Abstract
This study investigated the effects of different lead and cadmium salts (Pb(NO3)2, Cd(NO3)2, PbCl2, and CdCl2) on the photolytic degradation of two typical fluoroquinolones (levofloxacin (LVF) and norfloxacin (NOR)) under natural sunlight irradiation. Their half-life time and photolytic kinetic constants (k) were calculated at different molar ratios. The results indicated that the photolytic degradation curves of LVF and NOR followed apparent first-order kinetics. After 42 days of sunlight irradiation, approximately 48.3–69.4% of NOR was decomposed when the initial concentration increased from 0.006 to 0.06 mmol/L. In comparison, only 9.8–43.4% of LVF was decomposed. The k of NOR ranged from 0.79 × 10−3 to 1.30 × 10−3 h−1, and the k of LVF increased from 6.82 × 10−4 to 1.61 × 10−4 h−1. Compared with the control, the Pb2+ and Cd2+ participation tended to enhance the LVF and NOR photodegradation. The effects of Cd2+ on the photodegradation efficiency were more significant than those of Pb2+. It was inferred that the presence of aqueous NO3− obviously suppressed the NOR degradation, but Cl− had slight effects on these two fluoroquinolones’ photodegradation. These results are of importance toward the understanding of the persistence of FQs under natural sunlight irradiation in surface waters.
1. Introduction
Fluoroquinolones (FQs), such as norfloxacin (NOR) and levofloxacin (LVF), are a large class of antibiotics that are widely used in aquaculture, livestock husbandry, and human prescription for their broad activity spectrum and good oral intake properties [1,2]. Therefore, the widespread detection of FQs in terrestrial and aquatic systems has engendered significant scientific and regulatory concern. Previous studies showed that FQs are some of the most frequently detected antibiotics in surface ecosystems [3,4]. Relatively high residual FQ concentrations (ng/L to several μg/L) have been widely detected in seas [5,6], rivers [7,8], lakes [9,10], groundwater [11,12], and wastewater [13,14]. Ciprofloxacin (0–3.40 μg /L), norfloxacin (0.037–9.35 μg /L), and ofloxacin (0.047–8.64 μg /L) are the most commonly detected quinolone antibiotics in effluents [15], which could potentially pose a significant risk to ecosystems and human health.
The attenuation of FQs in the aquatic environment is mainly attributed to their biodegradation, photolytic degradation, and chemical degradation [2,16]. Of these degradation methods, photolytic degradation plays a significant role in FQs’ fate in some natural waters [17,18,19,20,21]. FQs can directly absorb sunlight and undergo photolysis, including direct photolysis and self-sensitized photooxidation via reactive oxygen species (ROS) such as hydroxyl radicals (•OH) and singlet oxygen (1O2). Moreover, the water constituents may also affect the photolytic degradation of FQs. Some studies have focused on the existing anions in the photodegradation of FQs [17,22]. In addition, the impact of coexisting metal cations on the environmental behaviors of FQs is a great of interest [17,23,24,25,26]. The co-existence of FQs with metal cations can form stable complexes and therefore this complexation interaction may alter FQs’ properties. Sciscenko et al. [26] found that iron complexation notably diminished enrofloxacin’s photodegradation. Ge et al. [17] also proved that Fe3+ inhibited the photodegradation of FQs. A possible reason was that Fe ions act as radiation filters and/or scavengers for ROS, which therefore inhibit the photolysis and/or photooxidation of FQs. With the development of modern agriculture and industry, increasing amounts of heavy metals along with FQs have been continuously discharged into surface water environments [9,27,28,29]. As reported in a survey in China, the total emissions of heavy metal(loid)s such as lead, cadmium, chromium, and arsenic in national wastewater reached 120 tons in 2019. Many studies have shown that rivers, surface water, and sewage contain heavy metals, in the range of ng/L~μg/L, such as Cd (0.025 μg/L) in Taihu Lake [30], Pb (1.21 μg/L) in the Yangtze River, and Pb (0.68 μg/L) Cd (0.07 μg/L) in the urban surface water of Su Zhou. Vinod Kumar et al. [31] collected 147 publications and proceedings on heavy metals in worldwide surface water bodies and found that the average concentration of Cd was 180 μg/L. It is necessary to investigate the environmental fate of FQs when they interact with heavy metals, particularly when the complex systems are exposed to natural sunlight in actual environmental scenarios. Nevertheless, research on this topic still remains limited and it needs further investigation.
As a result, this study focused on characterizing the influence of natural sunlight on the photodegradation of two typical FQs (LVF and NOR) with the interference of different Pb and Cd salts (Pb(NO3)2, PbCl2, Cd(NO3)2, and CdCl2) at different molar ratios. The results could facilitate an understanding of the FQs’ photolytic degradation in aquatic environments.
2. Materials and Methods
2.1. Materials and Chemicals
NOR was supplied by Hangzhou Minsheng Pharmaceutical Co., Ltd. (Hangzhou, China). LVF was obtained from Guangdong Eashu Pharmaceutical Co., Ltd. (Zhaoqing, China). Pb(NO3)2, Cd(NO3)2, PbCl2, and CdCl2 were purchased from Aladdin Industrial Co., Ltd. (Hong Kong, China). All the reagents were used without further purification.
2.2. Standard Curve of FQs
The FQ concentrations in the aqueous samples were measured using high-pressure liquid chromatography (HPLC, P1201, Dalian Elite Analytical Instruments Co., Ltd., Dalian, China) with a UV–vis detector and a UV–vis spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan). The absorbance values of NOR and LVF were recorded at 273 and 291 nm wavelengths, respectively (Figure S1). The standard concentrations of NOR and LVF (2.0–20 mg/L) were prepared by diluting the stock solution with deionized water. The calibration plot of NOR and LVF absorbance versus their concentrations shows a linear variation, with a correlation coefficient (R2) higher than 0.999 (Figure S1), indicating the reliable determination of this method.
2.3. Determination of Complexation Ratio
Complex formation between Pb/Cd ions and NOR/LVF was investigated using the molar ratio method by UV–vis spectroscopy. A 25 mL stock solution of FQs was transferred to a volumetric flask at 25 °C, and then a certain dosage of Pb2+/Cd2+ was added, causing the concentration ratio (CNOR/LVF/CPb/Cd) to range from 0.5 to 5. The absorbance of the complex solutions was plotted versus the concentration ratio (CNOR/LVF/CPb/Cd), and thus the concentration ratio curves were obtained.
2.4. Photolytic Degradation of FQs with or without Metal Salts
A series of NOR and LVF solutions (0.006, 0.012, and 0.06 mmol/L) were prepared by diluting the stock solution with deionized water. In the photodegradation experiments, 1 L of each solution was transferred to a 5 L beaker and all the beakers were placed in a glass box under natural sunlight irradiation. The experiment was conducted in June and July 2020, with an average temperature of 20 °C. At certain time intervals (ranging from 0 to 936 h), an aliquot of the solution was collected for the measurement. In addition, the Pb(NO3)2, Cd(NO3)2, PbCl2, and CdCl2 were added to different concentrations of NOR and LVF solutions at molar ratios (Pb/Cd to NOR/LVF) of 1:1, 1:2, and 1:3, respectively. Control tests were also conducted without Pb or Cd salt addition. The photodegradation experiments were the same as those described above. Each treatment and the control were prepared with three replicates.
The NOR or LVF concentrations in the sampling aliquots were analyzed through a UV–vis spectrophotometer and HPLC. All the analysis were conducted in triplicate, and the results reported in this study are the average values. After each measurement, the collected aliquot was returned to the corresponding beaker. Deionized water was supplemented at regular intervals to maintain the beakers at constant weights at the beginning of experiments.
3. Results and Discussion
3.1. Complexation Ratio Determination
The complexation ratio of NOR/LVF with Pb2+ and Cd2+ was determined by using UV–vis spectroscopy. As shown in Figure S2, the complexation ratio between FQs and Pb2+ or Cd2+ was around 1:2, which was consistent with previous studies [25]. Different heavy metal cations and molar ratios with FQs mean that different complexation structures will be formed, and their complexation structure may affect its photolytic degradation [25].
3.2. Effects of Initial FQ Concentrations
Figure 1 shows the effects of the initial concentrations (0.006, 0.012, and 0.06 mmol/L) on the photolytic degradation of NOR and LVF at different time intervals. The obtained pseudo-first-order kinetic parameters (correlation coefficients (R2), rate constants (k), half-life (t1/2)) and the degradation efficiencies (%) of FQs are presented in Table S1. The results indicated that the photolytic degradation of NOR followed the pseudo-first-order kinetics, with R2 values higher than 0.95 for all concentrations (Table S1). The photolytic degradation of LVF followed the pseudo-first-order kinetics at lower concentrations (0.006 and 0.012 mmol/L), with R2 values higher than 0.91 (Table S1). In general, our results were consistent with many previous studies, reporting that the FQs’ photodegradation followed apparent first-order kinetics when exposed to sunlight irradiation [17,20,32]. With an increase in the initial FQ concentration, a decrease trend in the photodegradation rate constant can be observed, particularly for LVF. After 42 days of sunlight irradiation, approximately 57.2%, 69.4%, and 48.3% of NOR was decomposed for 0.006, 0.012, and 0.06 mmol/L, respectively. In comparison, only 43.4%, 30.3%, and 9.8% of LVF was decomposed for 0.006, 0.012, and 0.06 mmol/L, respectively. Moreover, the rate constants of NOR were higher than those of LVF, and, correspondingly, the half-life values of NOR were smaller than those of LVF. These results indicated that the photodegradation ability of NOR was relatively higher than that of LVF.
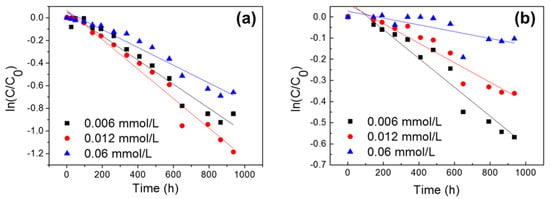
Figure 1.
Effects of initial FQ concentration on the attenuation of (a) NOR and (b) LVF (C, the concentration of FQs as a function of reaction time; C0, the initial concentration).
3.3. Effects of Lead and Cadmium Salts on the Photolytic Degradation of Two FQs
The effects of Pb and Cd salts on FQs’ photolytic degradation at different molar ratios are shown in Figure 2, Figure 3, Figure 4 and Figure 5. In all of the studied cases, the photolytic degradation data fitted the pseudo-first order model (lnc = lnc0 − kt), and the half-life times and decomposition rates for all samples are listed in Table S2.
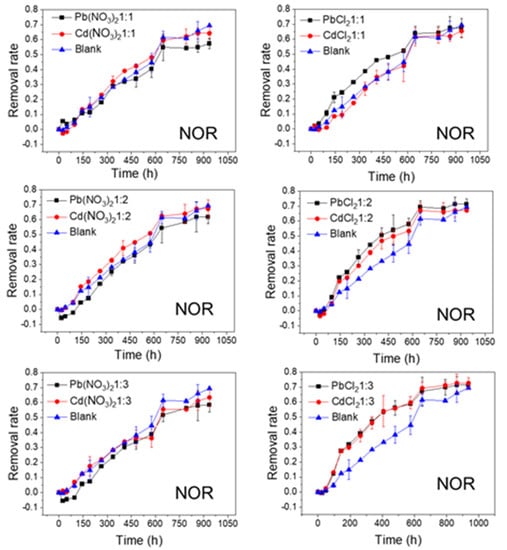
Figure 2.
Photolytic degradation of NOR (0.012 mmol/L) with the effects of Pb2+ and Cd2+ at different molar ratios; Blank means no heavy metal ions added.
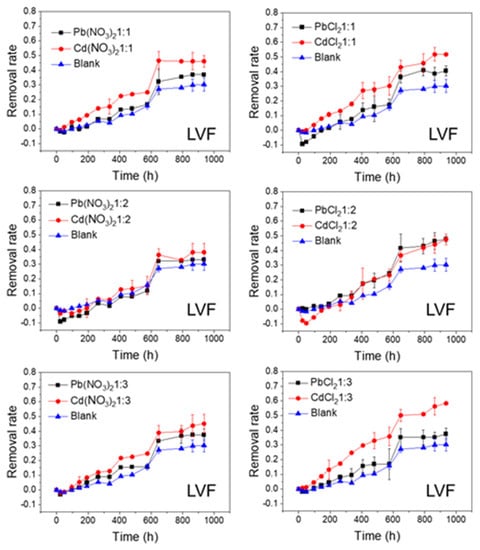
Figure 3.
Photolytic degradation of LVF (0.012 mmol/L) with the effects of Pb2+ and Cd2+ at different molar ratios; Blank means no heavy metal ions added.
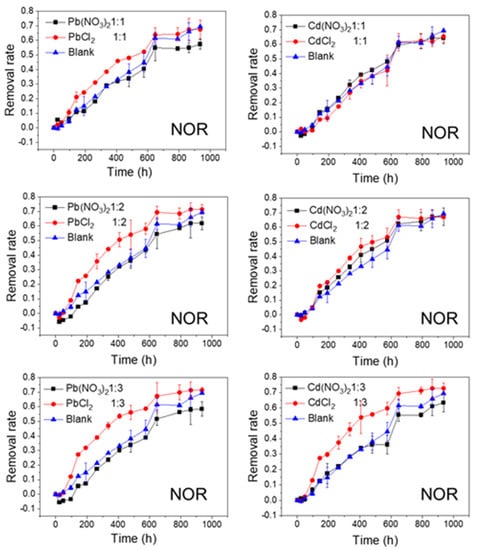
Figure 4.
Photolytic degradation of NOR (0.012 mmol/L) with the effects of NO3− and Cl− at different molar ratios; Blank means no heavy metal ions added.
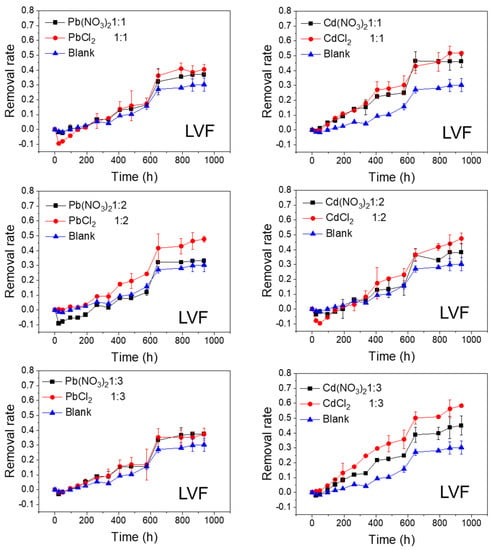
Figure 5.
Photolytic degradation of LVF (0.012 mmol/L) with the effects of NO3− and Cl− at different molar ratios; Blank means no heavy metal ions added.
As shown in Figure 2 and Figure 3, the FQs were prone to photolytic degradation under natural sunlight irradiation (42 days) without the addition of Pb or Cd salts, and NOR was more easily attenuated (69.4%) compared to LVF (30.3%). When the nitrate was mixed, the NOR removal efficiency ranged from 57.3% to 61.8% with Pb2+ as the positive ion, and it ranged from 63.4% to 67.3% with Cd2+ as the positive ion (Figure 2). When the chloride was mixed, the NOR removal efficiency changed to 67.5–71.4% with Pb2+ as the positive ion, and to 65.5–72.7% with Cd2+ as the positive ion (Figure 2). When the nitrate was mixed, the LVF removal efficiency ranged from 33.0% to 37.5% with Pb2+ as the positive ion, and ranged from 38.2% to 46.1% with Cd2+ as the positive ion (Figure 3). When the chloride was mixed, the LVF removal efficiency increased to 37.4–47.8% with Pb2+ as the positive ion, and to 47.5–58.3% with Cd2+ as the positive ion (Figure 3). In general, the removal efficiencies of both NOR and LVF were higher in FQs-Cd2+ systems than those in FQ-Pb2+ systems at each molar ratio. As shown in Table S2, for the same anion (NO3− or Cl−), the t1/2 values in FQ-Cd2+ systems were generally smaller than those in FQ-Pb2+ systems, and this phenomenon was more evident for LVF.
The effects of NO3− and Cl− on the photolytic degradation efficiency of the FQs are shown in Figure 4 and Figure 5. As shown in Figure 4, when the cations (Pb2+ or Cd2+) remained unchanged, the final removal rates of NOR tended to be higher when the anion was Cl−, whereas they were lower when the anion was NO3−, compared to the control test. In addition, this phenomenon was more evident at lower molar ratios (1:2 and 1:3). Moreover, the degradation rates of NOR in NOR-Cl− systems were faster in the first half of the reaction (until approximately 500 h). Similarly, when the cations (Pb2+ or Cd2+) remained unchanged, the final removal rates of LVF were higher when the anion was Cl− in comparison with NO3−. Moreover, this phenomenon was most obvious when the molar ratio was 1:2 for Pb2+ and 1:3 for Cd2+ (Figure 5). These results indicated that Cl− greatly accelerated the degradation of both NOR and LVF in the aqueous solution, without regard to the cations (Pb2+ or Cd2+). As shown in Table S2, for the same cation (Pb2+ or Cd2+), the t1/2 values of the FQ-NO3− systems were higher than those in the FQ-Cl− systems at each molar ratio. Almost all the t1/2 values of NOR-NO3− systems were higher than 22 days (the t1/2 of NOR blank sample), implying that NO3− retarded NOR degradation under natural sunlight irradiation. In comparison, almost every t1/2 of the NOR-Cl− system was less than 22 days, reflecting that Cl− tended to enhance the degradation of NOR. Meanwhile, for the LVF, all the t1/2 values of the LVF-NO3− and LVF-Cl− systems were obviously less than 65 days (the t1/2 of the blank sample of LVF), indicating that both nitrate and chloride played a positive role in the degradation of LVF. Moreover, each t1/2 value of the LVF-NO3− system was higher than that of the LVF-Cl− system at the same molar ratio.
The co-existence of FQs with metal ions in the aqueous environment leads to metal complexation, and this interaction may alter FQs’ properties. Previous studies reported the inconsistent effects of metal ions on FQs’ photodegradation efficiency. The photodegradation rate of RFX (a frequently found FQ) decreased in the presence of metal ions, but no changes occurred in the nature of the photoproducts [33]. The Cu(II)-CIP (a typical FQ) complexation inhibited the photodegradation of CIP and altered the photolytic pathways and products [34]. However, it was reported that metal ions (such as Cu, Zn, Fe, and Al) enhanced the photodegradation of moxifloxacin (a typical FQ) in aqueous solutions [35]. These findings indicate that the effect of the complexation reaction on FQs’ photodegradation greatly depends on the antibiotic molecular structure and metal type. Our results indicated that both Pb2+ and Cd2+ tend to enhance the photodegradation of these two FQs, and the promoting effect of Cd2+ was more significant than that of Pb2+, particularly for LVF. However, the effect of anions on the comprehensive photodegradation efficiency could not be neglected.
As reported previously, the presence of NO3− and Cl− might affect the photodegradation of FQs in aqueous solutions, but the findings were not unitary. Some studies proved that NO3− and Cl− decreased the photodegradation rates of FQs under VUV/UV irradiation, which could be attributed to their scavenging with ·OH and their VUV screening capacities [22,36]. Li et al. [37] found that NO3− may increase the photolysis efficiency of FQs under UV-254 and solar irradiation by producing ROS and nitrogen reactive species (i.e., NO, NO3−, and N2O4), but it also suppresses FQs’ photolysis under UV-254 and solar irradiation through competitive photoabsorption with FQs. Ge et al. [17] also proved that the coexisting NO3− inhibited the photodegradation of FQs, as it mainly acted as a radiation filter and/or scavenger for ROS. However, they claimed that Cl− had no significant impact on the photodegradation of FQs. Morimura et al. [38] also found that Cl− had no effect on the photodegradation of orbifloxacin. Our results also proved that the impacts of anions on FQs’ photodegradation varied with the types of anions and FQs. The NO3− was expected to play a significantly negative role in the degradation of FQs, particularly for NOR. In comparison, no obvious inhibitory effects of Cl− on both NOR and LVF were found.
As depicted in Figure 2, Figure 3, Figure 4 and Figure 5, the FQs’ degradation efficiency varied with the amounts of Pb and Cd salts added. It is likely that the FQ–heavy metal ion complexation altered the molecular orbital components of the excitation and orbital structures, resulting in different light absorption characteristics between FQ–metal complexes and FQ species alone. According to the results in Figure S2, when the molar ratio was 1:1, most of the heavy metal ions could coordinate with FQs, and there were free FQs in the aquatic solution. In comparison, there would be extra metal ions when the molar ratio was 1:3. However, there was no unitary photodegradation performance for all the molar ratios (FQs to heavy metal ions), but a higher molar ratio tends to favor the photodegradation of both NOR and LVF.
The photodegradation mechanisms, products, and pathways of FQs have been studied extensively. As mentioned earlier, FQs can absorb sunlight directly and undergo apparent photolysis. Fe3+, Cu2+, Ca2+, and nitrate can affect the self-sensitized photooxidation rate via changing the properties of hydroxyl radicals (·OH) and 1O2, and photocatalytic degradation occurs via four pathways: (I) defluorination, (II) hydroxylation in the quinolone ring, (III) dealkylation in the piperazine ring, and (IV) oxidation in the piperazine ring [39,40,41,42,43,44]. For danofloxacin, LEV, difloxacin, and enrofloxacin, N4 dealkylation was the main pathway, followed by decarboxylation and defluorination [17]. Wu et al. [45] found that AgI@Ag3PO4 enabled the photodegradation of norfloxacin and suggested that 1O2 radicals are the major active species in the visible-light-driven photodegradation of norfloxacin, with sustained attacks on the piperazine ring and the generation of intermediates, while OH radicals are not the major contributors. However, G e et al. [18] suggested that OH can oxidize almost all classes of organic chemicals because of the lower selectivity, oxidized through hydroxylated defluorination and piperazinyl hydroxylation. The photodegradation of antibiotics by different ions, photocatalysts, and adsorbents will produce different intermediates; there were seven products detected during LEV degradation by Mn(VII) [46]. During the photocatalytic degradation of CIP under different pH conditions, more than ten products or intermediates were identified [45,47]. Some studies found that the toxicities first decreased, then increased, and finally decreased, implying the generation of some more toxic intermediates than the parent compound [17]. Some researchers found that solar light effectively degraded FQs, but the photodegradation products retained significant biotoxicity [15,46,48]. However, Zhou et al. [49,50,51] reached the opposite conclusion about photodegradation products. As a result, the photo-modified toxicities for the water ecological environment need more attention. Heavy metal ions can complex with antibiotics and affect the photodegradation, antagonistic, and synergistic effects. Some metal ions serve as a natural photocatalyst in environmental water. Metal complexation can alter the 1O2 oxidation reaction pathways of antibiotics due to the rearrangement of atomic charges, and also alter the molecular orbital components of the excitation and orbital structures, causing different light absorption characteristics [34]. The oxygen-containing functional groups, namely carbonyl and carboxyl groups, of FQs are able to form complexes with metal ions, which is favorable for the complex compound to enter the triplet state, making the intersystem crossing transitions more efficient [43]. NO3− can inhibit the photodegradation of quinolone antibiotics in water by competitively absorbing solar photons or quenching ROS [17,52]. In the future, the degradation behavior and pathways of FQs in real wastewater should be further studied, due to the coexistence of various substances.
4. Conclusions
The effects of Pb2+, Cd2+, Cl−, and NO3− on the photolytic degradation of NOR and LVF in aqueous solutions were investigated under natural sunlight irradiation. Our results demonstrated that the photodegradation of FQs occurred in aquatic solutions under natural sunlight irradiation. Both NOR and LVF’s photolytic degradation followed the first-order kinetics, and lower initial concentrations of FQs corresponded to higher photolysis efficiency. Both heavy metal ions tended to enhance the FQs’ photodegradation. The effects of Cd2+ on the degradation efficiency were more significant than those of Pb2+ for both FQs. The presence of aqueous NO3− obviously suppressed the NOR degradation, but Cl− had slight effects on these two FQs’ photodegradation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20010323/s1, Table S1: The correlation coefficients (R2), rate constants (k) and half-life time (t1/2) of the two FQs; Table S2: The pseudo-first-order kinetics parameters of FQs at different molar ratios; Figure S1: UV spectrum of NOR and LVF. (a) NOR; (b) LVF; Figure S2: Determination of complexation ratio of NOR/LVF with Pb2+ and Cd2+.
Author Contributions
Conceptualization, methodology, validation, formal analysis, writing—original draft preparation, L.D.; writing—review and editing, H.Y.; supervision, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Program of China (grant number 2020YFF0218300), and the special fund from the Key Laboratory for Soft Chemistry and Functional Materials of Ministry of Education (Project No. 2021-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Siddique, M.B.A.; Kamal, A.; Coyne, M. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2017, 191, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; Zhang, Z.Y.; Li, P.; Zang, Y.G.; Liu, X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotox. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef]
- Du, J.; Zhao, H.X.; Liu, S.S.; Xie, H.J.; Wang, Y.; Chen, J.W. Antibiotics in the coastal water of the South Yellow Sea in China: Occurrence, distribution and ecological risks. Sci. Total Environ. 2017, 595, 521–527. [Google Scholar] [CrossRef]
- Zhang, R.L.; Kang, Y.R.; Zhang, R.J.; Han, M.W.; Zeng, W.B.; Wang, Y.H.; Yu, K.F.; Yang, Y. Occurrence, source, and the fate of antibiotics in mariculture ponds near the Maowei Sea, South China: Storm caused the increase of antibiotics usage. Sci. Total Environ. 2021, 752, 141882. [Google Scholar] [CrossRef]
- Jia, J.; Guan, Y.J.; Cheng, M.Q.; Chen, H.; He, J.F.; Wang, S.; Wang, Z.Z. Occurrence and distribution of antibiotics and antibiotic resistance genes in Ba River, China. Sci. Total Environ. 2018, 642, 1136–1144. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, D.L.; Xiao, X.T.; Cui, L.J.; Zhang, H.L. Occurrence of quinotone antibiotics and their impacts on aquatic environment in typical river-estuary system of Jiaozhou Bay, China. Ecotox. Environ. Saf. 2020, 190, 109993. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Zhang, Q.; Song, K.; Zhou, X.H.; Tang, Z.; Zhou, X. Occurrence and ecological risk assessment of selected antibiotics in the freshwater lakes along the middle and lower reaches of Yangtze River Basin. J. Environ. Manag. 2019, 249, 109396. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, J.M.; Zhang, G.D.; Lu, S.Y.; Liu, X.H.; Li, L.X.; Li, M. Occurrence of antibiotics and antibiotic resistance genes in the Fuxian Lake and antibiotic source analysis based on principal component analysis-multiple linear regression model. Chemosphere 2021, 262, 127741. [Google Scholar] [CrossRef]
- Boy-Roura, M.; Mas-Pla, J.; Petrovic, M.; Gros, M.; Soler, D.; Brusi, D.; Mencio, A. Towards the understanding of antibiotic occurrence and transport in groundwater: Findings from the Baix Fluvia alluvial aquifer (NE Catalonia, Spain). Sci. Total Environ. 2018, 612, 1387–1406. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.L.; Wang, Y.X.; Tong, L.; Deng, Y.M.; Li, Y.G.; Gan, Y.Q.; Guo, W.; Dong, C.J.; Duan, Y.H.; Zhao, K. Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers: A case study at Jianghan Plain, central China. Ecotox. Environ. Saf. 2017, 135, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Langbehn, R.K.; Michels, C.; Soares, H.M. Antibiotics in wastewater: From its occurrence to the biological removal by environmentally conscious technologies. Environ. Pollut. 2021, 275, 116603. [Google Scholar] [CrossRef]
- Spataro, F.; Ademollo, N.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Antibiotic residues and endocrine disrupting compounds in municipal wastewater treatment plants in Rome, Italy. Microchem. J. 2019, 148, 634–642. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered. 2021, 12, 7376–7416. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.F.; Wang, W.L. Photolysis of Enrofloxacin in aqueous systems under simulated sunlight irradiation: Kinetics, mechanism and toxicity of photolysis products. Chemosphere 2011, 85, 892–897. [Google Scholar] [CrossRef]
- Ge, L.K.; Chen, J.W.; Wei, X.X.; Zhang, S.Y.; Qiao, X.L.; Cai, X.Y.; Xie, Q. Aquatic photochemistry of fluoroquinolone antibiotics: Kinetics, pathways, and multivariate effects of main water constituents. Environ. Sci. Technol. 2010, 44, 2400–2405. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.K.; Halsall, C.; Chen, C.E.; Zhang, P.; Dong, Q.Q.; Yao, Z.W. Exploring the aquatic photodegradation of two ionisable fluoroquinolone antibiotics-Gatifloxacin and balofloxacin: Degradation kinetics, photobyproducts and risk to the aquatic environment. Sci. Total Environ. 2018, 633, 1192–1197. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Fasani, E.; Albini, A. Photochemical degradation of marbofloxacin and enrofloxacin in natural waters. Environ. Sci. Technol. 2010, 44, 4564–4569. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Profumo, A.; Fasani, E.; Albini, A.; Migliavacca, R.; Nucleo, E. Photodegradation of fluoroquinolones in surface water and antimicrobial activity of the photoproducts. Water Res. 2012, 46, 5575–5582. [Google Scholar] [CrossRef]
- Wammer, K.H.; Korte, A.R.; Lundeen, R.A.; Sundberg, J.E.; McNeill, K.; Arnold, W.A. Direct photochemistry of three fluoroquinolone antibacterials: Norfloxacin, ofloxacin, and enrofloxacin. Water. Res. 2013, 47, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Liang, Z.J.; Cui, F.Y.; Zhao, Z.W.; Yuan, C.; Du, J.Y.; Wang, C. Energy-saving photo-degradation of three fluoroquinolone antibiotics under VUV/UV irradiation: Kinetics, mechanism, and antibacterial activity reduction. Chem. Eng. J. 2020, 383, 123145. [Google Scholar] [CrossRef]
- Pan, B.; Qiu, M.; Wu, M.; Zhang, D.; Peng, H.; Wu, D.; Xing, B. The opposite impacts of Cu and Mg cations on dissolved organic matter–ofloxacin interaction. Environ. Pollut. 2012, 161, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, H.; Liao, S.; Sun, X.; Peng, H.; Zhang, D.; Pan, B. Cosorption of ofloxacin and Cu(II) in soils before and after organic matter removal. Sci. Total Environ. 2014, 481, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Cuprys, A.; Pulicharla, R.; Brar, S.K.; Drogui, P.; Verma, M.; Surampalli, R.Y. Fluoroquinolones metal complexation and its environmental impacts. Coordin. Chem. Rev. 2018, 376, 46–61. [Google Scholar] [CrossRef]
- Sciscenko, I.; Arques, A.; Varga, Z.; Bouchonnet, S.; Monfort, O.; Brigante, M.; Mailhot, G. Significant role of iron on the fate and photodegradation of enrofloxacin. Chemosphere 2021, 270, 129791. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhou, C.B.; Guo, C.S.; Wang, D.M.; Du, P.; Luo, Y.; Wan, J.; Meng, W. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci. Total Environ. 2014, 497, 267–273. [Google Scholar] [CrossRef]
- Lu, G.H.; Yang, X.F.; Li, Z.H.; Zhao, H.Z.; Wang, C. Contamination by metals and pharmaceuticals in northern Taihu Lake (China) and its relation to integrated biomarker response in fish. Ecotoxicology 2013, 22, 50–59. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.; Shi, Y.; Wang, C.; Li, B.; Li, Y.; Wu, S. Heavy metals in food crops, soil, and water in the Lihe River Watershed of the Taihu region and their potential health risks when ingested. Sci. Total Environ. 2018, 615, 141–149. [Google Scholar] [CrossRef]
- Zuo, J.X.; Fan, W.H.; Wang, X.L.; Ren, J.; Zhang, Y.; Wang, X.; Zhang, Y.; Yu, T.; Li, X. Trophic transfer of Cu, Zn, Cd, and Cr, and biomarker response for food webs in Taihu Lake, China. RSC Adv. 2018, 8, 3410–3417. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.Z.; Busetti, F.; Langsa, M.; Croué, J.P. Roles of singlet oxygen and dissolved organic matter in self-sensitized photo-oxidation of antibiotic norfloxacin under sunlight irradiation. Water Res. 2016, 106, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Cuquerella, M.C.; Belvedere, A.; Catalfo, A.; Miranda, M.A.; Scaiano, J.C.; de Guidi, G. Effects of bio-compatible metal ions on rufloxacin photochemistry, photophysics and photosensitization: Copper(II). J. Photoch. Photobio. B 2010, 101, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.X.; Chen, J.W.; Xie, Q.; Zhang, S.Y.; Li, Y.J.; Zhang, Y.F.; Xie, H.B. Photochemical behavior of antibiotics impacted by complexation effects of concomitant metals: A case for ciprofloxacin and Cu(II). Environ. Sci. Process. Impacts 2015, 17, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Hubicka, U.; Krzek, J.; Zuromska, B.; Walczak, M.; Zylewski, M.; Pawłowski, D. Determination of photostability and photodegradation products of moxifloxacin in the presence of metal ions in solutions and solid phase. Kinetics and identification of photoproducts. Photochem. Photobiol. Sci. 2012, 11, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chen, Y.; Ye, J.S.; Zhuang, L.; Zhang, H.L.; Ou, H. Degradation of ciprofloxacin by 185/254 nm vacuum ultraviolet: Kinetics, mechanism and toxicology. Environ. Sci. Wat. Res. 2019, 5, 564–576. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.F.; Shang, E.X.; Zheng, M.Y.; Luan, T.L. Effects of nitrate and humic acid on enrofloxacin photolysis in an aqueous system under three light conditions: Kinetics and mechanism. Environ. Chem. 2014, 11, 333–340. [Google Scholar] [CrossRef][Green Version]
- Morimura, T.; Kohno, K.; Nobuhara, Y.; Matsukura, H. Photoreaction and active oxygen generation by photosensitization of a new antibacterial fluoroquinolone derivative, orbifloxacin, in the presence of chloride ion. Chem. Pharm. Bull. 1997, 45, 1828–1832. [Google Scholar] [CrossRef][Green Version]
- Fisher, J.M.; Reese, J.G.; Pellechia, P.J.; Moeller, P.L.; Ferry, J.L. Role of Fe(III), phosphate, dissolved organic matter, and nitrate during the photodegradation of domoic acid in the marine environment. Environ. Sci. Technol. 2006, 40, 2200–2205. [Google Scholar] [CrossRef]
- Grannas, A.M.; Pagano, L.P.; Pierce, B.C.; Bobby, R.; Fede, A. Role of dissolved organic matter in ice photochemistry. Environ. Sci. Technol. 2014, 48, 10725–10733. [Google Scholar] [CrossRef]
- Mack, J.; Bolton, J.R. Photochemistry of nitrite and nitrate in aqueous solution: A review. J. Photochem. Photobiol. A 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Walse, S.S.; Morgan, S.L.; Kong, L.; Ferry, J.L. Role of dissolved organic matter, nitrate, and bicarbonate in the photolysis of aqueous fipronil. Environ. Sci. Technol. 2004, 38, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, X.; Liu, J.; Hao, C. Photophysical and photochemical insights of the photodegradation of norfloxacin: The rate-limiting step and the influence of Ca2+ ion. Chemosphere 2019, 219, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, T.; Du, P.; Liu, W.; Hu, J. Photocatalytic transformation fate and toxicity of ciprofloxacin related to dissociation species: Experimental and theoretical evidences. Water Res. 2020, 185, 116286. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, J.; Wang, W.; Xin, C.; Yu, X.; Tang, Y. High-performance photodegradation of norfloxacin enabled by AgI@Ag3PO4 nanostructures. J. Alloys Compd. 2022, 891, 161877. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Jiang, J.; Shen, Y.-M.; Pang, S.-Y.; Song, Y.; Guo, Q. A comparison study of levofloxacin degradation by peroxymonosulfate and permanganate: Kinetics, products and effect of quinone group. J. Hazard. Mater. 2021, 403, 123834. [Google Scholar] [CrossRef]
- Tang, L.; Wang, J.; Zeng, G.; Liu, Y.; Deng, Y.; Zhou, Y.; Tang, J.; Wang, J.; Guo, Z. Enhanced photocatalytic degradation of norfloxacin in aqueous Bi2WO6 dispersions containing nonionic surfactant under visible light irradiation. J. Hazard. Mater. 2016, 306, 295–304. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Tarantino, S.; Gualtieri, A.F.; Zema, M. Removal of fluoroquinolone contaminants from environmental waters on sepiolite and its photo-induced regeneration. Chemosphere 2016, 150, 686–693. [Google Scholar] [CrossRef]
- Santos, L.V.; Meireles, A.M.; Lange, L.C. Degradation of antibiotics norfloxacin by Fenton, UV and UV/H2O2. J. Environ. Manag. 2015, 154, 8–12. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Ferraro, F.; Silva-Agredo, J.; Torres-Palma, R.A. Degradation of highly consumed fluoroquinolones, penicillins and cephalosporins in distilled water and simulated hospital wastewater by UV254 and UV254/persulfate processes. Water Res. 2017, 122, 128–138. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W.; Sun, W.; Cai, M.; Ye, Z.; Yang, C.; Lu, Z.; Li, C. Medium pressure UV-activated peroxymonosulfate for ciprofloxacin degradation: Kinetics, mechanism, and genotoxicity. Chem. Eng. J. 2018, 345, 87–97. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, L.; Li, Y.; Zhao, J.; Lv, S.; Xiao, K. Effects of environmental factors on sulfamethoxazole photodegradation under simulated sunlight irradiation: Kinetics and mechanism. J. Environ. Sci. 2013, 25, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).