Frailty Screening in the Emergency Department: Comparing the Variable Indicative of Placement Risk, Clinical Frailty Scale and PRISMA-7
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Instruments
2.3. Data Collection
2.4. Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Yang, Y.; Schwebel, D.C.; Liu, Z.; Li, L.; Cheng, P.; Ning, P.; Hu, G. Population ageing and mortality during 1990–2017: A global decomposition analysis. PLoS Med. 2020, 17, e1003138. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-ananlysis of population-level studies. Age Ageing 2020, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, L.; O’Caoimh, R.; Rodríguez-Laso, Á.; Beltzer, N.; Ranhoff, A.H.; Van der Heyden, J.; Lamprini-Koula, M.; Ciutan, M.; López-Samaniego, L.; Liew, A.; et al. Incidence of frailty: A systematic review of scientific literature from a public health perspective. Ann. Dell’istituto Super. Di Sanita 2018, 54, 239–245. [Google Scholar]
- Sezgin, D.; O’Donovan, M.; Cornally, N.; Liew, A.; O’Caoimh, R. Defining frailty for healthcare practice and research: A qualitative systematic review with thematic analysis. Int. J. Nurs. Stud. 2019, 92, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Latham, L.P.; Ackroyd-Stolarz, S. Emergency department utilization by older adults: A descriptive study. Can. Geriatr. J. 2014, 17, 118. [Google Scholar] [CrossRef]
- Salvi, F.; Morichi, V.; Grilli, A.; Giorgi, R.; De Tommaso, G.; Dessi-Fulgheri, P. The elderly in the emergency department: A critical review of problems and solutions. Int. Emerg. Med. 2007, 2, 292–301. [Google Scholar] [CrossRef]
- Ukkonen, M.; Jämsen, E.; Zeitlin, R.; Pauniaho, S.L. Emergency department visits in older patients: A population-based survey. BMC Emerg. Med. 2019, 19, 20. [Google Scholar] [CrossRef]
- Hubbard, R.E.; Peel, N.M.; Samanta, M.; Gray, L.C.; Mitnitski, A.; Rockwood, K. Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing 2017, 46, 801–806. [Google Scholar] [CrossRef]
- Wallis, S.J.; Wall, J.B.; Biram, R.W.; Romero-Ortuno, R. Association of the clinical frailty scale with hospital outcomes. QJM 2015, 108, 943–949. [Google Scholar] [CrossRef]
- Hendry, A.; Vanhecke, E.; Carriazo, A.M.; López-Samaniego, L.; Espinosa, J.M.; Sezgin, D.; O’Donovan, M.; Hammar, T.; Ferry, P.; Vella, A.; et al. Integrated care models for managing and preventing frailty: A systematic review for the European Joint Action on Frailty Prevention (ADVANTAGE JA). Transl. Med. UniSa 2019, 19, 5–10. [Google Scholar]
- Carpenter, C.R.; Shelton, E.; Fowler, S.; Suffoletto, B.; Platts-Mills, T.F.; Rothman, R.E.; Hogan, T.M. Risk factors and screening instruments to predict adverse outcomes for undifferentiated older emergency department patients: A systematic review and meta-analysis. Acad. Emerg. Med. 2015, 22, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.; Hull, L.; Conroy, S.P. Frailty identification in the emergency department—A systematic review focussing on feasibility. Age Ageing 2017, 46, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.; Phelps, K.; Regen, E.; Conroy, S.P. Identifying frailty in the Emergency Department—Feasibility study. Age Ageing 2017, 46, 840–845. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Kennelly, S.; Ahern, E.; O’Keeffe, S.; Ortuño, R.R. COVID-19 and the Challenges of Frailty Screening in Older Adults. J. Frailty Aging 2020, 9, 185–186. [Google Scholar]
- Larner, A.J. Speed versus accuracy in cognitive assessment when using CSIs. Prog. Neurol. Psychiatr. 2015, 19, 21–24. [Google Scholar] [CrossRef]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; Holland, C. Predicting risk and outcomes for frail older adults: An umbrella review of frailty screening tools. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 1154. [Google Scholar] [CrossRef]
- Moloney, E.; Sezgin, D.; O’Donovan, M.; Adja, K.; McGrath, K.; Liew, A.; Lenzi, J.; Gori, D.; O’Connor, K.; Molloy, D.W.; et al. The Diagnostic Accuracy and Clinimetric Properties of Screening Instruments to Identify Frail Older Adults Attending Emergency Departments: A Protocol for a Mixed Methods Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1380. [Google Scholar] [CrossRef]
- Theou, O.; Squires, E.; Mallery, K.; Lee, J.S.; Fay, S.; Goldstein, J.; Armstrong, J.J.; Rockwood, K. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018, 18, 139. [Google Scholar] [CrossRef]
- Preston, L.; Chambers, D.; Campbell, F.; Cantrell, A.; Turner, J.; Goyder, E. What evidence is there for the identification and management of frail older people in the emergency department? A systematic mapping review. Health Serv. Delivery Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Vandewoude, M.; Geerts, C.; d’Hooghe, A.; Paridaens, K. A screening tool to identify older people at risk of adverse health outcomes at the time of hospital admission. Tijdschr. Voor Gerontol. En Geriatr. 2006, 37, 203–209. [Google Scholar]
- Raîche, M.; Hébert, R.; Dubois, M.-F. PRISMA-7: A case-finding tool to identify older adults with moderate to severe disabilities. Arch. Gerontol. Geriatr. 2008, 47, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mackway-Jones, K.; Marsden, J.; Windle, J. Emergency Triage: Manchester Triage Group; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kojima, G. Prevalence of frailty in nursing homes: A systematic review and meta-analysis. JAMDA 2015, 16, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Vandewoude, M.; Geerts, C.; Paridaens, K.; d’Hooghe, H. A screening tool for activating liaison geriatrics in general hospitals: The “variable indicative of placement risk”(VIP). Eur. J. Geriatr. 2008, 10, 120–126. [Google Scholar]
- O’Caoimh, R.; Costello, M.; Small, C.; Spooner, L.; Flannery, A.; O’Reilly, L.; Heffernan, L.; Mannion, E.; Maughan, A.; Joyce, A. Comparison of frailty screening instruments in the emergency department. Int. J. Environ. Res. Public Health 2019, 16, 3626. [Google Scholar] [CrossRef]
- Bédard, M.; Molloy, D.W.; Squire, L.; Dubois, S.; Lever, J.A.; O’Donnell, M. The Zarit Burden Interview: A new short version and screening version. Gerontol. 2001, 41, 652–657. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Stevernik, N.; Slaets, J.; Schuurmans, H.; Van Lis, M.; Steverink, N.; Slaets, J.; Lis, C. Measuring frailty: Development and testing the GFI (Groningen Frailty Indicator). Gerontol. 2001, 41, 236–237. [Google Scholar]
- Lilamand, M.; Kelaiditi, E.; Cesari, M.; Raynaud-Simon, A.; Ghisolfi, A.; Guyonnet, S.; Vellas, B.; Van Kan, G.A. Validation of the Mini Nutritional Assessment-Short Form in a population of frail elders without disability. Analysis of the Toulouse Frailty Platform population in 2013. J. Nutr. Health Aging 2015, 19, 570–574. [Google Scholar] [CrossRef]
- Brazier, J.; Walters, S.; Nicholl, J.; Kohler, B. Using the SF-36 and Euroqol on an elderly population. Qual. Life Res. 1996, 5, 195–204. [Google Scholar] [CrossRef]
- DeSalvo, K.B.; Fan, V.S.; McDonell, M.B.; Fihn, S.D. Predicting mortality and healthcare utilization with a single question. Health Serv. Res. 2005, 40, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Gagné, A.; Voyer, P.; Boucher, V.; Nadeau, A.; Carmichael, P.; Pelletier, M.; Gouin, E.; Berthelot, S.; Daoust, R.; Wilchesky, M.; et al. Performance of the French version of the 4AT for screening the elderly for delirium in the emergency department. CJEM 2018, 20, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Roe, C.M.; Powlishta, K.K.; Coats, M.A.; Muich, S.J.; Grant, E.; Miller, J.P.; Storandt, M.; Morris, J.C. The AD8 A brief informant interview to detect dementia. Neurology 2005, 65, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Buderer, N.M.F. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad. Emerg. Med. 1996, 3, 895–900. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol. Hepatol. Bed Bench 2013, 6, 14–17. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Yourman, L.C.; Lee, S.J.; Schonberg, M.A.; Widera, E.W.; Smith, A.K. Prognostic indices for older adults: A systematic review. JAMA 2012, 307, 182–192. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; Svendrovski, A.; Healy, E.; O’Connell, E.; O’Keeffe, G.; Cronin, U.; O’Herlihy, E.; Cornally, N.; Molloy, W.D. Screening for markers of frailty and perceived risk of adverse outcomes using the Risk Instrument for Screening in the Community (RISC). BMC Geriatr. 2014, 14, 104. [Google Scholar] [CrossRef]
- Lewis, E.T.; Dent, E.; Alkhouri, H.; Kellett, J.; Williamson, M.; Asha, S.; Holdgate, A.; Mackenzie, J.; Winoto, L.; Fajardo-Pulido, D.; et al. Which frailty scale for patients admitted via Emergency Department? A cohort study. Arch. Gerontol. Geriatr. 2019, 80, 104–114. [Google Scholar] [CrossRef]
- Jørgensen, R.; Brabrand, M. Screening of the frail patient in the emergency department: A systematic review. Eur. J. Intern. Med. 2017, 45, 71–73. [Google Scholar] [CrossRef]
- McFeely, A.; Corcoran, R.; Jusmanova, K.; Kearns, R.; Marks, E.; Ward, L.; O’Brien, S.; Fagan, O.; Nugent, M.; O’Dwyer, O. 288 Geriatric EMergency Services (GEMS): An Acute Floor Frailty Service Model. Age Ageing 2018, 47, 71–73. [Google Scholar] [CrossRef]
- Brolly, A.; Bergin, L.; McQuinn, P.; O′’Sullivan, S.; Naughton, C. 112 A Critical Evaluation of the VIP Tool in Identifying Frailty in the Older Adult in a Kerry Hospital. Age Ageing 2019, 48, iii17–iii65. [Google Scholar] [CrossRef]
- Sezgin, D.; O’Donovan, M.; Woo, J.; Bandeen-Roche, K.; Liotta, G.; Fairhall, N.; Rodríguez-Laso, A.; Apóstolo, J.; Clarnette, R.; Holland, C.; et al. Early identification of frailty: Developing an international delphi consensus on pre-frailty. Arch. Gerontol. Geriatr. 2021, 99, 104586. [Google Scholar] [CrossRef] [PubMed]
- Salvi, F.; Morichi, V.; Grilli, A.; Lancioni, L.; Spazzafumo, L.; Polonara, S.; Abbatecola, A.M.; De Tommaso, G.; Dessi-Fulgheri, P.; Lattanzio, F. Screening for frailty in elderly emergency department patients by using the Identification of Seniors at Risk (ISAR). J. Nutr. Health Aging 2012, 16, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56A, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Gambassi, G.; van Kan, G.A.; Vellas, B. The frailty phenotype and the frailty index: Different instruments for different purposes. Age Ageing 2014, 43, 10–12. [Google Scholar] [CrossRef]
- Parker, S.G.; McCue, P.; Phelps, K.; McCleod, A.; Arora, S.; Nockels, K.; Kennedy, S.; Roberts, H.; Conroy, S. What is comprehensive geriatric assessment (CGA)? An umbrella review. Age Ageing 2018, 47, 149–155. [Google Scholar] [CrossRef]
- Chen, H.H.; Sun, F.J.; Yeh, T.L.; Liu, H.E.; Huang, H.L.; Kuo, B.I.; Huang, H.Y. The diagnostic accuracy of the Ascertain Dementia 8 questionnaire for detecting cognitive impairment in primary care in the community, clinics and hospitals: A systematic review and meta-analysis. Fam. Pract. 2018, 35, 239–246. [Google Scholar] [CrossRef]
- Savva, G.M.; Donoghue, O.A.; Horgan, F.; O’Regan, C.; Cronin, H.; Kenny, R.A. Using timed up-and-go to identify frail members of the older population. J. Geront. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 441–446. [Google Scholar] [CrossRef]
- Kansagara, D.; Englander, H.; Salanitro, A.; Kagen, D.; Theobald, C.; Freeman, M.; Kripalani, S. Risk prediction models for hospital readmission: A systematic review. J. Am. Med. Assoc. 2011, 306, 1688–1698. [Google Scholar] [CrossRef]

| Characteristics | Total (n = 197) | Frail (n = 98) | Non-Frail (n = 99) | Frail vs. Non-Frail |
|---|---|---|---|---|
| Median (Q3–Q1 or %) | Median (Q3–Q1 or %) | Median (Q3–Q1 or %) | p-Value for Difference | |
| Age (Years) | 79 (83–73) | 80 (86–76) | 75 (82–72) | <0.001 |
| Sex (Female) | 45% | 49% | 41% | 0.257 |
| BMI | 25 (28–22) | 24 (28–21) | 26 (29–23) | 0.009 |
| MNA-SF | 12 (13–10) | 11 (13–9) | 13 (14–12) | <0.001 |
| AD8 | 1 (2–0) | 1 (3–0) | 0 (1–0) | <0.001 |
| 4AT | 0 (1–0) | 1 (2–0) | 0 (0–0) | <0.001 |
| CBS | 12 (18–3) | 15 (19–9) | 0 (10–0) | 0.001 |
| EQ-5D (VAS) | 60 (75–45) | 50 (50–30) | 70 (80–60) | <0.001 |
| GSRH (V. Good/Excellent) | 20% | 6% | 35% | <0.001 |
| FRAIL scale | 2 (3–1) | 2 (3–2) | 1 (2–0) | <0.001 |
| GFI | 4 (6–2) | 6 (8–5) | 2 (3–2) | <0.001 |
| CFS | 4 (6–3) | 6 (7–5) | 3 (4–2) | <0.001 |
| PRISMA-7 | 4 (5–2) | 5 (6–4) | 2 (3–2) | <0.001 |
| VIP 3 | 1 (2–0) | 2 (2–1) | 0 (1–0) | <0.001 |
| VIP 4 | 1 (2–0) | 2 (3–1) | 0 (1–0) | <0.001 |
| FRAIL (score ≥ 3) | 31% | 47% | 14% | <0.001 |
| GFI (score ≥ 4) | 60% | 96% | 24% | <0.001 |
| CFS (score ≥ 5) | 45% | 77% | 13% | <0.001 |
| PRISMA-7 (score ≥ 3) | 67% | 98% | 36% | <0.001 |
| VIP-3 (score ≥ 2) | 30% | 59% | 2% | <0.001 |
| VIP-4 (score ≥ 3) | 14% | 29% | 0% | <0.001 |
| Admitted | 66% | 82% | 49% | <0.001 |
| Length of stay (if admitted) in days | 7 (14–3) | 9 (16–5) | 4 (9–2) | 0.003 |
| Died (within 3 months of presenting) | 14 (7%) | 12 (12%) | 2 (2%) | 0.005 |
| 30-day re-admission (same hospital) | 14 (7%) | 9 (9%) | 5 (5%) | 0.283 |
| 90-day re-presentation ED /re-admission * | 55 (28%) | 32 (33%) | 23 (23%) | 0.155 |
| Agreement (rs) between Frailty Scales (Number of Criteria) | ||||||
| Frailty Scales (cut-offs) | CFS (Scale 1–9) | PRISMA-7 (Scale 0–7) | VIP-3 (Scale 0–3) | VIP-4 (Scale 0–4) | FRAIL (Scale 0–5) | GFI (Scale 0–15) |
| CFS (scale 1–9) | - | 0.70 ** | 0.56 ** | 0.58 ** | 0.52 ** | 0.62 ** |
| PRISMA-7 (scale 0–7) | 0.70 ** | - | 0.48 ** | 0.51 ** | 0.53 ** | 0.63 ** |
| VIP-3 (scale 0–3) | 0.56 ** | 0.48 ** | - | 0.98 ** | 0.44 * | 0.59 ** |
| FRAIL (scale 0–5) | 0.52 ** | 0.53 ** | 0.44 * | 0.47 * | - | 0.59 ** |
| GFI (scale 0–15) | 0.62 ** | 0.63 ** | 0.59 ** | 0.61 ** | 0.59 ** | - |
| Agreement (κ) between Frailty Cut-Offs and Comprehensive Geriatric Assessment | ||||||
| Frailty Scales (cut-offs) | CFS (Frail ≥ 5) | PRISMA-7 (Frail ≥ 3) | VIP-3 (Frail ≥ 2) | VIP-4 (Frail ≥ 3) | FRAIL (Frail ≥ 3) | GFI (Frail ≥ 4) |
| CFS (frail ≥ 5) | - | 0.48 ** | 0.46 ** | 0.25 ** | 0.26 ** | 0.50 ** |
| PRISMA-7 (frail ≥ 3) | 0.48 ** | - | 0.29 ** | 0.15 ** | 0.26 ** | 0.59 ** |
| VIP-3 (frail ≥ 2) | 0.46 ** | 0.29 ** | - | 0.56 ** | 0.16 * | 0.41 ** |
| FRAIL (frail ≥ 3) | 0.26 ** | 0.26 ** | 0.16 * | 0.21 * | - | 0.29 ** |
| GFI (frail ≥ 4) | 0.50 ** | 0.59 ** | 0.41 ** | 0.17 ** | 0.29 ** | - |
| CGA (frail/non-frail) | 0.64 ** | 0.62 ** | 0.56 ** | 0.29 ** | 0.32 ** | 0.72 ** |
| Frailty Screen Cut-Off | Youden’s Index | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | False Positive (95% CI) | False Negative (95% CI) | PLR † (95% CI) | NLR † (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| CFS | |||||||||
| ≥2 | 0.15 | 1 (0.92–1.0) | 0.15 (0.09–0.24) | 0.53 (0.46–0.61) | 1 (0.75–1.0) | 0.47 (0.39–0.54) | 0 (0–0.25) | 1.1 (0.93–1.4) | 0 (0.0–0.0) |
| ≥3 | 0.39 | 1 (0.95–1.0) | 0.39 (0.30–0.49) | 0.61 (0.53–0.69) | 1 (0.89–1.0) | 0.39 (0.31–0.47) | 0 (0–0.11) | 1.6 (1.3–2.0) | 0 (0–0) |
| ≥4 ^ | 0.66 | 0.95 (0.88–0.98) | 0.70 (0.60–0.79) | 0.76 (0.67–0.83) | 0.93 (0.84–0.98) | 0.25 (0.17–0.33) | 0.07 (0.02–0.16) | 3.1 (2.2–4.3) | 0.07 (0.03–0.17) |
| ≥5 * | 0.64 | 0.77 (0.67–0.85) | 0.87 (0.78–0.93) | 0.85 (0.76–0.92) | 0.80 (0.71–0.87) | 0.15 (0.08–0.24) | 0.20 (0.13–0.29) | 5.8 (3.5–9.6) | 0.25 (0.17–0.37) |
| ≥6 | 0.50 | 0.53 (0.42–0.63) | 0.97 (0.91–0.99) | 0.94 (0.84–0.99) | 0.68 (0.59–0.75) | 0.06 (0.01–0.16) | 0.32 (0.25–0.41) | 17 (5.6–51) | 0.47 (0.37–0.61) |
| ≥7 | 0.25 | 0.27 (0.19–0.37) | 0.98 (0.92–1.0) | 0.93 (0.75–0.99) | 0.58 (0.50–0.65) | 0.07 (0.01–0.25) | 0.42 (0.35–0.50) | 13 (3.4–49.6) | 0.72 (0.6–0.87) |
| ≥8 | 0.01 | 0.03 (0.01–0.09) | 0.98 (0.92–1.0) | 0.60 (0.17–0.93) | 0.51 (0.44–0.58) | 0.4 (0.07–0.83) | 0.49 (0.42–0.56) | 1.5 (0.41–5.5) | 0.96 (0.82–1.12) |
| PRISMA-7 | |||||||||
| ≥2 | 0.22 | 1 (0.95–1.0) | 0.23 (0.15–0.33) | 0.56 (0.48–0.63) | 1 (0.82–1.0) | 0.44 (0.37–0.52) | 0 (0.0–0.18) | 1.3 (1.0–1.6) | 0 (0–0) |
| ≥3* | 0.62 | 0.98 (0.92–0.99) | 0.64 (0.54–0.73) | 0.73 (0.64–0.80) | 0.97 (0.86–0.99) | 0.27 (0.20–0.36) | 0.03 (0.01–0.11) | 2.7 (2–3.6) | 0.03 (0.01–0.12) |
| ≥4 ^ | 0.68 | 0.87 (0.78–0.92) | 0.82 (0.73–0.89) | 0.83 (0.74–0.89) | 0.86 (0.77–0.92) | 0.17 (0.11–0.26) | 0.14 (0.08–0.23) | 4.7 (3.1–7.3) | 0.16 (0.1–0.26) |
| ≥5 | 0.56 | 0.64 (0.54–0.74) | 0.92 (0.84–0.96) | 0.89 (0.78–0.95) | 0.72 (0.64–0.80) | 0.11 (0.05–0.22) | 0.28 (0.20–0.36) | 7.9 (4.1–15.2) | 0.38 (0.29–0.51) |
| ≥6 | 0.28 | 0.31 (0.22–0.41) | 0.97 (0.91–0.99) | 0.91 (0.75–0.98) | 0.59 (0.51–0.66) | 0.09 (0.02–0.25) | 0.41 (0.34–0.49) | 10 (3.4–29.6) | 0.7 (0.6–0.85) |
| ≥7 | 0.04 | 0.04 (0.01–0.11) | 1 (0.95–1.0) | 1 (0.40–1.0) | 0.52 (0.44–0.59) | 0 (0–0.60) | 0.49 (0.41–0.56) | NA | 0.94 (0.8–1.1) |
| VIP-3 | |||||||||
| ≥1 | 0.43 | 0.87 (0.78–0.92) | 0.57 (0.46–0.66) | 0.66 (0.57–0.74) | 0.81 (0.70–0.89) | 0.34 (0.26–0.43) | 0.19 (0.11–0.30) | 2.0 (1.5–2.6) | 0.23 (0.14–0.38) |
| ≥2 * ^ | 0.57 | 0.58 (0.48–0.68) | 0.98 (0.92–0.99) | 0.97 (0.87–0.99) | 0.70 (0.62–0.78) | 0.03 (0.01–0.13) | 0.29 (0.22–0.38) | 28.5 (7.3–111) | 0.42 (0.33–0.55) |
| ≥3 | 0.15 | 0.14 (0.08–0.23) | 1 (0.95–1.0) | 1 (0.73–1.0) | 0.54 (0.47–0.61) | 0 (0–0.27) | 0.46 (0.39–0.53) | NA | 0.85 (0.72–1.0) |
| VIP-4 | |||||||||
| ≥1 | 0.42 | 0.87 (0.78–0.92) | 0.56 (0.45–0.65) | 0.66 (0.57–0.74) | 0.81 (0.69–0.89) | 0.34 (0.26–0.43) | 0.19 (0.11–0.31) | 1.9 (1.5–2.5) | 0.24 (0.14–0.39) |
| ≥2 ^ | 0.61 | 0.63 (0.53–0.73) | 0.98 (0.92–0.99) | 0.97 (0.88–0.99) | 0.73 (0.64–0.80) | 0.03 (0.01–0.12) | 0.27 (0.20–0.36) | 31 (8–121) | 0.37 (0.28–0.49) |
| ≥3 * | 0.29 | 0.29 (0.20–0.39) | 1 (0.95–1.0) | 1 (0.85–1.0) | 0.59 (0.51–0.66) | 0 (0–0.15) | 0.41 (0.34–0.49) | NA | 0.71 (0.59–0.85) |
| ≥4 | 0.05 | 0.05 (0.02–0.12) | 1 (0.95–1.0) | 1 (0.46–1.0) | 0.52 (0.44–0.59) | 0 (0–0.54) | 0.48 (0.41–0.55) | NA | 0.94 (0.8–1.1) |
| Diagnostic Approach | CFS | PRISMA-7 | VIP-3 | VIP-4 |
|---|---|---|---|---|
| AUC (95% confidence intervals) for differentiating frail from non-frail | ||||
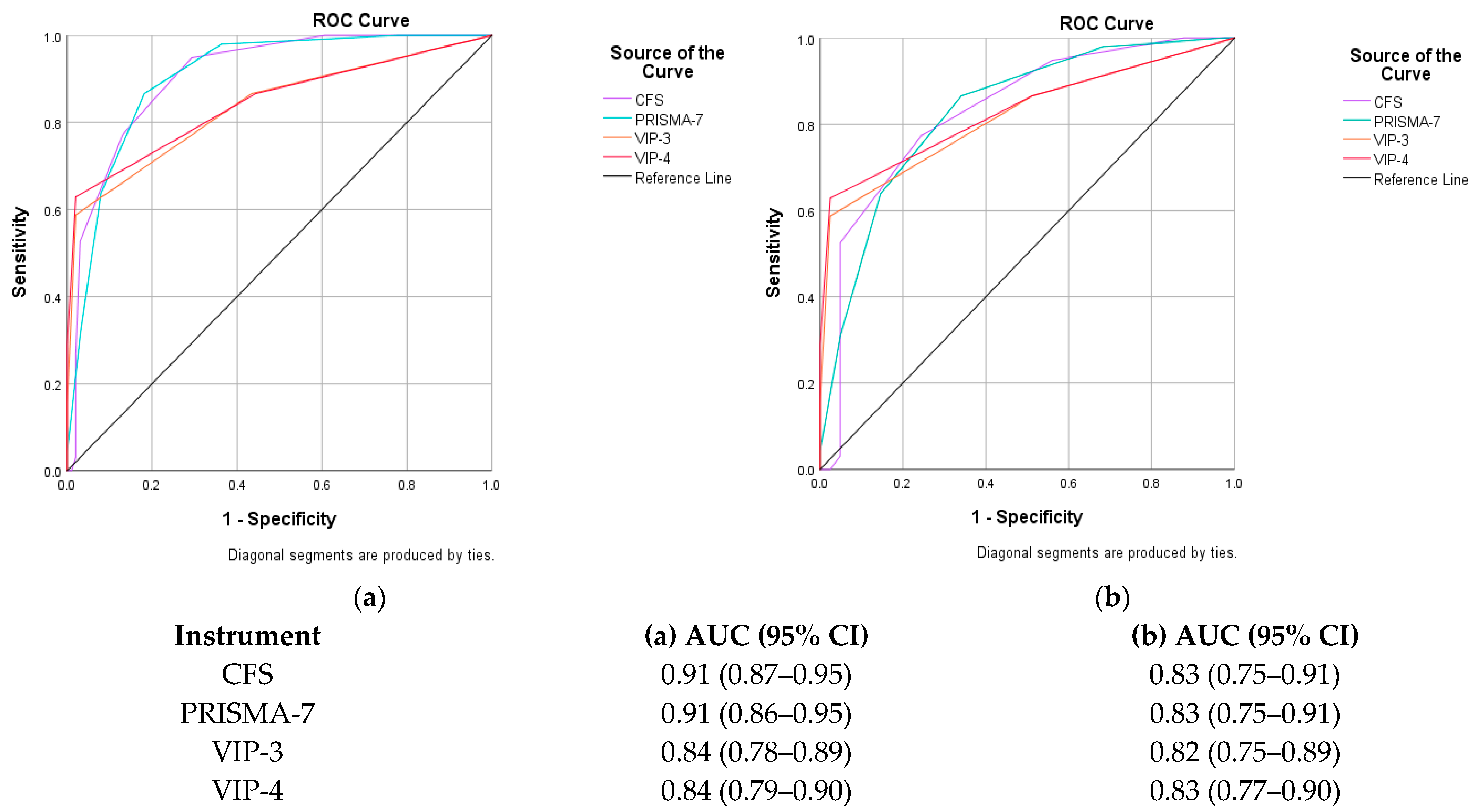
| CGA | 0.91 (0.87–0.95) p < 0.001 | 0.90 (0.86–0.95) p < 0.001 | 0.84 (0.78–0.89) p < 0.001 | 0.84 (0.79–0.90) p < 0.001 |
| FRAIL scale i.e., physical frailty (frail ≥ 3 criteria) | 0.69 (0.62–0.77) p < 0.001 | 0.70 (0.62–0.77) p < 0.001 | 0.63 (0.55–0.71) p = 0.004 | 0.66 (0.57–0.74) p < 0.001 |
| GFI (frail ≥ 4 criteria) | 0.82 (0.76–0.88) p < 0.001 | 0.85 (0.79–0.91) p < 0.001 | 0.78 (0.71–0.84) p < 0.001 | 0.78 (0.72–0.85) p < 0.001 |
| AUC (95% confidence intervals) for differentiating frail from pre-frail | ||||
| CGA | 0.83 (0.75–0.91) p < 0.001 | 0.83 (0.75–0.91) p < 0.001 | 0.82 (0.75–0.89) p < 0.001 | 0.83 (0.77–0.90) p < 0.001 |
| FRAIL scale (physical frailty) | 0.62 (0.53–0.71) p = 0.012 | 0.61 (0.52–0.70) p = 0.021 | 0.55(0.46–0.65) p = 0.275 | 0.59 (0.49–0.68) p = 0.077 |
| CGA (pre-frail/robust) | 0.81 (0.72–0.89) p < 0.001 | 0.82 (0.73–0.90) p < 0.001 | 0.57 (0.45–0.68) p = 0.260 | 0.56 (0.44–0.67) p = 0.325 |
| FRAIL scale (i.e., physical frailty) | 0.78 (0.69–0.86) p < 0.001 | 0.78 (0.70–0.86) p < 0.001 | 0.74 (0.66–0.82) p < 0.001 | 0.75 (0.67–0.83) p < 0.001 |
| AUC (95% confidence intervals) for predicting adverse health-related outcomes | ||||
| Outcomes (Yes/No) | CFS | PRISMA-7 | VIP-3 | VIP-4 |
| Admission | 0.78 (0.70–0.85) p < 0.001 | 0.65 (0.56–0.74) p < 0.001 | 0.59 (0.51–0.67) p = 0.032 | 0.60 (0.52–0.68) p = 0.026 |
| Length of Stay (>7 days, i.e., median LOS) | 0.60 (0.51–0.70) p = 0.044 | 0.61 (0.51–0.70) p = 0.036 | 0.66 (0.57–0.76) p = 0.001 | 0.68 (0.58–0.77) p < 0.001 |
| Readmission to hospital (within 30–90 days) | 0.69 (0.61–0.78) p = 0.005 | 0.60 (0.49–0.72) p = 0.130 | 0.60 (0.47–0.73) p = 0.149 | 0.60 (0.47–0.74) p = 0.125 |
| Died within 3 months of presentation | 0.71 (0.59–0.83) p = 0.008 | 0.71 (0.58–0.84) p = 0.009 | 0.66 (0.50–0.82) p = 0.050 | 0.67 (0.51–0.83) p = 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Caoimh, R.; McGauran, J.; O’Donovan, M.R.; Gillman, C.; O’Hea, A.; Hayes, M.; O’Connor, K.; Moloney, E.; Alcock, M. Frailty Screening in the Emergency Department: Comparing the Variable Indicative of Placement Risk, Clinical Frailty Scale and PRISMA-7. Int. J. Environ. Res. Public Health 2023, 20, 290. https://doi.org/10.3390/ijerph20010290
O’Caoimh R, McGauran J, O’Donovan MR, Gillman C, O’Hea A, Hayes M, O’Connor K, Moloney E, Alcock M. Frailty Screening in the Emergency Department: Comparing the Variable Indicative of Placement Risk, Clinical Frailty Scale and PRISMA-7. International Journal of Environmental Research and Public Health. 2023; 20(1):290. https://doi.org/10.3390/ijerph20010290
Chicago/Turabian StyleO’Caoimh, Rónán, Jane McGauran, Mark R. O’Donovan, Ciara Gillman, Anne O’Hea, Mary Hayes, Kieran O’Connor, Elizabeth Moloney, and Megan Alcock. 2023. "Frailty Screening in the Emergency Department: Comparing the Variable Indicative of Placement Risk, Clinical Frailty Scale and PRISMA-7" International Journal of Environmental Research and Public Health 20, no. 1: 290. https://doi.org/10.3390/ijerph20010290
APA StyleO’Caoimh, R., McGauran, J., O’Donovan, M. R., Gillman, C., O’Hea, A., Hayes, M., O’Connor, K., Moloney, E., & Alcock, M. (2023). Frailty Screening in the Emergency Department: Comparing the Variable Indicative of Placement Risk, Clinical Frailty Scale and PRISMA-7. International Journal of Environmental Research and Public Health, 20(1), 290. https://doi.org/10.3390/ijerph20010290







