Respiratory Muscle Interval Training Improves Exercise Capacity in Obese Adolescents during a 3-Week In-Hospital Multidisciplinary Body Weight Reduction Program
Abstract
:1. Introduction
2. Materials and methods
2.1. Population
2.2. Body Weight Reduction Program
2.3. Respiratory Muscle Interval Training
2.4. Spirometry
2.5. Exercise Testing
2.6. Measurements
2.7. Statistical Analysis
3. Results
3.1. Anthropometric and Pulmonary Outcomes
3.2. Cardiorespiratory Outcomes during the Incremental Exercise Test
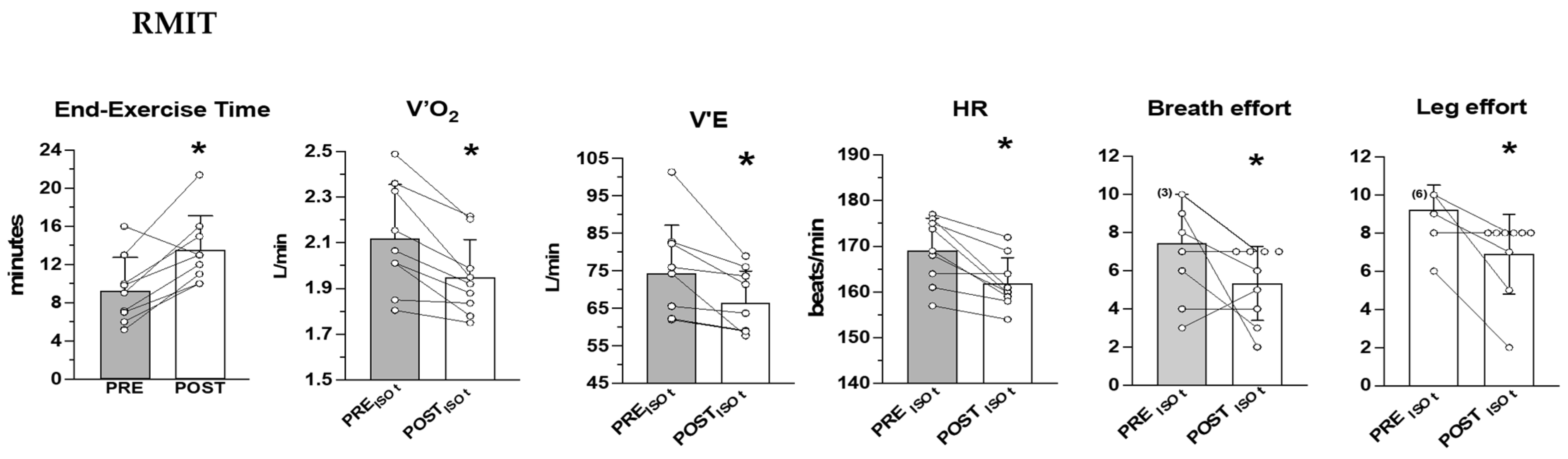
3.3. Cardiorespiratory Outcomes during the Heavy-Intensity Exercise Test
4. Discussion
4.1. Main Findings
4.2. Characteristics of the Interval Training Approach for the Respiratory Muscles
4.3. Metabolic Responses after Rmit and CTRL
4.4. Respiratory Responses and Exercise Tolerance after Rmit and CTRL
4.5. Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babb, T.G. Obesity: Challenges to ventilatory control during exercise—A brief review. Respir. Physiol. Neurobiol. 2013, 189, 364–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chlif, M.; Keochkerian, D.; Choquet, D.; Vaidie, A.; Ahmaidi, S. Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respir. Physiol. Neurobiol. 2009, 168, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.K.; Lin, C.C. Work of breathing and respiratory drive in obesity. Respirology 2012, 17, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Sood, A. Altered resting and exercise respiratory physiology in obesity. Clin. Chest Med. 2009, 30, 445–454. [Google Scholar] [CrossRef] [Green Version]
- LoMauro, A.; Cesareo, A.; Agosti, F.; Tringali, G.; Salvadego, D.; Grassi, B.; Sartorio, A.; Aliverti, A. Effects of a multidisciplinary body weight reduction program on static and dynamic thoraco-abdominal volumes in obese adolescents. Appl. Physiol. Nutr. Metab. 2016, 41, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, R.G.; Ondrak, K.S. Effects of being overweight on ventilatory dynamics of youth at rest and during exercise. Eur. J. Appl. Physiol. 2011, 111, 285–292. [Google Scholar] [CrossRef]
- Mendelson, M.; Michallet, A.-S.; Estève, F.; Perrin, C.; Levy, P.; Wuyam, B.; Flore, P. Ventilatory responses to exercise training in obese adolescents. Respir. Physiol. Neurobiol. 2012, 184, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wilhite, D.P.; Bhammar, D.M.; Martinez-Fernandez, T.; Babb, T.G. Mechanical effects of obesity on central and peripheral airway resistance in nonasthmatic early pubescent children. Pediatr. Pulmonol. 2022, 57, 2937–2945. [Google Scholar] [CrossRef]
- Bernhardt, V.; Wood, H.E.; Moran, R.B.; Babb, T.G. Dyspnea on exertion in obese men. Respir. Physiol. Neurobiol. 2013, 185, 241–248. [Google Scholar] [CrossRef] [Green Version]
- DeLorey, D.S.; Wyrick, B.L.; Babb, T.G. Mild-to-moderate obesity: Implications for respiratory mechanics at rest and during exercise in young men. Int. J. Obes. 2005, 29, 1039–1047. [Google Scholar] [CrossRef]
- Bhammar, D.M.; Babb, T.G. Effects of obesity on the oxygen cost of breathing in children. Respir. Physiol. Neurobiol. 2021, 285, 103591. [Google Scholar] [CrossRef] [PubMed]
- Chlif, M.; Keochkerian, D.; Feki, Y.; Vaidie, A.; Choquet, D.; Ahmaidi, S. Inspiratory muscle activity during incremental exercise in obese men. Int. J. Obes. 2007, 31, 1456–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezzoli, E.; Andreotti, D.; Pianta, L.; Mascheroni, M.; Piccinno, L.; Puricelli, L.; Cimolin, V.; Salvadori, A.; Codecasa, F.; Capodaglio, P. Motor control exercises of the lumbar-pelvic region improve respiratory function in obese men. A pilot study. Disabil. Rehabil. 2018, 40, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Bhammar, D.M.; Jones, H.N.; Lang, J.E. Inspiratory Muscle Rehabilitation Training in Pediatrics: What Is the Evidence? Can. Respir. J. 2022, 2022, 5680311. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [Green Version]
- Frank, I.; Briggs, R.; Spengler, C.M. Respiratory muscles, exercise performance, and health in overweight and obese subjects. Med. Sci. Sports Exerc. 2011, 43, 714–727. [Google Scholar] [CrossRef] [Green Version]
- Alemayehu, H.K.; Salvadego, D.; Isola, M.; Tringali, G.; De Micheli, R.; Caccavale, M.; Sartorio, A.; Grassi, B. Three weeks of respiratory muscle endurance training improve the O2 cost of walking and exercise tolerance in obese adolescents. Physiol. Rep. 2018, 6, e13888. [Google Scholar] [CrossRef] [Green Version]
- Salvadego, D.; Sartorio, A.; Agosti, F.; Tringali, G.; Patrizi, A.; Lo Mauro, A.; Aliverti, A.; Grassi, B. Respiratory muscle endurance training reduces the O2 cost of cycling and perceived exertion in obese adolescents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R487–R495. [Google Scholar] [CrossRef] [Green Version]
- Verges, S.; Boutellier, U.; Spengler, C.M. Effect of respiratory muscle endurance training on respiratory sensations, respiratory control and exercise performance: A 15-year experience. Respir. Physiol. Neurobiol. 2008, 161, 16–22. [Google Scholar] [CrossRef]
- Wüthrich, T.U.; Marty, J.; Benaglia, P.; Eichenberger, P.A.; Spengler, C.M. Acute Effects of a Respiratory Sprint-Interval Session on Muscle Contractility. Med. Sci. Sports Exerc. 2015, 47, 1979–1987. [Google Scholar] [CrossRef]
- Schaer, C.E.; Wüthrich, T.U.; Beltrami, F.G.; Spengler, C.M. Effects of Sprint-Interval and Endurance Respiratory Muscle Training Regimens. Med. Sci. Sports Exerc. 2019, 51, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical measurements to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, D.S.; Bray, G.A.; Gemayel, N.; Kaplan, K. Effects of obesity on bioelectrical impedance. Am. J. Clin. Nutr. 1989, 50, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hofsteenge, G.H.; Chinapaw, M.J.; Weijs, P.J. Fat-free mass prediction equations for bioelectric impedance analysis compared to dual energy X-ray absorptiometry in obese adolescents: A validation study. BMC Pediatr. 2015, 15, 158. [Google Scholar] [CrossRef] [Green Version]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, J.L.; Odencranz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Whipp, B.J. Principles of Exercise Testing and Interpretation, 3rd ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1999. [Google Scholar]
- Wilson, R.C.; Jones, P.W. Long-term reproducibility of Borg scale estimates of breathlessness during exercise. Clin. Sci. 1991, 80, 309–312. [Google Scholar] [CrossRef] [Green Version]
- Lamarra, N.; Whipp, B.J.; Ward, S.A.; Wasserman, K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J. Appl. Physiol. 1987, 62, 2003–2012. [Google Scholar] [CrossRef]
- Grassi, B.; Rossiter, H.B.; Zoladz, J.A. Skeletal muscle fatigue and decreased efficiency: Two sides of the same coin? Exerc. Sport Sci. Rev. 2015, 43, 75–83. [Google Scholar] [CrossRef]
- Jones, A.M.; Grassi, B.; Christensen, P.M.; Krustrup, P.; Bangsbo, J.; Poole, D.C. The slow component of O2 kinetics: Mechanistic bases and practical applications. Med. Sci. Sports Exerc. 2011, 43, 2046–2062. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Schaffartzik, W.; Knight, D.R.; Derion, T.; Kennedy, B.; Guy, H.J.; Prediletto, R.; Wagner, P.D. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J. Appl. Physiol. 1991, 71, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Carra, J.; Candau, R.; Keslacy, S.; Giolbas, F.; Borrani, F.; Millet, G.P.; Varray, A.; Ramonatxo, M. Addition of inspiratory resistance increases the amplitude of the slow component of O2 uptake kinetics. J. Appl. Physiol. 2003, 94, 2448–2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, T.J.; Morris, N.R.; Haseler, L.J.; Schneider, D.A.; Sabapathy, S. The influence of breathing mechanics on the development of the slow component of O2 uptake. Respir. Physiol. Neurobiol. 2010, 173, 125–131. [Google Scholar] [CrossRef]
- Cross, T.J.; Sabapathy, S.; Schneider, D.A.; Haseler, L.J. Breathing He-O2 attenuates the slow component of O2 uptake kinetics during exercise performed above the respiratory compensation threshold. Exp. Physiol. 2010, 95, 172–183. [Google Scholar] [CrossRef]
- Wetter TJHarms, C.A.; Nelson, W.B.; Pegelow, D.F.; Dempsey, J.A. Influence of respiratory muscle work on O2 and leg blood flow during submaximal exercise. J. Appl. Physiol. 1999, 87, 643–651. [Google Scholar] [CrossRef]
- Salvadego, D.; Sartorio, A.; Agosti, F.; Tringali, G.; Patrizi, A.; Mauro, A.L.; Aliverti, A.; Grassi, B. Acute respiratory muscle unloading by normoxic helium-O2 breathing reduces the O2 cost of cycling and perceived exertion in obese adolescents. Eur. J. Appl. Physiol. 2015, 115, 99–109. [Google Scholar] [CrossRef]
- Salvadego, D.; Lazzer, S.; Busti, C.; Galli, R.; Agosti, F.; Lafortuna, C.; Sartorio, A.; Grassi, B. Gas Exchange kinetics in obese adolescents. Inferences on exercise tolerance and prescription. Am. J. Physiol. Regul. Int. Comp. Physiol. 2010, 299, R1298–R1305. [Google Scholar] [CrossRef] [Green Version]
- Guenette, J.A.; Querido, J.S.; Eves, N.D.; Chua, R.; Sheel, A.W. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R166–R175. [Google Scholar] [CrossRef] [Green Version]
- Sheel, A.W.; Boushel, R.; Dempsey, J.A. Competition for blood flow distribution between respiratory and locomotor muscles: Implications for muscle fatigue. J. Appl. Physiol. 2018, 125, 820–831. [Google Scholar] [CrossRef]
- Sheel, A.W.; Dominelli, P.B. Breathing during exercise: There is no such thing as a free lunch. Exp. Physiol. 2019, 104, 1333–1334. [Google Scholar] [CrossRef] [PubMed]



| CTRL (n = 8) | |||||||
| Session 1 | |||||||
| Before | End I1 | End I2 | End I3 | End I4 | End I5 | End I6 | |
| HR bpm | 89.5 ± 8.4 | 89.9 ± 8.5 | 91.1 ± 8.6 | 91.4 ± 8.8 | 91.9 ± 8.2 | 91.6 ± 7.6 | 91.5 ± 6.8 |
| RPEB | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| SpO2 % | 95.9 ± 0.6 | - | - | - | - | - | 96.1 ± 0.6 |
| SBP mmHg | 115.6 ± 6.2 | - | - | - | - | - | 118.1 ± 3.7 |
| DBP mmHg | 81.2 ± 8.3 | - | - | - | - | - | 81.2 ± 6.4 |
| Session 12 | |||||||
| Before | End I1 | End I2 | End I3 | End I4 | End I5 | End I6 | |
| HR bpm | 87.7 ± 9.3 | 90.0 ± 7.9 | 90.4 ± 8.0 | 91.7 ± 9.2 | 90.6 ± 7.8 | 90.3 ± 7.6 | 90.3 ± 8.2 |
| RPEB | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| SpO2 % | 96.1 ± 0.6 | - | - | - | - | - | 96.4 ± 0.5 |
| SBP mmHg | 115.6 ± 4.9 | - | - | - | - | - | 118.7 ± 3.5 |
| DBP mmHg | 80.0 ± 2.7 | - | - | - | - | - | 82.5 ± 4.6 |
| RMIT (n = 9) | |||||||
| Session 1 | |||||||
| Before | End I1 | End I2 | End I3 | End I4 | End I5 | End I6 | |
| HR bpm | 89.9 ± 9.2 | 96.8 ± 9.5 | 105.8 ± 8.1 | 107.0 ± 10.4 | 106.4 ± 7.2 | 107.5 ± 8.5 | 105.6 ± 9.1 |
| RPEB | 0.0 ± 0.0 | 4.0 ± 1.9 | 4.0 ± 1.4 | 4.4 ± 1.7 | 5.2 ± 2.4 | 4.8 ± 1.9 | 4.8 ± 1.9 |
| SpO2 % | 95.0 ± 0.5 | - | - | - | - | - | 95.4 ± 0.8 |
| SBP mmHg | 120.6 ± 6.3 | - | - | - | - | - | 120.4 ± 3.2 |
| DBP mmHg | 85.0 ± 8.3 | - | - | - | - | - | 86.6 ± 8.2 |
| Session 12 | |||||||
| Before | End I1 | End I2 | End I3 | End I4 | End I5 | End I6 | |
| HR bpm | 91.6 ± 3.7 | 98.2 ± 8.0 | 104.0 ± 9.9 | 104.6 ± 9.8 | 106.9 ± 9.9 | 108.7 ± 8.7 | 104.9 ± 9.8 |
| RPEB | 0.0 ± 0.0 | 0.5 ± 0.9 | 0.7 ± 1.3 | 0.7 ± 1.3 | 0.8 ± 1.3 | 1.1 ± 1.7 | 1.3 ± 2.1 |
| SpO2 % | 95.4 ± 1.0 | - | - | - | - | - | 95.8 ± 1.1 |
| SBP mmHg | 120.0 ± 3.9 | - | - | - | - | - | 120.8 ± 4.9 |
| DBP mmHg | 81.6 ± 4.1 | - | - | - | - | - | 81.9 ± 4.5 |
| CTRL (n = 8) | RMIT (n = 9) | |||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Age (years) | 17.0 ± 5.7 | 17.0 ± 5.7 | 1.00 | 17.9 ± 4.9 | 17.9 ± 4.9 | 1.00 |
| Stature (m) | 1.72 ± 0.06 | 1.72 ± 0.06 | 1.00 | 1.72 ± 0.08 | 1.72 ± 0.08 | 1.00 |
| Body mass (kg) | 109.3 ± 8.9 | 105.1 ± 9.1 | 0.0002 | 113.8 ± 16.3 | 110.0 ± 16.2 | 0.0001 |
| BMI (kg∙m−2) | 37.0 ± 3.2 | 35.6 ± 3.8 | 0.0003 | 38.6 ± 5.5 | 37.3 ± 5.3 | 0.0001 |
| FFM (kg) | 65.8 ± 6.9 | 62.4 ± 6.1 | 0.009 | 69.8 ± 12.9 | 68.2 ± 12.7 | 0.11 |
| FM (kg) | 43.5 ± 3.3 | 42.7 ± 4.2 | 0.23 | 44.0 ± 3.9 | 41.9 ± 5.1 | 0.02 |
| FM (% of body mass) | 40.0 ± 1.3 | 40.3 ± 1.0 | 0.99 | 39.0 ± 2.7 | 38.3 ± 3.5 | 0.71 |
| FVC (L) | 4.82 ± 0.25 | 4.80 ± 0.35 | 0.74 | 4.55 ± 0.63 | 5.01 ± 0.50 | 0.01 |
| FVC (% of predicted) | 102.2 ± 5.3 | 101.8 ± 7.4 | 0.79 | 93.8 ± 13.0 | 103.3 ± 10.3 | 0.006 |
| FEV1 (L) | 4.05 ± 0.32 | 4.00 ± 0.31 | 0.80 | 3.80 ± 0.37 | 4.22 ± 0.55 | 0.02 |
| FEV1 (% of predicted) | 100.7 ± 7.9 | 99.5 ± 7.7 | 0.88 | 92.1 ± 8.9 | 102.3 ± 12.3 | 0.02 |
| FEV1/FVC (%) | 83.9 ± 5.2 | 82.8 ± 4.4 | 0.93 | 84.1 ± 6.7 | 83.7 ± 6.7 | 0.86 |
| FEF25–75% (L∙min−1) | 4.35 ± 0.79 | 4.25 ± 0.58 | 0.58 | 3.99 ± 0.62 | 4.57 ± 0.99 | 0.12 |
| FEF25–75% (% of predicted) | 100.1 ± 18.2 | 97.8 ± 13.3 | 0.66 | 89.3 ± 13.9 | 102.2 ± 22.1 | 0.10 |
| PEF (L∙s−1) | 7.39 ± 1.49 | 7.57 ± 1.36 | 0.67 | 6.64 ± 0.79 | 8.10 ± 1.40 | 0.01 |
| PEF (% of predicted) | 87.1 ± 19.6 | 89.2 ± 16.0 | 0.68 | 78.4 ± 11.9 | 97.6 ± 18.8 | 0.004 |
| CTRL (n = 8) | RMIT (n = 9) | |||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Work rate (watt) | 148.7 ± 13.6 | 157.5 ± 17.5 | 0.005 | 154.4 ± 21.3 | 164.4 ± 22.4 | 0.001 |
| HR (b∙min−1) | 173.7 ± 6.9 | 169.1 ± 6.8 | 0.04 | 179.3 ± 3.6 | 172.8 ± 6.6 | 0.003 |
| E (L∙min−1) | 77.4 ± 9.2 | 84.8 ± 11.7 | 0.25 | 80.4 ± 23.9 | 84.3 ± 13.3 | 0.62 |
| VT (L) | 2.02 ± 0.46 | 2.10 ± 0.46 | 0.39 | 2.05 ± 0.38 | 2.25 ± 0.56 | 0.01 |
| fR (br∙min−1) | 39.4 ± 6.6 | 41.3 ± 5.9 | 0.78 | 39.4 ± 9.6 | 38.6 ± 6.1 | 0.96 |
| O2 (L∙min−1) | 2.22 ± 0.34 | 2.40 ± 0.34 | 0.0007 | 2.34 ± 0.32 | 2.46 ± 0.30 | 0.02 |
| O2/BM (mL∙kg−1∙min−1) | 20.5 ± 4.0 | 22.0 ± 3.9 | 0.0007 | 20.5 ± 2.3 | 21.9 ± 2.5 | 0.02 |
| O2/FFM (mL∙kg−1∙min−1) | 33.5 ± 8.1 | 38.8 ± 7.8 | 0.0001 | 33.8 ± 3.9 | 36.3 ± 5.2 | 0.003 |
| CO2 (L∙min−1) | 2.40 ± 0.31 | 2.52 ± 0.31 | 0.07 | 2.48 ± 0.42 | 2.65 ± 0.32 | 0.14 |
| R | 1.08 ± 0.10 | 1.05 ± 0.08 | 0.42 | 1.05 ± 0.10 | 1.08 ± 0.07 | 0.50 |
| E/O2 | 35.2 ± 6.6 | 35.6 ± 5.0 | 0.99 | 33.4 ± 10.0 | 34.8 ± 5.8 | 0.85 |
| E/CO2 | 31.9 ± 3.1 | 33.7 ± 2.1 | 0.36 | 31.2 ± 6.1 | 32.1 ± 3.5 | 0.94 |
| PETO2 (mmHg) | 95.6 ± 4.5 | 96.1 ± 3.4 | 0.99 | 92.8 ± 7.4 | 95.0 ± 4.3 | 0.32 |
| PETCO2 (mmHg) | 34.7 ± 3.2 | 33.4 ± 2.0 | 0.55 | 36.4 ± 5.1 | 34.8 ± 3.4 | 0.35 |
| RPER | 7.5 ± 1.8 | 7.0 ± 1.2 | 0.99 | 8.0 ± 1.7 | 6.9 ± 2.6 | 0.36 |
| RPEL | 8.9 ± 1.1 | 9.1 ± 1.0 | 0.90 | 9.3 ± 0.9 | 9.4 ± 0.5 | 0.99 |
| GET (L∙min−1) | 1.33 ± 0.28 | 1.43 ± 0.23 | 0.25 | 1.57 ± 0.24 | 1.50 ± 0.21 | 0.95 |
| GET (watt) | 87.5 ± 11.6 | 87.5 ± 11.6 | 1.00 | 96.3 ± 7.4 | 90.0 ± 15.0 | 0.41 |
| RCT (L∙min−1) | 2.15 ± 0.42 | 2.35 ± 0.29 | 0.01 | 2.29 ± 0.40 | 2.41 ± 0.35 | 0.59 |
| RCT (watt) | 143.7 ± 16.0 | 152.5 ± 18.3 | 0.12 | 146.3 ± 26.1 | 158.6 ± 25.5 | 0.20 |
| CTRL (n = 8) | RMIT (n = 9) | |||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Work rate (watt) | 113.7 ± 15.1 | 113.7 ± 15.1 | 1.00 | 122.3 ± 12.3 | 122.3 ± 12.3 | 1.00 |
| Time to exhaustion (min) | 14.4 ± 5.6 | 15.3 ± 5.4 | 0.62 | 9.2 ± 3.5 * | 13.5 ± 3.7 | 0.0003 |
| HR (b∙min−1) | 168.7 ± 9.2 | 164.4 ± 8.3 | 0.30 | 169.3 ± 6.9 | 169.5 ± 5.9 | 0.99 |
| E (L∙min−1) | 68.0 ± 11.1 | 69.5 ± 7.7 | 0.88 | 73.9 ± 13.7 | 73.1 ± 11.7 | 0.80 |
| VT (L) | 1.72 ± 0.36 | 1.80 ± 0.34 | 0.31 | 1.98 ± 0.28 | 1.96 ± 0.49 | 0.99 |
| fR (br min−1) | 40.6 ± 9.3 | 39.6 ± 7.7 | 0.72 | 37.7 ± 7.2 | 38.5 ± 6.6 | 0.98 |
| VT/TI (L∙s−1) | 2.48 ± 0.28 | 2.54 ± 0.36 | 0.98 | 2.64 ± 0.38 | 2.56 ± 0.40 | 0.76 |
| Resting O2 (L∙min−1) | 0.42 ± 0.05 | 0.44 ± 0.03 | 0.90 | 0.44 ± 0.08 | 0.44 ± 0.07 | 0.99 |
| O2 (L∙min−1) | 1.87 ± 0.20 | 1.96 ± 0.11 | 0.27 | 2.11 ± 0.25 | 2.08 ± 0.17 | 0.90 |
| O2 slow component (L∙min−1) | 0.19 ± 0.06 | 0.25 ± 0.08 | 0.51 | 0.24 ± 0.05 | 0.25 ± 0.15 | 0.99 |
| O2/BM (mL∙kg−1∙min−1) | 17.1 ± 2.9 | 18.0 ± 2.8 | 0.21 | 18.6 ± 2.15 | 18.5 ± 1.2 | 0.96 |
| O2/FFM (mL∙kg−1∙min−1) | 28.3 ± 5.9 | 31.7 ± 5.2 | 0.03 | 30.8 ± 4.6 | 31.2 ± 3.7 | 0.88 |
| CO2 (L∙min−1) | 1.86 ± 0.19 | 1.88 ± 0.14 | 0.94 | 2.10 ± 0.23 | 2.06 ± 0.21 | 0.44 |
| R | 1.00 ± 0.07 | 0.97 ± 0.05 | 0.62 | 1.00 ± 0.10 | 0.99 ± 0.04 | 0.70 |
| E/O2 | 33.8 ± 4.9 | 32.9 ± 4.0 | 0.59 | 32.5 ± 7.8 | 32.5 ± 4.3 | 0.99 |
| E/CO2 | 33.7 ± 3.4 | 34.1 ± 2.1 | 0.84 | 32.1 ± 3.9 | 32.9 ± 3.0 | 0.64 |
| PETO2 (mmHg) | 95.1 ± 4.5 | 94.8 ± 3.5 | 0.92 | 92.9 ± 4.7 | 94.0 ± 3.4 | 0.62 |
| PETCO2 (mmHg) | 33.1 ± 3.6 | 32.2 ± 2.0 | 0.53 | 34.9 ± 3.1 | 33.6 ± 2.6 | 0.15 |
| RPER | 6.6 ± 2.8 | 4.9 ± 1.2 | 0.10 | 7.8 ± 2.8 | 7.7 ± 2.5 | 0.99 |
| RPEL | 8.0 ± 1.9 | 7.6 ± 2.8 | 0.99 | 9.0 ± 1.7 | 9.6 ± 0.9 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvadego, D.; Tringali, G.; De Micheli, R.; Sartorio, A. Respiratory Muscle Interval Training Improves Exercise Capacity in Obese Adolescents during a 3-Week In-Hospital Multidisciplinary Body Weight Reduction Program. Int. J. Environ. Res. Public Health 2023, 20, 487. https://doi.org/10.3390/ijerph20010487
Salvadego D, Tringali G, De Micheli R, Sartorio A. Respiratory Muscle Interval Training Improves Exercise Capacity in Obese Adolescents during a 3-Week In-Hospital Multidisciplinary Body Weight Reduction Program. International Journal of Environmental Research and Public Health. 2023; 20(1):487. https://doi.org/10.3390/ijerph20010487
Chicago/Turabian StyleSalvadego, Desy, Gabriella Tringali, Roberta De Micheli, and Alessandro Sartorio. 2023. "Respiratory Muscle Interval Training Improves Exercise Capacity in Obese Adolescents during a 3-Week In-Hospital Multidisciplinary Body Weight Reduction Program" International Journal of Environmental Research and Public Health 20, no. 1: 487. https://doi.org/10.3390/ijerph20010487
APA StyleSalvadego, D., Tringali, G., De Micheli, R., & Sartorio, A. (2023). Respiratory Muscle Interval Training Improves Exercise Capacity in Obese Adolescents during a 3-Week In-Hospital Multidisciplinary Body Weight Reduction Program. International Journal of Environmental Research and Public Health, 20(1), 487. https://doi.org/10.3390/ijerph20010487








