Abstract
Palbociclib and ribociclib are indicated in the first-line treatment of hormonal-receptor-positive HER-2 negative (HR+/HER-2 negative) advanced breast cancer. Despite their clinical benefit, they can increase healthcare expenditure. Yet, there are no comparative pharmacoeconomic evaluations for them in developing countries, the Middle East, or Gulf countries. This study compared the cost-effectiveness of palbociclib and ribociclib in Qatar. A 10-year within-cycle-corrected Markov’s model was developed using TreeAge Pro® software. The model consisted of three main health states: progression-free (PFS), progressed-disease (PD), and death. Costs were obtained from the actual hospital settings, transition probabilities were calculated from individual-patient data, and utilities were summarized from the published literature. The incremental cost-effectiveness ratio (ICER) and the incremental cost-utility ratio (ICUR) were calculated and compared to three gross-domestic-products per capita. Deterministic and probabilistic sensitivity analyses were performed. Ribociclib dominated palbociclib in terms of costs, life-years gained, and quality-adjusted life-years gained. The conclusions remained robust in the different cases of the deterministic sensitivity analyses. Taking all combined uncertainties into account, the confidence in the base-case conclusion was approximately 60%. Therefore, in HR+/HER-2 negative stage IV breast cancer patients, the use of ribociclib is considered cost-saving compared to palbociclib.
1. Introduction
Breast cancer is one of the most common non-communicable diseases worldwide, ranking second amongst all cancers with a total estimated number of 627,000 deaths (6.2% of total cancer-related deaths and 15% in women’s cancer-related deaths) in 2018 [1,2]. Stage IV breast cancer, including advanced breast cancer (ABC) and metastatic breast cancer (MBC), is challenging due to being incurable with low survival rates [3]. The majority of stage IV patients are observed to be hormone receptor-positive (HR+)/human epidermal receptor 2-negative (HER2-) [4]. For these patients, there are several first-line treatments depending on the patient’s case. Generally, endocrine therapy is considered the mainstay first-line treatment for the majority of patients with HR+ advanced breast cancer. Chemotherapy can also be used as a first-line treatment for patients who have a life-threatening disease or who require early relief of symptoms due to significant visceral organ involvement with endocrine [5]. Nonetheless, cyclin-dependent kinase 4 and 6 enzyme inhibitors (CDK4/6 inhibitors) are a relatively new class of medications that were approved in first-line treatment alongside endocrine therapy for HR+/HER-2 negative stage IV breast cancer patients after proving their clinical superiority compared to endocrine monotherapy [5]. To date, there are three CDK4/6 inhibiting agents approved by the Food and Drug Administration (FDA): palbociclib, ribociclib, and abemaciclib [6]. CDK4/6 inhibitors are expensive, and they need frequent close monitoring due to their side effects, which include blood-related side effects (such as neutropenia, febrile neutropenia, thrombocytopenia, anemia, and leukopenia), heart-related side effects (such as affecting the QT interval and induced abnormalities in electrocardiography), gastric side effects (such as diarrhea and constipation), and generalized fatigue and neurological pain [7,8]. Therefore, although CDK4/6 inhibitors were proven to add clinical benefit as per the available randomized controlled trials and observational studies [9,10,11,12,13,14,15,16], they can increase healthcare expenditure and healthcare costs. Thus, their use should be guided by reliable pharmacoeconomic evidence.
As for the pharmacoeconomic evidence, on one hand, to date, there are a few existing comparative cost-effectiveness studies for the cost-effectiveness of palbociclib and ribociclib in stage IV HR+/HER2-negative breast cancer patients [17,18,19,20,21]. The settings and perspectives of these studies varied from different countries, including the Spanish National Health System perspective in Spain [17], the third-party payer perspective in the United States of America (USA) [18,19], the National Health Services and Personal Social Services perspective in the United Kingdom (UK) [20], and the private healthcare system perspective in Brazil [21]. All of them concluded that the treatment with ribociclib plus letrozole was cost-effective compared to palbociclib plus letrozole, except for one study that concluded that both palbociclib plus letrozole and ribociclib plus letrozole combinations are not cost-effective compared to letrozole monotherapy [19]. Of note, all of these evaluations included only a single combination with the treatment of interest, palbociclib plus letrozole versus ribociclib plus letrozole, without taking any of the other FDA-indicated combinations into consideration, such as fulvestrant or tamoxifen. In addition, these analyses were based on secondary data obtained from phase III clinical trials that were mainly MONALEESA-2 [9], PALOMA-1 [16], and PALOMA-2 [12]. On the other hand, a recent 3-year cost-minimization analysis from Russia using more real-world data concluded that palbociclib is a cost-saving option compared to ribociclib, assuming equal clinical benefit [22]. Currently, only one comparative cost-effectiveness analysis compared the three CDK4/6 inhibiting agents in combination with fulvestrant in the USA [23]. It concluded that abemaciclib was a more cost-effective option than ribociclib, but it was less cost-effective than palbociclib [23]. Therefore, it can be concluded that the conclusion regarding the cost-effectiveness of CDK4/6 inhibitors varies depending on the different settings and perspectives from which the pharmacoeconomic analysis is carried out.
Qatar is an Arab country with a diverse population, where more than 80% of the population is not Qatari and comes from different ethnicities [24]. In Qatar, breast cancer remains challenging, as it is the most common type of cancer, accounting for 31% of the total new cases of cancer in 2018 [25]. The healthcare system in Qatar is a nonprofit healthcare system in which it is the main payer for healthcare services to all citizens and residents [26]. Cancer care is mainly provided by the National Center for Cancer Care and Research (NCCCR), which is the premier hospital for managing cancer in the state of Qatar and one of the main hospitals under Hamad Medical Corporation [27]. For stage IV HR+/HER-2 negative breast cancer patients, CDK4/6 inhibitors are used in the treatment of these patients; however, only palbociclib and ribociclib are authorized in the formulary so far. Due to the nature of the healthcare system in Qatar, where the government pays 100% of the cancer care on behalf of patients, and due to the fact that CDK4/6 inhibitors can increase healthcare costs, it is important to have strong cost-effectiveness evidence to guide their optimal use. To date, there are no cost-effectiveness analyses comparing palbociclib and ribociclib conducted in Qatar, nor in countries with similar healthcare systems and economic situations to Qatar, such as the Gulf countries—the regional, intergovernmental, political, and economic countries union comprising Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates, or the Middle East. The existing cost-effectiveness analyses conducted worldwide may be misleading when adopted to Qatar due to the different perspectives, populations, and economic profiles. Therefore, this study aimed to compare the cost-effectiveness of palbociclib and ribociclib with their approved FDA combinations in stage IV HR+/HER-2 negative breast cancer females in the state of Qatar, which can also serve as a guide for countries with similar healthcare and economic profiles to Qatar.
2. Materials and Methods
2.1. Overview
A 10-year within-cycle-corrected Markov decision analytical model was developed to estimate overall costs, effectiveness (represented in life years gained), and quality-adjusted life years gained for the targeted population. The model was carried out from the healthcare-payer perspective, Hamad Medical Corporation, NCCCR. The incremental cost-effectiveness ratio (ICER) was compared to a willingness-to-pay (WTP) threshold of fewer than three times the national annual gross domestic product (GDP) per capita, as per the World Health Organization (WHO) for cost-effective interventions [28]. As a result, a treatment regimen of an incremental cost of less than QAR 576,150 per QALY gained was considered to be cost-effective and very cost-effective if it was less than QAR 192,050 per QALY, based on the Qatari GDP/capita of USD 52,751 (USD 1 = QAR 3.65, 2020 financial year) [29]. All costs and outcomes were discounted by an annual discounting rate of 3.5%.
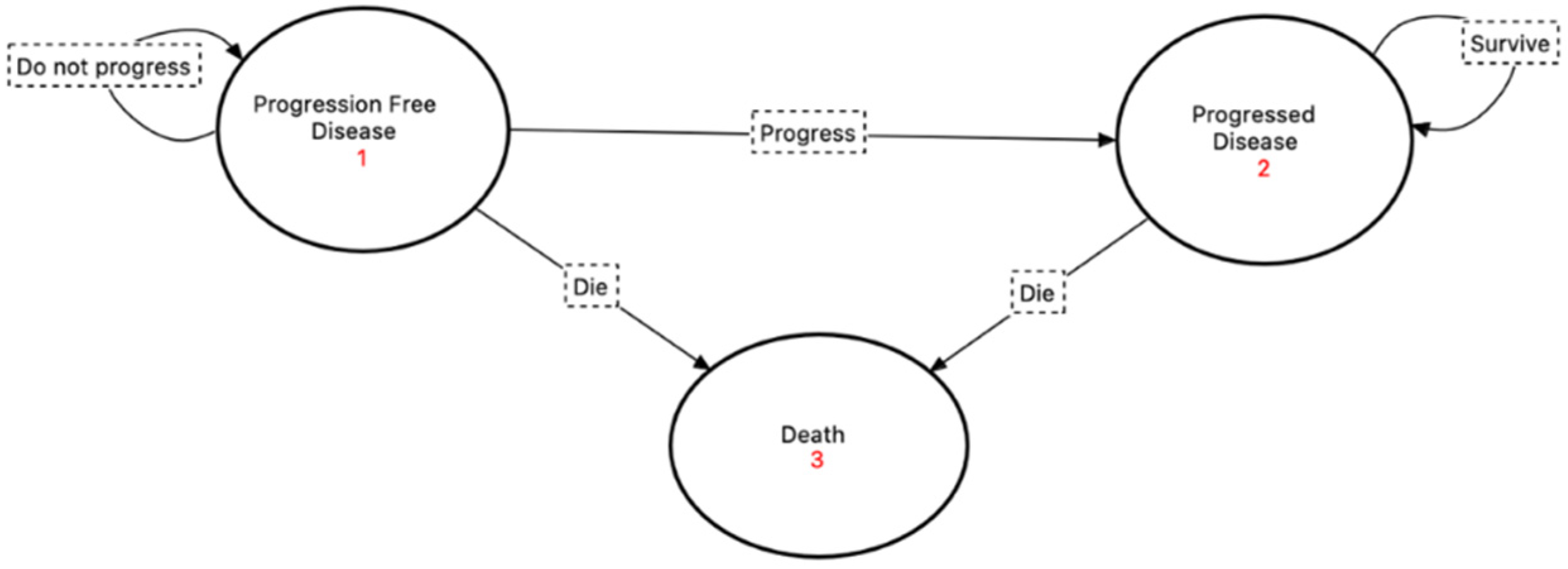
The model was composed of three health states: ‘progression-free disease’, which was defined in this model as the length of time in months that a patient can live with breast cancer while receiving palbociclib or ribociclib but without dying from tumor progression or following adverse events of the treatment; ‘progressed disease’, which is the length of time a patient can live with breast cancer after developing any increase in tumor size or new development of lymphadenopathy or distant metastasis; and ‘death’, which is the absorbing state in this model. All patients were assumed to enter the model in the ‘progression-free disease’ state. The transition between the health states followed a unidirectional transition, where at the end of each cycle, a patient could stay in the same state, move to the next state, or move directly to death, with no back transition to the previous state; this is due to the disease nature, since stage IV breast cancer is not curable. The Markov cycle length was assumed to be one month since that is the normal evaluation for the event development as per clinical guidelines. A visualization of Markov’s model for this study is illustrated in Figure 1. The model was developed and analyzed using the TreeAge Pro 2020.2.1® software.
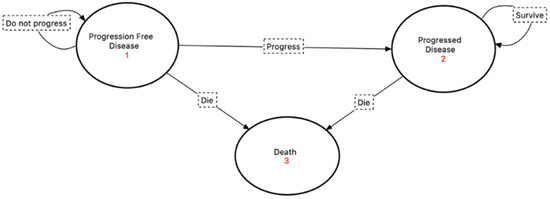
Figure 1.
Visualization of the Markov model implemented in the study with the state diagram and their transitions’ pathways.
2.2. Model Inputs
The model inputs included costs, transition probabilities between health states, and effectiveness parameters (life months and utilities). These were estimated based on individual patient data and based on the published literature to feed the model as follows:
- Costs
The total direct medical costs per patient per month for each health state in each treatment strategy were calculated based on individual patient data inputs. The unit costs were obtained from the department of accounting and finance in Hamad Medical Corporation based on the 2019/2020 financial year. All costs were calculated in the local currency, Qatar riyals (USD 1 = QAR 3.65). For each of the two comparators, palbociclib and ribociclib, all cost components were analyzed based on the following components: drug acquisition cost, combination drug acquisition cost, laboratory tests needed throughout the treatment period complete blood count (CBC), blood chemistry tests (comprehensive metabolic panel, liver function test, magnesium and phosphorus levels), endocrinology tests (25-hydroxyvitamin D, TSH receptor antibody, follicle-stimulating hormone, vitamin B12), tumor markers and catechol amines (thyroglobulin and carcinoembryonic antigen, CEA), coagulation tests, urine analysis tests, clinical radiology costs (X-ray, ultrasound, mammogram, magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography scan (PET scan), and the bone density DEXA scan), the required cardiac procedure for the CDK4/6 inhibitors costs (electrocardiogram (ECG) and echocardiogram), costs of the outpatient visits, and hospitalization costs.
- Effectiveness-based transition probabilities
The monthly transition probabilities were calculated from the real evidence of individual patient data based on HR+/HER2- stage IV breast cancer patients who were taking palbociclib or ribociclib from January 2016 to January 2020 at the NCCCR in Qatar. Firstly, cumulative probabilities of each of the events of interest for the two comparators were calculated based on Equation (1), where P is the probability and A is the event of interest. Secondly, the cumulative probabilities were converted into a rate as per the below Equation (2), where P is the cumulative probability, r is the rate, and t is the time in years [30]. Lastly, the rate was converted back to a 1-month transition probability as per Equation (3) [30]. Therefore, three unique transition probabilities with two complementary ones for each arm were generated as follows: monthly transition probability from PFS to PD, monthly transition probability from PFS to death, their complementary probability of staying in the PFS, monthly transition probability from PD to death, and its complimentary monthly probability of staying PFS for both groups. All transition probabilities, utility values [31,32,33], and discounting rates [34] are summarized in Table 1.

Table 1.
Inputs of the Markov model.
Cumulative probability of event (A) for a patient cohort.
Constant rate from probability.
Fixed time probability.
- Utilities
Quality of life was used to investigate the impact of the quality of life (QoL) on every additional year gained by the treatment of palbociclib and ribociclib to generate quality-adjusted life years (QALYs). QoL values were obtained from the published literature [31,32,33]. QoL values for the ‘progression-free disease’ health state were summarized from the published literature from findings from the PALOMA-2 trial for the palbociclib group [31] and from the MONALEESA-3 trial for the ribociclib group [32]. For the ‘progressed disease’ health state, it was assumed that there was no difference in terms of the quality of life between palbociclib and ribociclib. This assumption was based on the fact that when a patient develops a progression, she is managed according to the same hospital guidelines depending on the progression type she had and regardless of the CDK4/6 inhibiting drug that she received before. Therefore, the same utility value of 0.45 was used as per a published literature systematic review [33]. All utility values and other model inputs are summarized in Table 1.
2.3. Sensitivity Analysis
To address the impact of any uncertainties regarding the model inputs on the conclusion of the cost-effectiveness or cost-utility, a univariate deterministic sensitivity analysis was implemented with a single scenario for each variable assessment. The variables assessed through the deterministic sensitivity analysis were costs, transition probabilities from ‘progression-free disease’ to ‘progressed disease’, and quality of life for each health state. In order to ensure the robustness of the output, each of the variables of interest were varied separately while fixing the other model inputs, and ICER and ICUR were calculated accordingly. Then, a tornado analysis was generated to determine the variables that had the maximum effect on the cost-effectiveness conclusion. Deterministic sensitivity analysis inputs and source of the inputs’ boundaries are summarized in Table 2.

Table 2.
Univariate deterministic sensitivity analysis (DSA) inputs.
Additionally, a probabilistic sensitivity analysis (PSA) was implemented using the Monte-Carlo simulation analysis based on 10,000 unique simulations. Therein, the parameters were input as probability distributions rather than just fixed values, and the different distributions of the different parameters were varied together to generate 10,000 different scenarios with possible outcomes. The detailed inputs of the Monte-Carlo analysis are summarized in Table 3. In addition, the incremental cost-effectiveness scatterplot (ICE), also known as incremental cost-effectiveness plane, was generated to illustrate the ratio of the simulations favoring ribociclib treatment versus palbociclib treatment and to present the overall uncertainty surrounding the base-case conclusion cost-effectiveness results.

Table 3.
Probabilistic sensitivity analysis (PSA) inputs.
3. Results
As per Markov’s model, the 10-year cost of the palbociclib treatment strategy was QAR 372,663.3 per patient. In accordance, it yielded a gain of 71.62 life months (5.968 life years (LYs)) and, overall, gained quality-adjusted life years (QALYs) of 3.058 per patient (36.70 quality-adjusted life months), whereas, for the ribociclib treatment arm, the estimated 10-year cost was QAR 333,584.4 per patient. Similarly, the model produced a gain of 75.96 life months (6.330 gained life years) and 37.93 quality-adjusted life months per patient, with 3.160 QALYs per patient in the ribociclib treatment arm. The cost and effectiveness values were incremented to compare the two treatment options. When compared to palbociclib, ribociclib appeared to be more effective in terms of both LYs and QALYs gained, and it was less costly. The detailed values of the base-case overall cost and effectiveness are shown in Table 4.

Table 4.
Base-case results for palbociclib and ribociclib treatment groups.
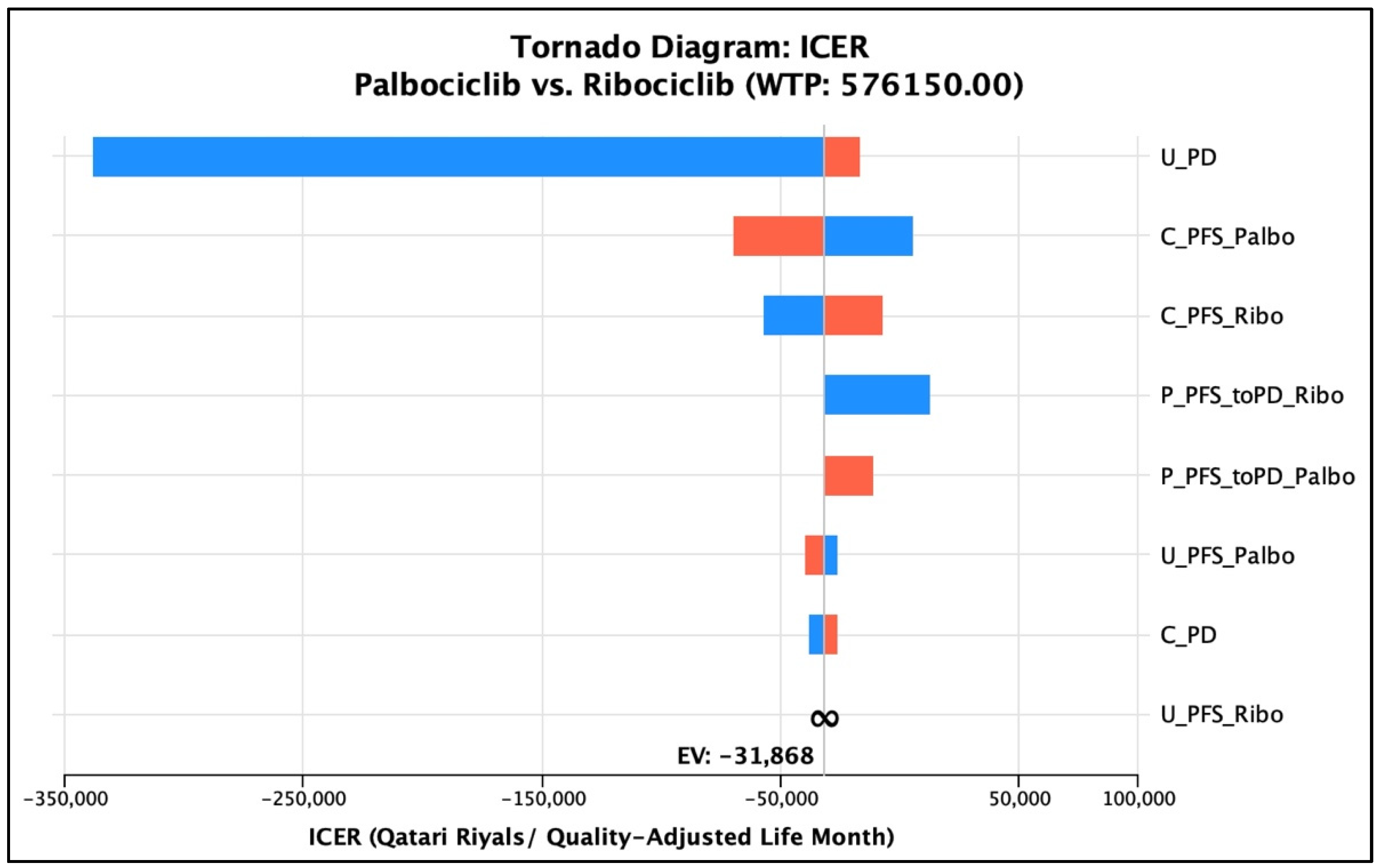
Regarding the univariate deterministic sensitivity analysis, the base-case conclusion of having ribociclib as a cost-effective option remained robust against the variation of the cost of PFS for the palbociclib group. However, it was shown that palbociclib was not dominated as described previously at a cost of PFS that equaled QAR 9612.9 (17.33% reduction of the input cost), but ribociclib was still a cost-effective option compared to palbociclib in total (ICUR = QAR 5572.79/QALY gained per patient). Similarly, the conclusion of the cost-effectiveness and cost-utility of the two medications remained the same, keeping ribociclib dominant over palbociclib with ±20% adjustment in the total PFS cost of ribociclib, with costs ranging from QAR 302,750 to QAR 364,418 and 3.160 QALYs. As for the transition probabilities of PFS to PD in the palbociclib arm to vary within a range between 0.0459 and 0.05035, the conclusion of having ribociclib dominant over palbociclib remained robust. On the other hand, for the monthly probability of PFS to PD for the ribociclib group, ribociclib remained more cost-effective than palbociclib when the probability was varied according to the MONALEESA-2 trial; nonetheless, it was dominant only when the probability was more than or equal to 0.04537 (25% variation from the base-case probability). The uncertainty regarding the probability of PFS to PD in the ribociclib treatment arm was associated with an ICUR ranging from QAR 12,894.35/QALY to a dominance range suggesting that ribociclib is either a cost-effective option or a dominant option. Lastly, the utility associated with the PFS of palbociclib was varied according to the 95% CI range of the base case (0.7387–0.7627). The conclusion of the cost-effectiveness of ribociclib over palbociclib remained robust over that range of uncertainty, where ribociclib was dominant over palbociclib in all the uncertainty ranges. Similarly, the conclusion of the cost-effectiveness of ribociclib over palbociclib remained robust when the utility of PFS in the ribociclib group was varied by 18.5%, according to the standard deviation associated with the utility value as obtained from the literature. That is, ribociclib was dominant over palbociclib all over the uncertainty range; however, it did not dominate palbociclib at utility values less than 0.622, but it was cost-effective with an ICUR range from QAR 54,664.07/QALY to QAR 198,120.36/QALY. Lastly, the utility of progressed disease varied at ±20%, and the conclusion of the domination of ribociclib over palbociclib remained robust all over the range that ICER ranged from. A tornado diagram was generated to illustrate the effect of the individual factors’ uncertainty on the overall cost-effectiveness conclusion (Figure 2). For further clarification, the deterministic sensitivity analysis parameter, cost outputs, QALYs generated, and the overall cost-effectiveness decision are summarized in Table 5.
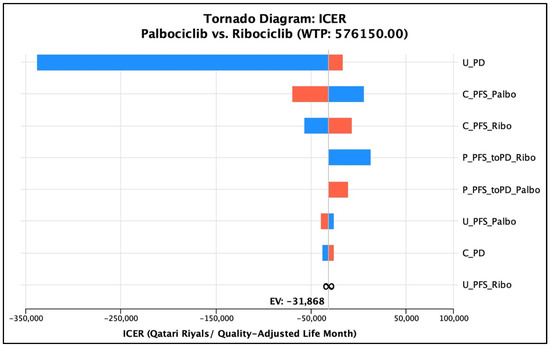
Figure 2.
Tornado diagram of the univariate sensitivity analyses and their impact on ICER. The blue color represents the parameter, whereas the red color represents the ICER. U_PD: utility value of the ‘progressed disease’ health state; C_PFS_Palbo: cost of the ‘progression-free survival’ health state for the ‘palbociclib’ treatment group; C_PFS_Ribo: cost of the ‘progression-free survival’ health state for the ‘ribociclib’ treatment group; P_PFS_toPD_Ribo: transition probability from the ‘progression-free survival’ health state to the ‘progressed disease’ health state for the ‘ribociclib’ treatment group; P_PFS_toPD_Palbo: transition probability from the ‘progression-free survival’ health state to the ‘progressed disease’ health state for the ‘palbociclib’ treatment group; U_PFS_Palbo: utility value of the ‘progression-free survival’ health state for the ‘palbociclib’ treatment group; U_PFS_Ribo: utility value of the ‘progression-free survival’ health state for the ‘ribociclib’ treatment group; C_PD: cost of the ‘progressed disease’ health state.

Table 5.
DSA outputs for palbociclib and ribociclib groups at each of the uncertainty parameters with the overall cost-effectiveness conclusions.
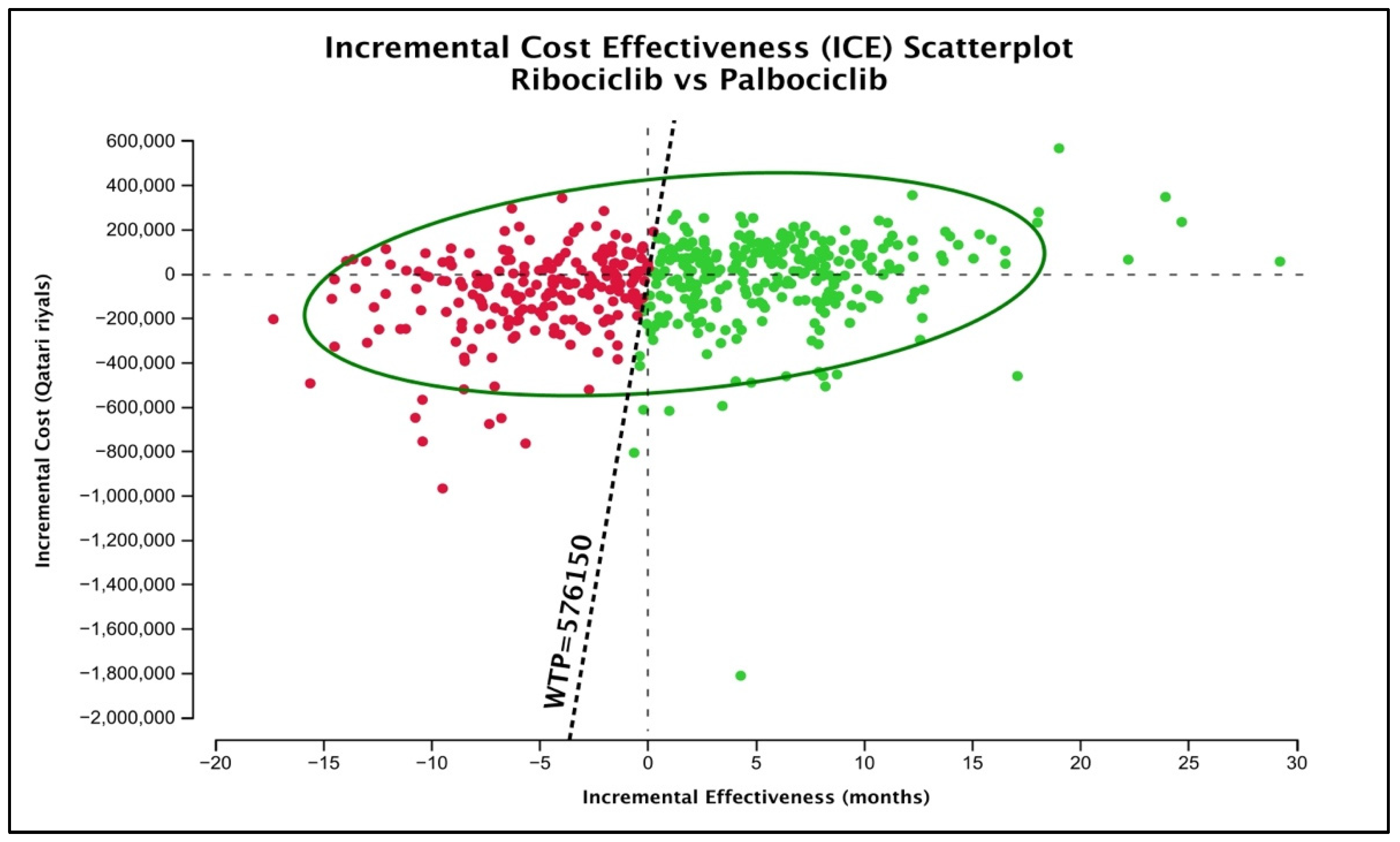
Lastly, as for the probabilistic sensitivity analysis by the Monte-Carlo simulation, the mean (SD) lifetime cost of palbociclib was QAR 386,778.1 (196,998.1) with an average (SD) gained QALYs of 3.135 (0.8725). For the ribociclib treatment group, the mean (SD) lifetime cost according to the generated simulations was QAR 354,057.03 (152,369), with an average (SD) gained QALYs of 3.246 (1.0425). To graphically test the uncertainty around the base-case conclusion of preferring ribociclib over palbociclib, the ICE plot of ribociclib versus palbociclib was generated (Figure 3). According to the figure, 59.01% of the generated scenarios (presented as green dots) still favored ribociclib over palbociclib. Ribociclib was still dominant in 26.14%, and it was higher in cost but more effective (cost-effective) in 32.87% of the cases. Nonetheless, it was less costly but less effective than in 24.65% of the cases (cost-saving) and inferior to palbociclib in 15.16% of the cases.
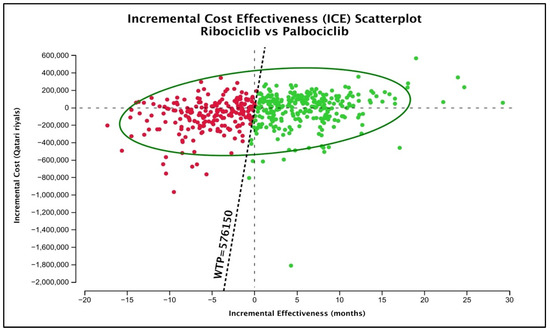
Figure 3.
ICE plot of ribociclib versus palbociclib. Green dots represent the scenarios favoring ribociclib over palbociclib at a 3 GDP WTP of QAR 576,150.
4. Discussion
In this research, we aimed at identifying the long-term cost, long-term effectiveness, and incremental cost-effectiveness of two treatment strategies that are used in the first-line treatment of HR+/HER-2 negative stage IV breast cancer. Therefore, a 10-year Markov model was run to summarize the long-term cost (QARs), effectiveness (LYs), and utility (QALYs) for each of the two treatment strategies. Overall, in our base-case analysis, the treatment with ribociclib was dominant over palbociclib in terms of both ICER and ICUR. The finding of ribociclib being more cost-effective than palbociclib remained robust against all the one-way sensitivity analyses at the 3 GDP WTP threshold (cost-effectiveness threshold). For the 1 GDP WTP threshold (very cost-effectiveness threshold), only the uncertainty regarding one factor, the utility of PFS status in the ribociclib treatment arm, yielded an ICUR above 1 GDP (QAR 198,120.3/QALY), suggesting that ribociclib is not a very cost-effective option. However, compared to the recommended 3 GDP WTP threshold as per the WHO, ribociclib is still a cost-saving option compared to palbociclib, even with the uncertainty associated with that factor. Of note, our conclusion remained robust against the probabilistic sensitivity analysis that was associated with the combined uncertainties of all factors. Approximately 60% of the yielded hypothetical 10,000 scenarios in the Monte-Carlo simulation suggested that ribociclib is cost-effective compared to palbociclib.
To date, and to our knowledge, there are four comparative pharmacoeconomic evaluations regarding the cost-effectiveness and the cost-utility of palbociclib and ribociclib. The findings of our study were consistent with three of them. That is, in a study conducted in Spain by Galve-Clavo et al. (2018) to evaluate the ICER and ICUR of ribociclib plus letrozole versus palbociclib plus letrozole, the former was associated with an ICER of EUR 1007.69 (QAR 4360.56) per every additional life year gained and an ICUR of EUR 1543.62 (QAR 6,679.69) per each QALY gained, at a threshold of EUR 30,000/QALY (129,818.59 QAR) [17]. Therefore, this study revealed that ribociclib was also cost-effective and cost-useful compared to palbociclib from the Spanish National Health System perspective [17]. In another study by Mistry R. et al. (2018) conducted in the USA also comparing ribociclib plus letrozole versus palbociclib plus letrozole versus letrozole monotherapy, ribociclib was dominant over palbociclib with a cost-saving of USD 43,037 and was still cost-effective compared to the letrozole monotherapy option [18]. That pharmacoeconomic analysis was conducted from the USA private third-party payer perspective at a WTP threshold of USD 198,000/QALY (QAR 720,918.06/QALY) [18]. Our findings were also consistent with one more pharmacoeconomic analyses by Suri G. et al. (2019) conducted in the UK, where ribociclib plus letrozole was also compared to palbociclib plus letrozole [20]. Their study reported that ribociclib plus letrozole was a cost-effective treatment strategy from the National Health Services (NHS) and Personal Social Services (PSS) perspective in the UK at a WTP threshold of EUR 30,000/QALY [20]. In only one cost-effectiveness study conducted in the USA, neither palbociclib nor ribociclib were cost-effective options, and the reason for this is that the ICER was calculated for each of the two comparators versus letrozole monotherapy [19]. There was no incremental cost-effectiveness ratio between the two CDK4/6 inhibitors, and therefore, none of them were cost-effective compared to letrozole monotherapy [19].
Although the previous pharmacoeconomic analyses were all of high quality, we could not rely on their findings to generate conclusions applicable to Qatar settings for multiple reasons. First, the generalizability of pharmacoeconomic analyses across countries is sometimes impaired due to the different sources of price weights among different countries [35] and due to different perspectives from which the pharmacoeconomic analyses take place [36]. Second, all used the published phase III clinical trials as the source for their simulated cohort, probabilities, effectiveness, and utility endpoints. Despite the success of the analysis, in the end, the use of these phase III trials themself is associated with some limitations since they were not designed to catch both clinical and economic endpoints. That is, in many of the pharmacoeconomic analyses based on RCTs, they tried to summarize the economic outcomes from the pre-collected primary clinical outcomes; thus, the sources of the economic data were not primary [35]. Both the MONALEESA and the PALOMA trials from which the four pharmacoeconomic analyses took their data were not predesigned to catch economic data. Therefore, we used a predesigned source of data to rely on for our economic analysis, the observational study conducted by our team earlier in 2021. Third, the four pharmacoeconomic analyses all compared the use of palbociclib versus ribociclib with only one of the indicated combinations, letrozole. This is because they used the same published phase III trial cohorts and interventions for their data. We sought a more thorough pharmacoeconomic analysis considering all the FDA-approved treatment combinations, especially since the COX regression conducted in phase I concluded no statistically significant differences in efficacy between the different treatment combinations. As a result, our analysis filled these gaps, providing a powerful pharmacoeconomic analysis that can be doubtlessly used for decision-makers in Qatar and other countries with similar health economic considerations.
Our study had several strengths. To begin, it was the first pharmacoeconomic evaluation focusing on the cost-effectiveness and the cost-utility of CDK4/6 inhibitors in Qatar, the Gulf region, and the Middle East and North Africa (MENA) region in general. Therefore, the findings of our current pharmacoeconomic analysis can be used locally and regionally in countries that have similar economic profiles and healthcare systems, with mild modifications to fit their context. Moreover, it is the first pharmacoeconomic analysis regarding these two treatments that was based on real-world evidence rather than just a simulation from clinical trials, avoiding all the disadvantages of modeling from clinical trials. Besides, it is the first pharmacoeconomic evaluation that compared palbociclib and ribociclib with all their FDA-indicated combinations; other analyses compared only the CDK4/6 inhibitors plus letrozole. In addition, it included pre/post-menopausal females in the cohort, unlike the other analyses that included only post-menopausal females as their cohort. Lastly, we performed an internal critical appraisal for our pharmacoeconomic study using the Quality of Health Economic Study (QHES) evaluation tool to assure the quality of the produced analysis; thus, we can assume that our results are assured of validity with minimal bias. However, our research had the main limitation that the base-case results were generated using observational real-world evidence, which itself has some limitations and potential uncertainties. However, we addressed this limitation by incorporating both deterministic and probabilistic sensitivity analyses that relied on phase III published RCTs. Our pharmacoeconomic conclusions remained robust against the uncertainties in both the deterministic and the probabilistic sensitivity analyses.
Based on our study findings, we have several recommendations for the current practice and future research. First, since ribociclib had a lower overall cost than palbociclib, although it had a higher acquisition cost in general, further evaluation of the consumption of the related resources needs to be conducted in the future with a larger sample size for both treatment arms. Second, all the published pharmacoeconomic agents included only palbociclib and ribociclib in their analyses. Abemaciclib is another CDK4/6 inhibitor that is under-addressed by pharmacoeconomic evaluations. Thus, more comparative pharmacoeconomic evaluations need to be conducted about this medication along with the other two medications in the same CDK4/6 inhibiting family.
5. Conclusions
Since their introduction to the market, the use of CDK4/6 inhibitors is increasing due to their proven clinical efficacy. Nonetheless, they can increase health expenditure due to their high acquisition cost and monitoring cost. Therefore, we underwent a thorough cost-effectiveness analysis using a well-designed Markov model. Ribociclib was more cost-effective than palbociclib at a 3 GDP threshold and at a 1 GDP threshold, suggesting that ribociclib should be a more favorable option over palbociclib to use in practice whenever applicable. This conclusion remained robust against the different single uncertainties as well as combined uncertainties. As a result, ribociclib was proven to be generally more cost-effective than palbociclib in the state of Qatar. This finding can be generalizable to countries with similar economic profiles, considerations, and cost drivers to Qatar. More similar pharmacoeconomic analyses that include the three CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) need to be conducted for more robust comparisons.
Author Contributions
All authors contributed to the work. Conceptualization, M.I.M.I. and M.F.A.; methodology, N.H.A.-Z., M.F.A., and A.A.S.; software, N.H.A.-Z.; validation, M.F.A., A.A.S., A.H., and M.I.M.I.; formal analysis, N.H.A.-Z.; investigation, N.H.A.-Z.; resources, M.I.M.I. and M.F.A.; data curation, N.H.A.-Z.; writing―original draft preparation, N.H.A.-Z.; writing―review and editing, M.I.M.I., M.F.A., S.E., A.A.S., and A.H.; visualization, N.H.A.-Z., M.I.M.I., S.E., M.F.A., A.A.S., and A.H.; supervision, M.I.M.I., M.F.A., and S.E.; project administration, N.H.A.-Z.; funding acquisition, M.I.M.I. and S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Qatar University, by financial grant #No. QUST-1-CPH-2019-12.
Institutional Review Board Statement
The study was firstly ethically approved by the Medical Research Center (MRC) in Hamad Medical Corporation on 30 January 2020, under the protocol approval number: MRC-01-19-318, followed by the approval from the Qatar University International Review Board (QU-IRB) on 10 February 2020, under the approval number: QU-IRB-1231-E/20.
Informed Consent Statement
Not Applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge Qatar University for the funding of this study. In addition, Qatar University has supported the APC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. International Agency for Research on Cancer. Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018. Available online: https://www.iarc.who.int/featured-news/latest-global-cancer-data-cancer-burden-rises-to-18-1-million-new-cases-and-9-6-million-cancer-deaths-in-2018 (accessed on 15 August 2021).
- World Health Organization. Cancer Report 2020—Global Profile 2020. Available online: https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=4-cancer-country-profiles-2020&alias=51561-global-cancer-profile-2020&Itemid=270&lang=fr (accessed on 15 August 2021).
- American Cancer Society. Survival Rates for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html (accessed on 25 February 2021).
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US Incidence of Breast Cancer Subtypes Defined by Joint Hormone Receptor and HER2 Status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines Version 5. 2020. Available online: https://www.tri-kobe.org/nccn/guideline/breast/english/breast.pdf (accessed on 15 December 2021).
- Shah, M.; Nunes, M.R.; Stearns, V. CDK4/6 inhibitors: Game changers in the management of hormone receptor—Positive advanced breast cancer? Oncology 2018, 32, 216. [Google Scholar] [PubMed]
- FDA. Highlights of Prescribing Information: Ibrance (Palbociclib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212436lbl.pdf (accessed on 20 September 2021).
- FDA. Highlights of prescribing information: Kisqali (ribociclib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209092s000lbl.pdf (accessed on 20 September 2021).
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib as Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Galve-Calvo, E.; González-Haba, E.; Gostkorzewicz, J.; Martínez, I.; Pérez-Mitru, A. Cost-effectiveness analysis of ribociclib versus palbociclib in the first-line treatment of HR+/HER2– advanced or metastatic breast cancer in Spain. Clin. Econ. Outcomes Res. 2018, 10, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.; May, J.R.; Suri, G.; Young, K.; Brixner, D.; Oderda, G.; Biskupiak, J.; Tang, D.; Bhattacharyya, S.; Mishra, D.; et al. Cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole and letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2-advanced or metastatic breast cancer: A US payer perspective. J. Manag. Care Spec. Pharm. 2018, 24, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Long, E.F. Cost-effectiveness analysis of palbociclib or ribociclib in the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. Breast Cancer Res. Treat. 2019, 175, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Suri, G.; Chandiwana, D.; Lee, A.; Mistry, R. Cost-effectiveness Analysis of Ribociclib plus Letrozole versus Palbociclib plus Letrozole in the United Kingdom. J. Health Econ. Outcomes Res. 2019, 6, 20–31. [Google Scholar] [CrossRef]
- Buehler, A.M.; Castilho, G.; Dionne, P.-A.; Stefani, S. Cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole or letrozole as monotherapy in first-line treatment of postmenopausal women with HR+/HER2- locally advanced or metastatic breast cancer: A Brazilian private payer perspective. Ther. Adv. Med. Oncol. 2021, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Avxentyev, N.A.; Lubennikova, E.V.; Frolov, M.Y. Pharmacoeconomic analysis of using cyclin-dependent kinase 4 and 6 inhibitors in the first line treatment of HR-positive HER2-negative advanced breast cancer. Farmakoekon. Mod. Pharm. Pharmacoepidemiol. 2020, 12, 279–290. [Google Scholar] [CrossRef]
- Wang, Y.; Rui, M.; Guan, X.; Cao, Y.; Chen, P. Cost-Effectiveness Analysis of Abemaciclib Plus Fulvestrant in the Second-Line Treatment of Women With HR+/HER2– Advanced or Metastatic Breast Cancer: A US Payer Perspective. Front. Med. 2021, 8, 658747. [Google Scholar] [CrossRef] [PubMed]
- Qatar Population 2021 (Demographics, Maps, Graphs). Available online: https://worldpopulationreview.com/countries/qatar-population (accessed on 15 December 2021).
- Qatar Source: Globocan 2018. Available online: https://gco.iarc.fr/today/data/factsheets/populations/634-qatar-fact-sheets.pdf (accessed on 27 August 2020).
- Ministry of Development Planning and Statistics. Qatar Second National Development Strategy 2018~2022. Available online: https://www.psa.gov.qa/en/knowledge/Documents/NDS2Final.pdf (accessed on 2 October 2021).
- National Center for Cancer Care and Research. Available online: https://www.hamad.qa/EN/Hospitals-and-services/NCCCR/Pages/default.aspx (accessed on 27 August 2021).
- Tan-Torres Edejer, T.; Baltussen, R.; Adam, T.; Hutubessy, R.; Acharya, A.; Evans, D.B.; Murray, D.B.; Murray, C.J.L. WHO Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- International Monetary Fund World Economic Outlook. World GDP per capita Ranking 2020. Available online: http://statisticstimes.com/economy/projected-world-gdp-capita-ranking.php (accessed on 22 January 2021).
- Briggs, A.; Sculpher, M.; Claxton, K. Decision Modelling for Health Economic Evaluations; Oxford University Press: Oxford, UK; p. 51.
- Rugo, H.; Diéras, V.; Gelmon, K.; Finn, R.; Slamon, D.; Martin, M.; Neven, P.; Shparyk, Y.; Mori, A.; Lu, D.; et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial. Ann. Oncol. 2018, 29, 888–894. [Google Scholar] [CrossRef]
- Fasching, P.A.; Beck, J.T.; Chan, A.; De Laurentiis, M.; Esteva, F.J.; Jerusalem, G.; Neven, P.; Pivot, X.; Bianchi, G.V.; Martin, M.; et al. Ribociclib plus fulvestrant for advanced breast cancer: Health-related quality-of-life analyses from the MONALEESA-3 study. Breast 2020, 54, 148–152. [Google Scholar] [CrossRef]
- Lloyd, A.; Nafees, B.; Narewska, J.; Dewilde, S.; Watkins, J. Health state utilities for metastatic breast cancer. Br. J. Cancer 2006, 95, 683–690. [Google Scholar] [CrossRef]
- Attema, A.E.; Brouwer, W.B.F.; Claxton, K. Discounting in Economic Evaluations. PharmacoEconomics 2018, 36, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Glick, H.A.; Doshi, J.A.; Sonnad, S.S.; Polsky, D. Economic Evaluation in Clinical Trials, 2nd ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Mason, J.M.; Mason, A.R. The generalisability of pharmacoeconomic studies: Issues and challenges ahead. PharmacoEconomics 2006, 24, 937–945. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).