez, F.; Ciolac, E.G.; Caamaño-Navarrete, F.; Delgado-Floody, P. Characterizing the Interindividual Postexercise Hypotension Response for Two Order Groups of Concurrent Training in Patients with Morbid Obesity. Front. Physiol. 2022, 2033, 913645. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Hernández-Quiñones, P.A.; Tordecilla-Sanders, A.; Álvarez, C.; Ramírez-Campillo, R.; Izquierdo, M.; Correa-Bautista, J.E.; Garcia-Hermoso, A.; Garcia, R.G. Effectiveness of HIIT Compared to Moderate Continuous Training in Improving Vascular Parameters in Inactive Adults. Lipids Health Dis. 2019, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Robertson, L.D. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs; Human Kinetics: Champaign, IL, USA, 2004; ISBN 0736048642. [Google Scholar]
- Agostoni, P.; Bianchi, M.; Moraschi, A.; Palermo, P.; Cattadori, G.; La Gioia, R.; Bussotti, M.; Wasserman, K. Work-rate Affects Cardiopulmonary Exercise Test Results in Heart Failure. Eur. J. Heart Fail. 2005, 7, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, T.-I.; Tveitå, E.K.; Natvig, G.K.; Tonstad, S.; Hjelmesæth, J. Validity of the SF-36 in Patients with Morbid Obesity. Obes. Facts 2011, 4, 346–351. [Google Scholar] [CrossRef]
- McGregor, G.; Powell, R.; Kimani, P.; Underwood, M. Does Contemporary Exercise-Based Cardiac Rehabilitation Improve Quality of Life for People with Coronary Artery Disease? A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e036089. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The Validity of the Hospital Anxiety and Depression Scale. An Updated Literature Review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Green, D.J.; Eijsvogels, T.; Bouts, Y.M.; Maiorana, A.J.; Naylor, L.H.; Scholten, R.R.; Spaanderman, M.E.A.; Pugh, C.J.A.; Sprung, V.S.; Schreuder, T. Exercise Training and Artery Function in Humans: Nonresponse and Its Relationship to Cardiovascular Risk Factors. J. Appl. Physiol. 2014, 117, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Floody, P.; Chirosa-Ríos, L.; Caamaño-Navarrete, F.; Valdés-Badilla, P.; Herrera-Valenzuela, T.; Monsalves-Álvarez, M.; Núñez-Espinosa, C.; Castro-Sepulveda, M.; Guzmán-Muñoz, E.; Andrade, D.C. Concurrent Training and Interindividual Response in Women with a High Number of Metabolic Syndrome Risk Factors. Front. Physiol. 2022, 1922, 934038. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [Green Version]
- Sandercock, G.; Hurtado, V.; Cardoso, F. Changes in Cardiorespiratory Fitness in Cardiac Rehabilitation Patients: A Meta-Analysis. Int. J. Cardiol. 2013, 167, 894–902. [Google Scholar] [CrossRef]
- Kokkinos, P.; Narayan, P. Cardiorespiratory Fitness in Cardiometabolic Diseases; Springer: Cham, Switzerland, 2019; pp. 49–56. [Google Scholar]
- O’Neill, J.O.; Young, J.B.; Pothier, C.E.; Lauer, M.S. Peak Oxygen Consumption as a Predictor of Death in Patients with Heart Failure Receiving β-Blockers. Circulation 2005, 111, 2313–2318. [Google Scholar] [CrossRef] [Green Version]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of Peak Exercise Oxygen Consumption for Optimal Timing of Cardiac Transplantation in Ambulatory Patients with Heart Failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Higginbotham, M.B.; Morris, K.G.; Williams, R.S.; McHale, P.A.; Coleman, R.E.; Cobb, F.R. Regulation of Stroke Volume during Submaximal and Maximal Upright Exercise in Normal Man. Circ. Res. 1986, 58, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Brubaker, P.H.; Kitzman, D.W. Chronotropic Incompetence: Causes, Consequences, and Management. Circulation 2011, 123, 1010–1020. [Google Scholar] [CrossRef] [Green Version]
- Dresing, T.J.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Marwick, T.H.; Lauer, M.S. Usefulness of Impaired Chronotropic Response to Exercise as a Predictor of Mortality, Independent of the Severity of Coronary Artery Disease. Am. J. Cardiol. 2000, 86, 602–609. [Google Scholar] [CrossRef]
- Hinkle, L.E.; Carver, S.T.; Plakun, A. Slow Heart Rates and Increased Risk of Cardiac Death in Middle-Aged Men. Arch. Intern. Med. 1972, 129, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, A.; Piras, F.; Chiappori, P.; Vitelli, S.; Caria, M.A.; Lobina, A.; Milia, R.; Tocco, F.; Concu, A.; Melis, F. Estimating Stroke Volume from Oxygen Pulse during Exercise. Physiol. Meas. 2007, 28, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Accalai, E.; Vignati, C.; Salvioni, E.; Pezzuto, B.; Contini, M.; Cadeddu, C.; Meloni, L.; Agostoni, P. Non-Invasive Estimation of Stroke Volume during Exercise from Oxygen in Heart Failure Patients. Eur. J. Prev. Cardiol. 2020, 28, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Lomivorotov, V.V.; Efremov, S.M.; Kirov, M.Y.; Fominskiy, E.V.; Karaskov, A.M. Low-Cardiac-Output Syndrome after Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 291–308. [Google Scholar] [CrossRef]
- Duncan, A.E.; Kartashov, A.; Robinson, S.B.; Randall, D.; Zhang, K.; Luber, J.; James, R.A.; Halvorson, S.; Bokesch, P. Risk Factors, Resource Use, and Cost of Postoperative Low Cardiac Output Syndrome. J. Thorac. Cardiovasc. Surg. 2022, 163, 1890–1898. [Google Scholar] [CrossRef]
- Savage, P.D.; Rengo, J.L.; Menzies, K.E.; Ades, P.A. Cardiac Rehabilitation after Heart Valve Surgery: Comparison with Coronary Artery Bypass Grafting Patients. J. Cardiopulm. Rehabil. Prev. 2015, 35, 231. [Google Scholar] [CrossRef]
- Wasserman, K.; Whipp, B.J. Exercise Physiology in Health and Disease. Am. Rev. Respir. Dis. 1975, 112, 219–249. [Google Scholar]
- Dubach, P.; Myers, J.; Dziekan, G.; Goebbels, U.; Reinhart, W.; Muller, P.; Buser, P.; Stulz, P.; Vogt, P.; Ratti, R. Effect of High Intensity Exercise Training on Central Hemodynamic Responses to Exercise in Men with Reduced Left Ventricular Function. J. Am. Coll. Cardiol. 1997, 29, 1591–1598. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Ma, W.; Song, H.; Gong, Z.; Wang, Q.; Che, L.; Xu, W.; Jiang, J.; Xu, J.; et al. VE/VCO2 Slope and Its Prognostic Value in Patients with Chronic Heart Failure. Exp. Ther. Med. 2015, 9, 1407. [Google Scholar] [CrossRef] [Green Version]
- Lavie, C.J.; Milani, R. V Effects of Cardiac Rehabilitation and Exercise Training in Obese Patients with Coronary Artery Disease. Chest 1996, 109, 52–56. [Google Scholar] [CrossRef]
- El Missiri, A.; Abdel Halim, W.A.; Almaweri, A.S.; Mohamed, T.R. Effect of a Phase 2 Cardiac Rehabilitation Program on Obese and Non-Obese Patients with Stable Coronary Artery Disease. Egypt. Heart J. 2021, 73, 1–8. [Google Scholar] [CrossRef]
- Von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle Wasting and Cachexia in Heart Failure: Mechanisms and Therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Valentino, G.; Galgani, J.E.; Álamos, M.; Orellana, L.; Adasme, M.; Berríos, A.; Acevedo, M. Anthropometric and Blood Pressure Changes in Patients with or without Nutritional Counselling during Cardiac Rehabilitation: A Retrospective Study. J. Hum. Nutr. Diet. 2021, 34, 402–412. [Google Scholar] [CrossRef]
- Marzolini, S.; Oh, P.I.; Brooks, D. Effect of Combined Aerobic and Resistance Training versus Aerobic Training Alone in Individuals with Coronary Artery Disease: A Meta-Analysis. Eur. J. Prev. Cardiol. 2012, 19, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pozuelo, L.; Brennan, D.M.; Hoar, B.; Hoogwerf, B.J. Association of SF-36 with Coronary Artery Disease Risk Factors and Mortality: A PreCIS Study. Prev. Cardiol. 2010, 13, 122–129. [Google Scholar] [CrossRef]
- De Melo Ghisi, G.L.; Chaves, G.S.S.; Ribeiro, A.L.; Oh, P.; Britto, R.R.; Grace, S.L. Comprehensive Cardiac Rehabilitation Effectiveness in a Middle-Income Setting: A Randomized Controlled Trial. J. Cardiopulm. Rehabil. Prev. 2020, 40, 399–406. [Google Scholar] [CrossRef]
- Saeidi, M.; Mostafavi, S.; Heidari, H.; Masoudi, S. Effects of a Comprehensive Cardiac Rehabilitation Program on Quality of Life in Patients with Coronary Artery Disease. ARYA Atheroscler. 2013, 9, 179–185. [Google Scholar] [CrossRef]
- Godfrey, C.; Harrison, M.B.; Medves, J.; Tranmer, J.E. The Symptom of Pain with Heart Failure: A Systematic Review. J. Card. Fail. 2006, 12, 307–313. [Google Scholar] [CrossRef]
- Lissåker, C.T.; Norlund, F.; Wallert, J.; Held, C.; Olsson, E.M.G. Persistent Emotional Distress after a First-Time Myocardial Infarction and Its Association to Late Cardiovascular and Non-Cardiovascular Mortality. Eur. J. Prev. Cardiol. 2019, 26, 1510–1518. [Google Scholar] [CrossRef]
- Gan, T.J.; Habib, A.S.; Miller, T.E.; White, W.; Apfelbaum, J.L. Incidence, Patient Satisfaction, and Perceptions of Post-Surgical Pain: Results from a US National Survey. Curr. Med. Res. Opin. 2014, 30, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Gremeaux, V.; Drigny, J.; Nigam, A.; Juneau, M.; Guilbeault, V.; Latour, E.; Gayda, M. Long-Term Lifestyle Intervention with Optimized High-Intensity Interval Training Improves Body Composition, Cardiometabolic Risk, and Exercise Parameters in Patients with Abdominal Obesity. Am. J. Phys. Med. Rehabil. 2012, 91, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Rankinen, T. Individual Differences in Response to Regular Physical Activity. Med. Sci. Sport Exerc. 2001, 33, S446–S451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Almela, S.; Way, K.L.; Terada, T.; Tulloch, H.E.; Keast, M.L.; Pipe, A.L.; Chirico, D.; Reed, J.L. Sex Differences in Physical and Mental Health Following Highintensity Interval Training in Adults with Cardiovascular Disease Who Completed Cardiac Rehabilitation. Appl. Physiol. Nutr. Metab. 2022, 47, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sibilitz, K.L.; Berg, S.K.; Rasmussen, T.B.; Risom, S.S.; Thygesen, L.C.; Tang, L.; Hansen, T.B.; Johansen, P.P.; Gluud, C.; Lindschou, J. Cardiac Rehabilitation Increases Physical Capacity but Not Mental Health after Heart Valve Surgery: A Randomised Clinical Trial. Heart 2016, 102, 1995–2003. [Google Scholar] [CrossRef]


| Variable | HVS | HFrEF | Post-AMI | CAD |
|---|---|---|---|---|
| (n=) | 32 | 23 | 40 | 45 |
| Age, years mean (SD) | 60.0 (13.2) | 53.0 (14.3) | 58.0 (10.6) | 63.2 (8.4) |
| Male, n (%) | 20 (62.5) | 16 (69.6) | 31 (77.5) | 40 (88.9) |
| Family history of CVD, n (%) | 13 (40.6) | 12 (52.2) | 21 (52.5) | 22 (48.9) |
| Ex-smokers, n (%) | 26 (81.3) | 19 (82.6) | 33 (82.5) | 37 (82.2) |
| Ejection fraction % mean (SD) | 63.1 (14.6) | 28.7 (7.0) | 57.2 (12.9) | 55.5 (15.6) |
| Myocardial akinesia, n (%) | 0 (0.0) | 1 (4.4) | 4 (10.0) | 1 (2.2) |
| Myocardial hypokinesia, n (%) | 0 (0.0) | 2 (8.7) | 1 (2.5) | 1 (2.2) |
| Myocardial dyskinesia, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) |
| Comorbidities, n (%) | ||||
| DMNID | 3 (9.4) | 2 (8.7) | 10 (25.0) | 10 (22.2) |
| DMID | 4 (12.5) | 4 (17.4) | 4 (10.0) | 6 (13.3) |
| Dyslipidemia | 10 (31.3) | 6 (26.1) | 32 (57.5) | 32 (71.1) |
| Arterial hypertension | 17 (53.1) | 11 (47.8) | 27 (67.5) | 36 (80.0) |
| Asthma | 0 (0.0) | 0 (0.0) | 1 (2.5) | 1 (2.2) |
| Stroke | 1 (3.1) | 0 (0.0) | 0 (0.0) | 1 (2.2) |
| COPD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) |
| CKD | 0 (0.0) | 0 (0.0) | 1 (2.5) | 2 (4.4) |
| Medical interventions, n (%) | ||||
| Arterial bypass | 2 (6.3) | 1 (4.4) | 12 (30.0) | 38 (84.4) |
| PTCA | 1 (3.1) | 1 (4.4) | 25 (62.5) | 7 (15.6) |
| Stent | 1 (3.1) | 0 (0.0) | 13 (32.50) | 5 (11.1) |
| Thrombolysis | 0 (0.0) | 0 (0.0) | 3 (7.5) | 1 (2.2) |
| Pacemaker | 3 (9.4) | 2 (8.7) | 0 (0.0) | 1 (2.2) |
| AICD | 0 (0.0) | 4 (17.39) | 0 (0.0) | 0 (0.0) |
| IRD | 0 (0.0) | 2 (8.7) | 0 (0.0) | 0 (0.0) |
| LVAD | 0 (0.0) | 1 (4.4) | 0 (0.0) | 0 (0.0) |
| Mitral valve surgery | 7 (219) | 2 (8.7) | 1 (2.5) | 2 (4.4) |
| Aorta valve surgery | 25 (78.1) | 1 (4.4) | 1 (2.5) | 6 (13.3) |
| Drugs, n (%) | ||||
| NSAIDs | 19 (59.4) | 13 (56.5) | 28 (70.0) | 32 (71.1) |
| Analgesic | 9 (28.1) | 8 (34.8) | 16 (40.0) | 16 (35.6) |
| ACE inhibitors | 15 (46.9) | 11 (47.8) | 19 (47.5) | 17 (37.8) |
| ARB | 16 (50.0) | 9 (39.1) | 20 (50.0) | 21(46.7) |
| Betablockers | 26 (81.3) | 17 (73.9) | 28 (70.0) | 35 (77.8) |
| Ca+2 blocker receptor | 2 (6.5) | 1 (4.4) | 4 (10.0) | 5 (11.1) |
| Diuretics | 8 (25) | 22 (96%) | 6 (15%) | 1 (2%) |
| Slow K+ | 1 (3.1) | 4 (17.4) | 0 (0.0) | 0 (0.0) |
| Phosphodiesterase inhibitor | 1 (3.1) | 1 (4.4) | 0 (0.0) | 2 (4.4) |
| Antiarrhythmic | 5 (15.6) | 7 (30.4) | 6 (15.0) | 7 (15.6) |
| Anticoagulants | 11 (34.4) | 5 (21.7) | 14 (35.0) | 9 (20.0) |
| Antiplatelet | 6 (18.8) | 2 (8.7) | 8 (20.0) | 12 (26.7) |
| Antithrombotic | 3 (9.4) | 2 (8.7) | 6 (15.0) | 11 (24.4) |
| Hypoglycemic agents | 9 (28.1) | 8 (34.8) | 9 (22.5) | 12 (26.7) |
| Anti-cholesterol drugs | 27 (84.4) | 18 (78.3) | 33 (82.5) | 41 (91.1) |
| Time | HVS | HFrEF | Post-AMI | CAD | F( ), p Value, η2 | |
|---|---|---|---|---|---|---|
| Anthropometric and body composition | ||||||
| Body mass (kg) | Pre | 71.0 ± 15.7 | 73.2 ± 9.9 | 76.3 ± 14.3 | 75.1 ± 14.6 | F(0.68), p = 0.563, 0.01 |
| Post | 71.5 ± 14.6 | 74.2 ± 10.1 | 77.7 ± 13.7 | 75.5 ± 14.3 | ||
| p-value | p = 0.443 | p = 0.232 | p = 0.024 | p = 0.500 | ||
| ∆ | +0.5 | +1.0 | +1.4 | +0.4 | ||
| BMI (kg·m−2) | Pre | 26.6 ± 4.7 | 26.7 ± 4.7 | 28.2 ± 4.4 | 27.3 ± 3.2 | F(5.99), p = 0.016, 0.05 |
| Post | 27.3 ± 54.5 | 27.1 ± 4.5 | 28.0 ± 4.2 | 27.5 ± 3.3 | ||
| p-value | p = 0.245 | p = 0.482 | p = 0.068 | p = 0.137 | ||
| ∆ | +0.7 | +0.4 | –0.2 | +0.2 | ||
| Visceral body fat (%) | Pre | 11.0 ± 4.3 | 9.3 ± 3.9 | 11.8 ± 4.4 | 10.3 ± 3.2 | F(2.69), p = 0.104, 0.02 |
| Post | 10.9 ± 4.3 | 10.7 ± 7.1 | 10.8 ± 4.1 | 9.5 ± 3.0 | ||
| p-value | p = 0.229 | p = 0.632 | p = 0.317 | p = 0.506 | ||
| ∆ | –0.1 | +1.4 | –1.0 | –0.8 | ||
| Waist hip ratio | Pre | 0.93 ± 0.0 | 0.91 ± 0.1 | 0.95 ± 0.0 | 0.93 ± 0.0 | F(0.59), p = 0.442, 0.006 |
| Post | 0.93 ± 0.1 | 0.93 ± 0.1 | 0.95 ± 0.0 | 0.93 ± 0.0 | ||
| p-value | p = 0.597 | p = 0.830 | p = 0.716 | p = 0.073 | ||
| ∆ | 0.0 | +0.02 | 0.0 | 0.0 | ||
| Cardiorespiratory performance | ||||||
| METsmax | Pre | 3.5 ± 1.3 | 3.8 ± 1.4 | 4.2 ± 1.5 | 3.6 ± 1.1 | F(1152), p < 0.001, 0.52 |
| Post | 5.3 ± 1.6 ** | 4.5 ± 1.3 ** | 5.6 ± 1.7 ** | 5.0 ± 1.2 ** | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| ∆ | +1.8 | +0.7 | +1.4 | +1.4 | ||
| O2pulsemax (ml·beat−1) | Pre | 7.6 ± 2.6 | 8.1 ± 3.5 | 9.3 ± 3.4 | 8.8 ± 5.3 | F(5.99), p = 0.016, 0.05 |
| Post | 10.7 ± 3.7 * | 9.3 ± 2.8 | 11.4 ± 3.9 ** | 10.7 ± 3.1 ** | ||
| p-value | p = 0.024 | p = 0.505 | p < 0.001 | p < 0.001 | ||
| ∆ | +3.1 | +1.2 | +2.1 | +1.9 | ||
| VO2AT-pred (%) | Pre | 33.5 ± 7.9 | 31.0 ± 10.0 | 37.4 ± 10.9 | 32.8 ± 9.2 | F(47.8), p < 0.001, 0.31 |
| Post | 47.2 ± 12.7 ** | 35.0 ± 9.3 * | 46.0 ± 12.2 * | 43.5 ± 13.3 ** | ||
| p-value | p < 0.001 | p = 0.037 | p = 0.004 | p < 0.001 | ||
| ∆ | +13.7 | +4.0 | +8.6 | +10.7 | ||
| VO2peak-pred (%) | Pre | 49.6 ± 12.6 | 45.8 ± 15.5 | 58.7 ± 16.8 | 51.1 ± 13.3 | F(122.4), p < 0.001, 0.53 |
| Post | 74.5 ± 15.1 ** | 56.5 ± 14.6 ** | 74.9 ± 17.7 ** | 69.9 ± 16.3 ** | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| ∆ | +24.9 | +10.7 | +16.2 | +18.8 | ||
| Quality of life | ||||||
| General Health | Pre | 62.3 ± 17.2 | 59.3 ± 10.9 | 63.5 ± 11.0 | 61.8 ± 11.3 | F(038), p = 0.766, 0.10 |
| Post | 73.5 ± 14.4 ** | 66.2 ± 12.6 | 71.3 ± 12.4 | 68.0 ± 18.3 * | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.01 | ||
| ∆ | +11.2 | +6.9 | +7.8 | +6.2 | ||
| Mental health | Pre | 61.7 ± 16.7 | 57.6 ± 17.9 | 64.5 ± 16.1 | 64.6 ± 18.0 | F(076), p = 0.518, 0.20 |
| Post | 78.1 ± 16.2 ** | 75.0 ± 14.5 ** | 84.5 ± 13.1 ** | 82.9 ± 19.1 ** | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| ∆ | +16.4 | +17.4 | +20.0 | +18.3 | ||
| Physical functioning | Pre | 69.8 ± 15.4 | 69.8 ± 11.9 | 75.5 ± 10.4 | 73.0 ± 12.2 | F(214.9), p = 0606, 0.67 |
| Post | 86.8 ± 7.4 ** | 81.9 ± 9.7 ** | 85.3 ± 15.9 ** | 84.2 ± 16.4 ** | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| ∆ | +17.0 | +12.1 | +9.8 | +11.2 | ||
| Role physical | Pre | 40.5 ± 28.1 | 36.3 ± 22.8 | 52.0 ± 25.1 | 42.0 ± 23 | F(151.1), p = 0.929, 0.59 |
| Post | 68.9 ± 19.0 ** | 63.1 ± 24.4 ** | 77.6 ± 18.3 ** | 67.3 ± 22.9 ** | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| ∆ | +28.4 | +26.8 | +25.6 | +25.3 | ||
| Bodily pain | Pre | 65.0 ± 22.6 | 76.4 ± 22.4 | 74.4 ± 192 | 70.5 ± 16 | F(8.92), p = 0.729, 0.07 |
| Post | 96.3 ± 17.7 | 96.8 ± 10.8 | 93.3 ± 14.6 | 96.2 ± 16.1 | ||
| p-value | p = 0.066 | p = 0.071 | p = 0.063 | p = 0.814 | ||
| ∆ | +31.3 | +20.4 | +18.9 | +25.7 | ||
| Vitality | Pre | 57.5 ± 15.9 | 53.3 ± 11.8 | 61.0 ± 15.2 | 61.2 ± 12.2 | F(93.1), p = 0.892, 0.47 |
| Post | 75.9 ± 15.1 ** | 67.6 ± 13.4 ** | 75.2 ± 14.2 ** | 71.8 ± 18.6 ** | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| ∆ | +18.4 | +14.3 | +14.2 | +10.6 | ||
| Social functioning | Pre | 57.6 ± 24.2 | 52.1 ± 20.4 | 61.4 ± 20.4 | 57.8 ± 22.4 | F(86.2), p = 0.532, 0.45 |
| Post | 78.0 ± 22.1 ** | 77.4 ± 21.9 ** | 81.8 ± 20.6 ** | 72.6 ± 25.5 ** | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| ∆ | +20.4 | +25.3 | +20.4 | +14.8 | ||
| Emotional health | ||||||
| Anxiety | Pre | 9.9 ± 2.1 | 9.9 ± 2.5 | 8.9 ± 2.1 | 9.1 ± 2.4 | F(79.9), p = 0.657, 0.43 |
| Post | 6.3 ± 3.0 ** | 7.6 ± 3.4 * | 5.9 ± 2.0 ** | 6.0 ± 2.2 ** | ||
| p-value | p < 0.001 | p = 0.003 | p < 0.001 | p < 0.001 | ||
| ∆ | –3.6 | –2.3 | –3.0 | –3.1 | ||
| Depression | Pre | 6.3 ± 3.0 | 7.6 ± 3.4 | 6.7 ± 3.2 | 5.8 ± 0.9 | F(148.), p = 0.890, 0.58 |
| Post | 3.5 ± 2.2 ** | 4.2 ± 2.2 ** | 3.5 ± 2.2 ** | 3.5 ± 2.2 ** | ||
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| ∆ | −2.8 | −3.4 | −3.2 | −2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuesta, M.; Alvarez, C.; Pedemonte, O.; Araneda, O.F.; Manríquez-Villarroel, P.; Berthelon, P.; Reyes, A. Average and Interindividual Effects to a Comprehensive Cardiovascular Rehabilitation Program. Int. J. Environ. Res. Public Health 2023, 20, 261. https://doi.org/10.3390/ijerph20010261
Tuesta M, Alvarez C, Pedemonte O, Araneda OF, Manríquez-Villarroel P, Berthelon P, Reyes A. Average and Interindividual Effects to a Comprehensive Cardiovascular Rehabilitation Program. International Journal of Environmental Research and Public Health. 2023; 20(1):261. https://doi.org/10.3390/ijerph20010261
Chicago/Turabian StyleTuesta, Marcelo, Cristian Alvarez, Oneglio Pedemonte, Oscar F. Araneda, Pablo Manríquez-Villarroel, Paulina Berthelon, and Alvaro Reyes. 2023. "Average and Interindividual Effects to a Comprehensive Cardiovascular Rehabilitation Program" International Journal of Environmental Research and Public Health 20, no. 1: 261. https://doi.org/10.3390/ijerph20010261





