Associations between Frailty and Ambient Temperature in Winter: Findings from a Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Frailty Assessment
2.3. Ambient Temperature in Winter Assessment
2.4. Covariates
2.5. Statistical Analysis
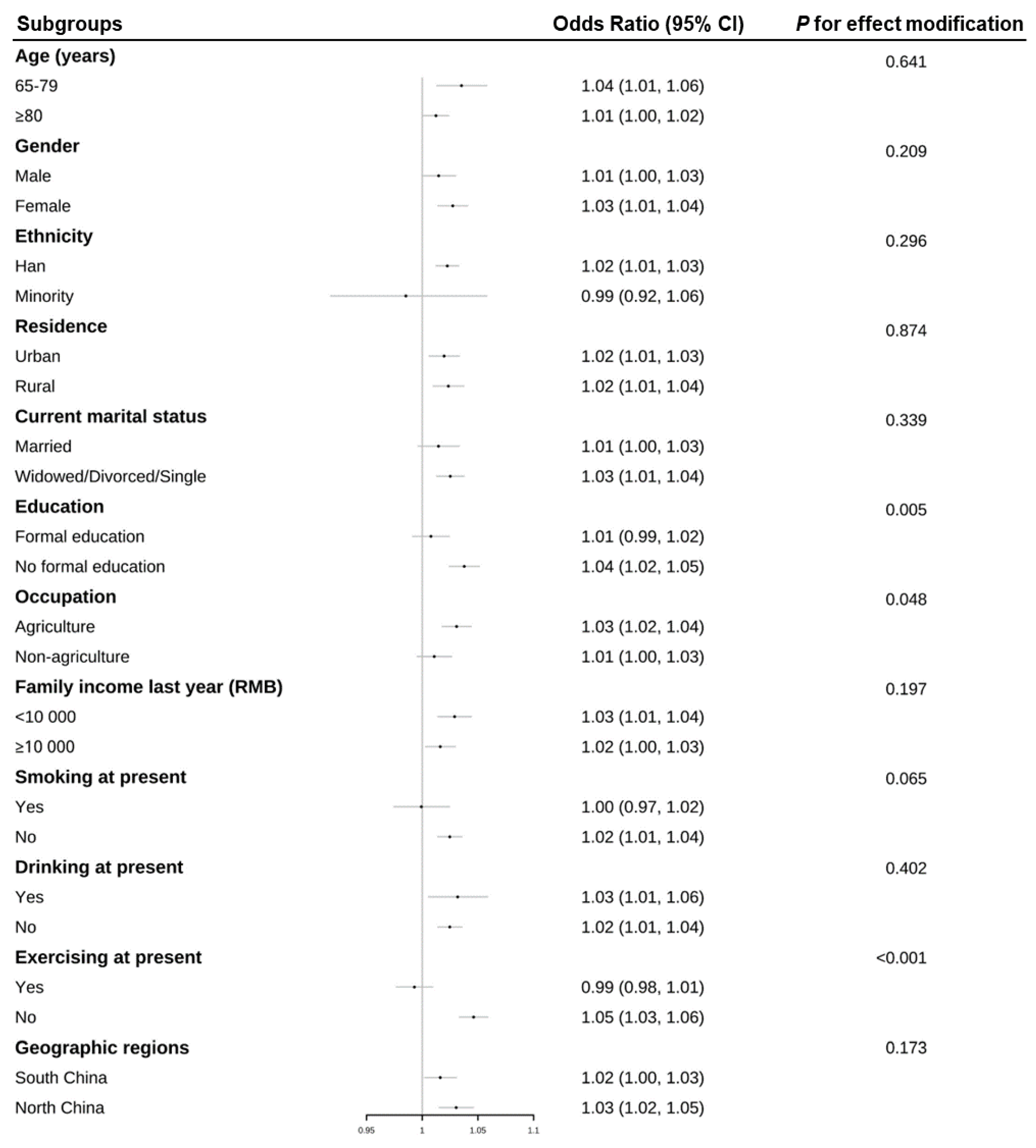
3. Results
3.1. Descriptive Analysis
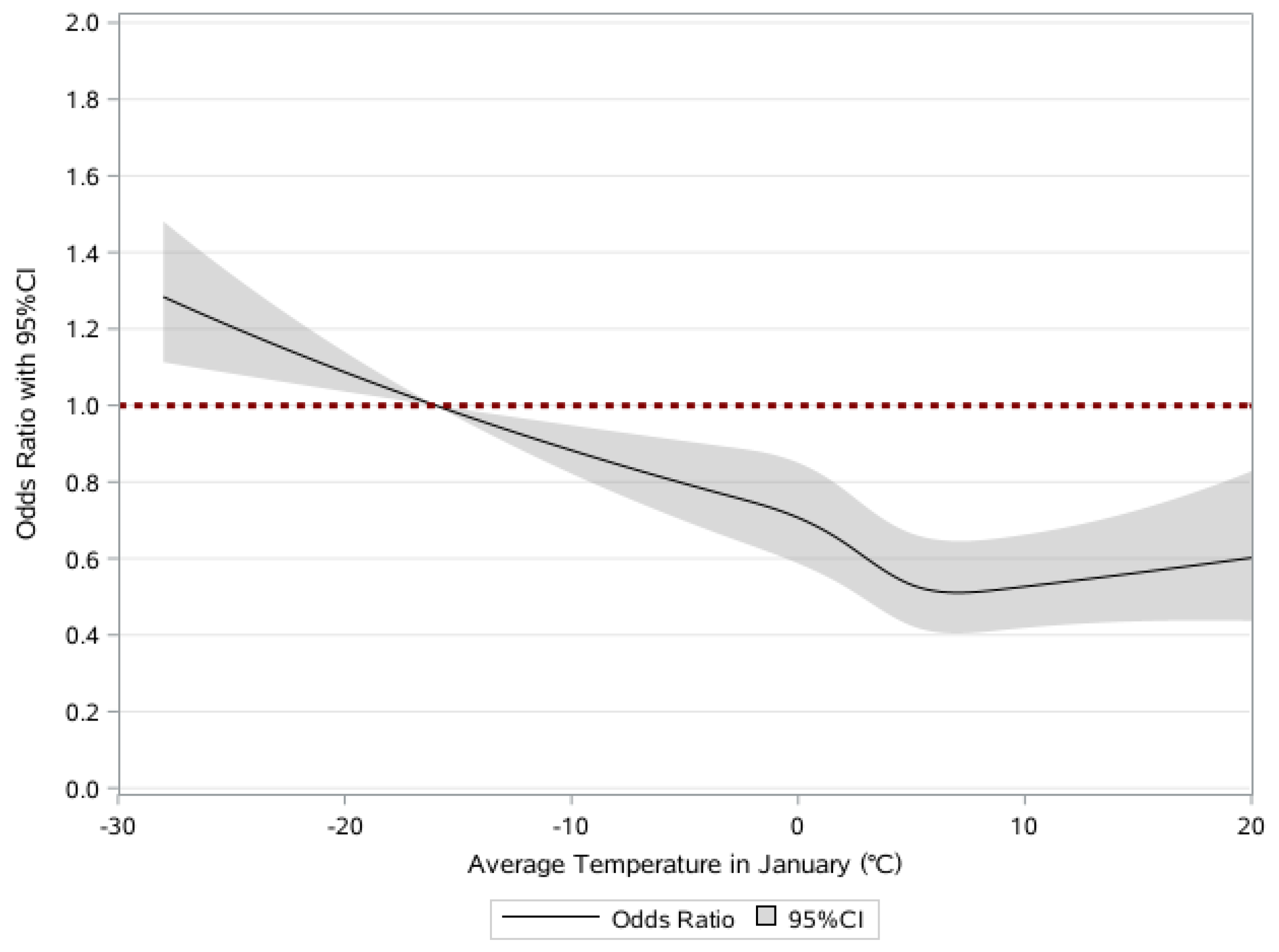
3.2. Associations of Frailty and Average Temperature in January
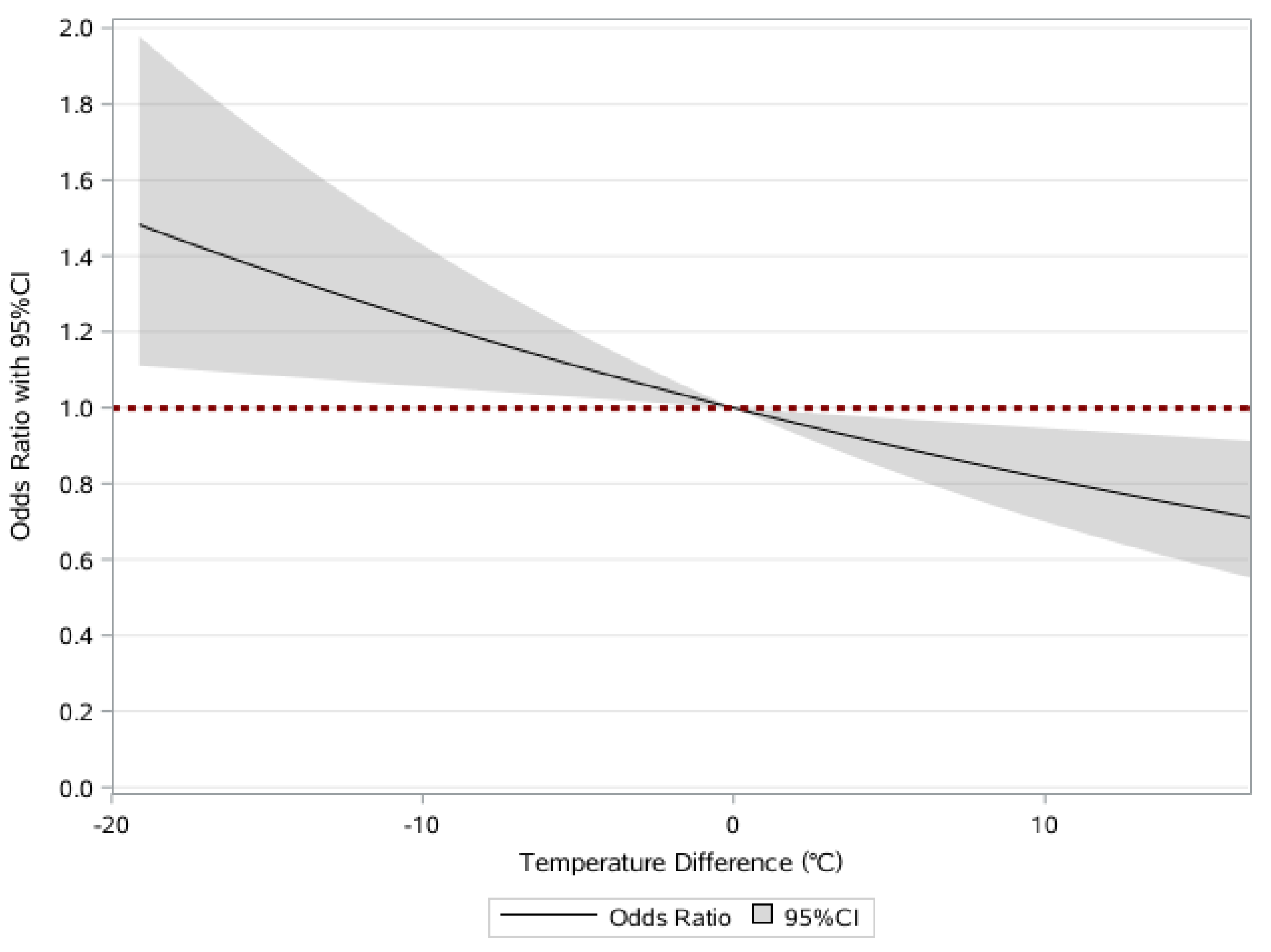
3.3. Associations of Frailty and Temperature Change of Average Temperature in January
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Auyeung, T.W.; Leung, J.; Kwok, T.; Woo, J. Transitions in frailty states among community-living older adults and their associated factors. J. Am. Med. Dir. Assoc. 2014, 15, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Shi, J.; Song, X.; Mitnitski, A.; Tang, Z.; Wang, C.; Yu, P.; Rockwood, K. Frailty in relation to the risk of falls, fractures, and mortality in older Chinese adults: Results from the Beijing Longitudinal Study of Aging. J. Nutr. Health Aging 2012, 16, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Suzuki, T. Impact of physical frailty on disability in community-dwelling older adults: A prospective cohort study. BMJ Open 2015, 5, e008462. [Google Scholar] [CrossRef] [Green Version]
- Markle-Reid, M.; Browne, G. Conceptualizations of frailty in relation to older adults. J. Adv. Nurs. 2003, 44, 58–68. [Google Scholar] [CrossRef]
- Graham, J.E.; Snih, S.A.; Berges, I.M.; Ray, L.A.; Markides, K.S.; Ottenbacher, K.J. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology 2009, 55, 644–651. [Google Scholar] [CrossRef]
- Rockwood, K.; Andrew, M.; Mitnitski, A. A comparison of two approaches to measuring frailty in elderly people. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 738–743. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Mitnitski, A.; Song, X.; Steen, B.; Skoog, I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J. Am. Geriatr. Soc. 2006, 54, 975–979. [Google Scholar] [CrossRef]
- He, B.; Ma, Y.; Wang, C.; Jiang, M.; Geng, C.; Chang, X.; Ma, B.; Han, L. Prevalence and Risk Factors for Frailty among Community-Dwelling Older People in China: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2019, 23, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Bountziouka, V.; Nelson, C.P.; Codd, V.; Wang, Q.; Musicha, C.; Allara, E.; Kaptoge, S.; Di Angelantonio, E.; Butterworth, A.S.; Thompson, J.R.; et al. Association of shorter leucocyte telomere length with risk of frailty. J. Cachexia Sarcopenia Muscle 2022, 13, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Myers, L.; Wyckoff, J.; Cherry, K.E.; Jazwinski, S.M. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience 2017, 39, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Muhammad, T. Socioeconomic vulnerability and frailty among community-dwelling older adults: Cross-sectional findings from longitudinal aging study in India, 2017–2018. BMC Geriatr. 2022, 22, 201. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Goggins, W.; Sham, A.; Ho, S.C. Social determinants of frailty. Gerontology 2005, 51, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Arai, H.; Sonoda, T.; Aoyama, T. Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. J. Am. Med. Dir. Assoc. 2012, 13, 507–511. [Google Scholar] [CrossRef]
- Fried, L.P. Interventions for Human Frailty: Physical Activity as a Model. Cold Spring Harb. Perspect. Med. 2016, 6, a025916. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Gu, D.; Purser, J.; Hoenig, H.; Christakis, N. Associations of environmental factors with elderly health and mortality in China. Am. J. Public Health 2010, 100, 298–305. [Google Scholar] [CrossRef]
- Palinkas, L.A. Mental and cognitive performance in the cold. Int. J. Circumpolar Health 2001, 60, 430–439. [Google Scholar] [CrossRef]
- Jones, G.R.; Brandon, C.; Gill, D.P. Physical activity levels of community-dwelling older adults are influenced by winter weather variables. Arch. Gerontol. Geriatr. 2017, 71, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Bunker, A.; Wildenhain, J.; Vandenbergh, A.; Henschke, N.; Rocklöv, J.; Hajat, S.; Sauerborn, R. Effects of Air Temperature on Climate-Sensitive Mortality and Morbidity Outcomes in the Elderly; a Systematic Review and Meta-analysis of Epidemiological Evidence. eBioMedicine 2016, 6, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, R.M.; Hayen, A.; Dunsmuir, W.T.; Finch, C.F. Air temperature and the incidence of fall-related hip fracture hospitalisations in older people. Osteoporos. Int. 2011, 22, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, U.; Oksa, J.; Skelton, D.A.; Beyer, N.; Klenk, J.; Zscheile, J.; Becker, C. Effect of cold indoor environment on physical performance of older women living in the community. Age Ageing 2014, 43, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Sugie, M.; Harada, K.; Takahashi, T.; Nara, M.; Fujimoto, H.; Kyo, S.; Ito, H. Effectiveness of a far-infrared low-temperature sauna program on geriatric syndrome and frailty in community-dwelling older people. Geriatr. Gerontol. Int. 2020, 20, 892–898. [Google Scholar] [CrossRef]
- Mullins, J.T.; White, C. Temperature and mental health: Evidence from the spectrum of mental health outcomes. J. Health Econ. 2019, 68, 102240. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.M.; Finlay, J.M.; Clarke, P.; Sol, K.; Melendez, R.; Judd, S.; Gronlund, C.J. Association between temperature exposure and cognition: A cross-sectional analysis of 20,687 aging adults in the United States. BMC Public Health 2021, 21, 1484. [Google Scholar] [CrossRef] [PubMed]
- Van Hoof, J.; Bennetts, H.; Hansen, A.; Kazak, J.K.; Soebarto, V. The Living Environment and Thermal Behaviours of Older South Australians: A Multi-Focus Group Study. Int. J. Environ. Res. Public Health 2019, 16, 935. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Ou, C.Q.; Ding, Y.; Zhou, Y.X.; Chen, P.Y. Daily temperature and mortality: A study of distributed lag non-linear effect and effect modification in Guangzhou. Environ. Health 2012, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Yao, Z.; Zhang, Y.; Xu, Y.; Xu, X.; Liu, T.; Lin, H.; Lao, X.; Rutherford, S.; Chu, C.; et al. Short-term effects of the 2008 cold spell on mortality in three subtropical cities in Guangdong Province, China. Environ. Health Perspect. 2013, 121, 210–216. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, Y.; Li, G.; Zeng, W.; Lin, H.; Rutherford, S.; Xu, Y.; Luo, Y.; Xu, X.; Chu, C.; et al. Temperature-mortality relationship in four subtropical Chinese cities: A time-series study using a distributed lag non-linear model. Sci. Total Environ. 2013, 449, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.; Deng, F.; Li, Y.; Zhao, J. Identification of gut microbiome signatures associated with longevity provides a promising modulation target for healthy aging. Gut Microbes 2019, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Prospects 2022. Available online: https://www.un.org/en/desa/world-population-reach-8-billion-15-november-2022 (accessed on 20 September 2022).
- Kulminski, A.; Yashin, A.; Arbeev, K.; Akushevich, I.; Ukraintseva, S.; Land, K.; Manton, K. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: Results from analyses of the National Long Term Care Survey. Mech. Ageing Dev. 2007, 128, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladyshev, V.N. Aging: Progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell 2016, 15, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Cai, Y.; Barratt, B.; Lyu, B.; Chan, Q.; Hansell, A.L.; Xie, W.; Zhang, D.; Kelly, F.J.; Tong, Z. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013–2017: An ecological analysis. Lancet Planet. Health 2019, 3, e270–e279. [Google Scholar] [CrossRef] [Green Version]
- Veronese, N.; Custodero, C.; Cella, A.; Demurtas, J.; Zora, S.; Maggi, S.; Barbagallo, M.; Sabbà, C.; Ferrucci, L.; Pilotto, A. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 72, 101498. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.L.; Schwartz, J.; Dockery, D.W. The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air 2014, 24, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Tamerius, J.; Perzanowski, M.; Acosta, L.; Jacobson, J.; Goldstein, I.; Quinn, J.; Rundle, A.; Shaman, J. Socioeconomic and Outdoor Meteorological Determinants of Indoor Temperature and Humidity in New York City Dwellings. Weather Clim. Soc. 2013, 5, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Feng, Q. Frailty still matters to health and survival in centenarians: The case of China. BMC Geriatr. 2015, 15, 159. [Google Scholar] [CrossRef]
- Gu, D. General Data Quality Assessment of the CLHLS. In Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions; Yi, Z., Poston, D.L., Vlosky, D.A., Gu, D., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 39–60. [Google Scholar]
- Deng, Y.; Gao, Q.; Yang, D.; Hua, H.; Wang, N.; Ou, F.; Liu, R.; Wu, B.; Liu, Y. Association between biomass fuel use and risk of hypertension among Chinese older people: A cohort study. Environ. Int. 2020, 138, 105620. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y. Towards Deeper Research and Better Policy for Healthy Aging—Using the Unique Data of Chinese Longitudinal Healthy Longevity Survey. China Econ. J. 2012, 5, 131–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, A.; Yan, L.; Wu, C.; Ji, J.S. Residential Greenness and Frailty Among Older Adults: A Longitudinal Cohort in China. J. Am. Med. Dir. Assoc. 2020, 21, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Kulminski, A.M.; Ukraintseva, S.V.; Akushevich, I.V.; Arbeev, K.G.; Yashin, A.I. Cumulative index of health deficiencies as a characteristic of long life. J. Am. Geriatr. Soc. 2007, 55, 935–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Li, W.; Ma, Y.; Chen, H.; Zeng, Y.; Yu, X.; Hofman, A.; Wang, H. Cognitive decline and mortality among community-dwelling Chinese older people. BMC Med. 2019, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.B.; Gao, X.; Yin, Z.X.; Chen, H.S.; Luo, J.S.; Brasher, M.S.; Kraus, V.B.; Li, T.T.; Zeng, Y.; Shi, X.M. Revisiting the association of blood pressure with mortality in oldest old people in China: Community based, longitudinal prospective study. BMJ 2018, 361, k2158. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wu, Y.; Liu, E. Influencing Factors of Undermet Care Needs of the Chinese Disabled Oldest Old People When Their Children Are Both Caregivers and Older People: A Cross-Sectional Study. Healthcare 2020, 8, 365. [Google Scholar] [CrossRef]
- Fan, J.; Yu, C.; Guo, Y.; Bian, Z.; Sun, Z.; Yang, L.; Chen, Y.; Du, H.; Li, Z.; Lei, Y.; et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: A prospective cohort study. Lancet Public Health 2020, 5, e650–e660. [Google Scholar] [CrossRef]
- The Eurowinter Group. Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet 1997, 349, 1341–1346. [Google Scholar] [CrossRef]
- Xiao, J.; Peng, J.; Zhang, Y.; Liu, T.; Rutherford, S.; Lin, H.; Qian, Z.; Huang, C.; Luo, Y.; Zeng, W.; et al. How much does latitude modify temperature-mortality relationship in 13 eastern US cities? Int. J. Biometeorol. 2015, 59, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Keenan, K.; Hale, J.M.; Börger, T. The association between city-level air pollution and frailty among the elderly population in China. Health Place 2020, 64, 102362. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Zhang, M.; Sun, X. Sex-Specific Association Between Socioeconomic Status, Lifestyle, and the Risk of Frailty Among the Elderly in China. Front. Med. 2021, 8, 775518. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Dai, L.; Wang, Y.; Zanobetti, A.; Choirat, C.; Schwartz, J.D.; Dominici, F. Association of short-term exposure to air pollution with mortality in older adults. JAMA 2017, 318, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, J.; Zhou, M.; Yin, P.; Chen, Z.; Zhao, Q.; Hu, K.; Liu, Q.; Ou, C.Q. Hourly temperature variability and mortality in 31 major Chinese cities: Effect modification by individual characteristics, season and temperature zone. Environ. Int. 2021, 156, 106746. [Google Scholar] [CrossRef]
- Nakajima, Y.; Schmidt, S.M.; Malmgren Fänge, A.; Ono, M.; Ikaga, T. Relationship between Perceived Indoor Temperature and Self-Reported Risk for Frailty among Community-Dwelling Older People. Int. J. Environ. Res. Public Health 2019, 16, 613. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yin, P.; Zhou, M.; Ou, C.Q.; Li, M.; Li, J.; Liu, X.; Gao, J.; Liu, Y.; Qin, R.; et al. The burden of stroke mortality attributable to cold and hot ambient temperatures: Epidemiological evidence from China. Environ. Int. 2016, 92, 232–238. [Google Scholar] [CrossRef]
- Collins, K.J.; Easton, J.C.; Belfield-Smith, H.; Exton-Smith, A.N.; Pluck, R.A. Effects of age on body temperature and blood pressure in cold environments. Clin. Sci. 1985, 69, 465–470. [Google Scholar] [CrossRef]
- Budd, G.M. Ergonomic aspects of cold stress and cold adaptation. Scand. J. Work Environ. Health 1989, 15 (Suppl. S1), 15–26. [Google Scholar]
- Hwang, R.L.; Chen, C.P. Field study on behaviors and adaptation of elderly people and their thermal comfort requirements in residential environments. Indoor Air 2010, 20, 235–245. [Google Scholar] [CrossRef]
- Avakian, E.V.; Horvath, S.M.; Colburn, R.W. Influence of age and cold stress on plasma catecholamine levels in rats. J. Auton. Nerv. Syst. 1984, 10, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kappes, B.; Mills, W.; O’Malley, J. Psychological and psychophysiological factors in prevention and treatment of cold injuries. Alaska Med. 1993, 35, 131–140. [Google Scholar] [PubMed]
- Friston, K.; Schwartenbeck, P.; FitzGerald, T.; Moutoussis, M.; Behrens, T.; Dolan, R.J. The anatomy of choice: Dopamine and decision-making. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130481. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Schmidt, S.M.; Malmgren Fänge, A.; Hoshi, T.; Ikaga, T. Lower Physical Performance in Colder Seasons and Colder Houses: Evidence from a Field Study on Older People Living in the Community. Int. J. Environ. Res. Public Health 2017, 14, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmussen, E.; BØJe, O. Body temperature and capacity for work. Acta Physiol. Scand. 1945, 10, 1–22. [Google Scholar] [CrossRef]
- Chai, G.; He, H.; Su, Y.; Sha, Y.; Zong, S. Lag effect of air temperature on the incidence of respiratory diseases in Lanzhou, China. Int. J. Biometeorol. 2020, 64, 83–93. [Google Scholar] [CrossRef]
- Fukuda, T.; Ohashi, N.; Doi, K.; Matsubara, T.; Kitsuta, Y.; Nakajima, S.; Yahagi, N. Impact of seasonal temperature environment on the neurologic prognosis of out-of-hospital cardiac arrest: A nationwide, population-based cohort study. J. Crit. Care 2014, 29, 840–847. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Hong, Y.C.; Rha, J.H.; Lee, J.T.; Ha, E.H.; Kwon, H.J.; Kim, H. Ischemic stroke associated with decrease in temperature. Epidemiology 2003, 14, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, C.; Wang, H.; Lang, L.; Yue, T.; Lin, H. Ischemic stroke hospital admission associated with ambient temperature in Jinan, China. PLoS ONE 2013, 8, e80381. [Google Scholar] [CrossRef]
- Lv, Y.; Zhou, J.; Kraus, V.B.; Li, T.; Sarnat, J.A.; Wang, J.; Liu, Y.; Chen, H.; Brasher, M.S.; Mao, C. Long-term exposure to PM2. 5 and incidence of disability in activities of daily living among oldest old. J. Environ. Pollut. 2020, 259, 113910. [Google Scholar] [CrossRef]
- O’Neill, M.S.; Zanobetti, A.; Schwartz, J. Modifiers of the temperature and mortality association in seven US cities. Am. J. Epidemiol. 2003, 157, 1074–1082. [Google Scholar] [CrossRef] [Green Version]
- Galobardes, B.; Shaw, M.; Lawlor, D.A.; Lynch, J.W.; Smith, G.D. Indicators of socioeconomic position (part 1). J. Epidemiol. Community Health 2006, 60, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Ek, K.; Söderholm, P. Households’ switching behavior between electricity suppliers in Sweden. Util. Policy 2008, 16, 254–261. [Google Scholar] [CrossRef]
- Lloyd, E.L. The role of cold in ischaemic heart disease: A review. Public Health 1991, 105, 205–215. [Google Scholar] [CrossRef]
- Public Health England. The Cold Weather Plan for England: Protecting Health and Reducing Harm from Cold Weather. Available online: https://www.gov.uk/government/publications/cold-weather-plan-cwp-for-england (accessed on 21 September 2022).
- Keatinge, W.R.; Donaldson, G.C. Winter mortality in elderly people in Britain: Action on outdoor cold stress is needed to reduce winter mortality. BMJ 2004, 329, 976. [Google Scholar] [CrossRef] [Green Version]
- Cesari, M.; Gambassi, G.; van Kan, G.A.; Vellas, B. The frailty phenotype and the frailty index: Different instruments for different purposes. Age Ageing 2014, 43, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Joseph, B.; Pandit, V.; Zangbar, B.; Kulvatunyou, N.; Hashmi, A.; Green, D.J.; O’Keeffe, T.; Tang, A.; Vercruysse, G.; Fain, M.J.; et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: A prospective analysis. JAMA Surg. 2014, 149, 766–772. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.R.; Walter, L.C.; Ensrud, K.E.; Suskind, A.M.; Newman, J.C.; Ricke, W.A.; Liu, T.T.; McVary, K.T.; Covinsky, K. Assessment of Frailty and Association with Progression of Benign Prostatic Hyperplasia Symptoms and Serious Adverse Events Among Men Using Drug Therapy. JAMA Netw. Open 2021, 4, e2134427. [Google Scholar] [CrossRef]
| Characteristics | Frailty Status | Total | |||
|---|---|---|---|---|---|
| Robust | Prefrail | Frail | p Value | ||
| N (%) | 3843 (40.79) | 3818 (40.53) | 1760 (18.68) | 9421 | |
| Age *, mean ± SD, years | 77.29 ± 9.68 | 84.05 ± 10.42 | 92.17 ± 9.37 | <0.001 | 82.81 ± 11.32 |
| Age *, years, N (%) | <0.001 | ||||
| 65–79 | 2300 (59.85) | 1263 (33.08) | 193 (10.97) | 3756 (39.87) | |
| ≥80 | 1543 (40.15) | 2555 (66.92) | 1567 (89.03) | 5665 (60.13) | |
| Sex *, N (%) | <0.001 | ||||
| Man | 2163 (56.28) | 1575 (41.25) | 469 (26.65) | 4207 (44.66) | |
| Women | 1680 (43.72) | 2243 (58.75) | 1291 (73.35) | 5214 (55.34) | |
| Ethnicity, N (%) | 0.878 | ||||
| Han | 3613 (94.02) | 3612 (94.60) | 1660 (94.32) | 8885 (94.31) | |
| Minority | 230 (5.98) | 206 (5.40) | 100 (5.68) | 536 (5.69) | |
| Residence *, N (%) | 0.015 | ||||
| Urban | 1542 (40.12) | 1522 (39.86) | 786 (44.66) | 3850 (40.87) | |
| Rural | 2301 (59.88) | 2296 (60.14) | 974 (55.34) | 5571 (59.13) | |
| Current marital status *, N (%) | <0.001 | ||||
| Married | 2131 (55.45) | 1403 (36.75) | 339 (19.26) | 3873 (41.11) | |
| Widowed/Divorced/Single | 1712 (44.55) | 2415 (63.25) | 1421 (80.74) | 5548 (58.89) | |
| Education *, N (%) | <0.001 | ||||
| Formal education | 2116 (55.06) | 1461 (38.27) | 431 (24.49) | 4008 (42.54) | |
| No formal education | 1727 (44.94) | 2357 (61.73) | 1329 (75.51) | 5413 (57.46) | |
| Occupation, N (%) | 0.650 | ||||
| Agriculture | 2449 (63.73) | 2458 (64.38) | 1093 (62.10) | 6000 (63.69) | |
| Non-agriculture | 1394 (36.27) | 1360 (35.62) | 667 (37.90) | 3421 (36.31) | |
| Family income last year *, RMB, N (%) | 0.002 | ||||
| <10,000 | 2706 (70.41) | 2730 (71.50) | 1159 (65.85) | 6595 (70.00) | |
| ≥10,000 | 1137 (29.59) | 1088 (28.50) | 601 (34.15) | 2826 (30.00) | |
| Smoking at present *, N (%) | <0.001 | ||||
| Yes | 1076 (28.00) | 736 (19.28) | 176 (10.00) | 1988 (21.10) | |
| No | 2767 (72.00) | 3082 (80.72) | 1584 (90.00) | 7433 (78.90) | |
| Drinking at present *, N (%) | <0.001 | ||||
| Yes | 1039 (27.04) | 744 (19.49) | 209 (11.88) | 1992 (21.14) | |
| No | 2804 (72.96) | 3074 (80.51) | 1551 (88.13) | 7429 (78.86) | |
| Exercising at present *, N (%) | <0.001 | ||||
| Yes | 1560 (40.59) | 1380 (36.14) | 311 (17.67) | 3251 (34.51) | |
| No | 2283 (59.41) | 2438 (63.86) | 1449 (82.33) | 6170 (65.49) | |
| Social and leisure activity index *, median (IQR) | 2.61 (2.06) | 1.92 (1.88) | 0.60 (1.59) | <0.001 | 2.00 (2.14) |
| Yearly rainfall *, mm, N (%) | <0.001 | ||||
| <800 | 1289 (33.54) | 1279 (33.50) | 720 (40.91) | 3288 (34.90) | |
| ≥800 | 2554 (66.46) | 2539 (66.50) | 1040 (59.09) | 6133 (65.10) | |
| Geographic regions *, N (%) | <0.001 | ||||
| South China | 2651 (68.98) | 2621 (68.65) | 1061 (60.28) | 6333 (67.22) | |
| North China | 1192 (31.02) | 1197 (31.35) | 699 (39.72) | 3088 (32.78) | |
| Average temperature in January *, N (%) | <0.001 | ||||
| Q1 | 851 (22.14) | 863 (22.60) | 485 (27.56) | 2199 (23.34) | |
| Q2 | 886 (23.05) | 953 (24.96) | 493 (28.01) | 2332 (24.75) | |
| Q3 | 1053 (27.40) | 1072 (28.08) | 449 (25.51) | 2574 (27.32) | |
| Q4 | 1053 (27.40) | 930 (24.36) | 333 (18.92) | 2316 (24.58) | |
| Variables | Age Adjusted OR (95%CI) | Fully Adjusted OR (95%CI) | ||
|---|---|---|---|---|
| Prefrail | Frail | Prefrail | Frail | |
| Quartiles of AT in January | ||||
| Q4 | Reference | Reference | Reference | Reference |
| Q3 | 1.23 (1.12, 1.34) *** | 1.29 (1.15, 1.45) *** | 1.17 (1.07, 1.29) *** | 1.14 (1.01, 1.30) * |
| Q2 | 1.27 (1.16, 1.40) *** | 1.72 (1.53, 1.93) *** | 1.22 (1.10, 1.36) *** | 1.24 (1.07, 1.43) ** |
| Q1 | 1.34 (1.22, 1.47) *** | 2.26 (2.01, 2.54) *** | 1.35 (1.17, 1.57) *** | 1.61 (1.32, 1.95) *** |
| 1-unit decrease of AT in January | 1.01 (1.00, 1.01) *** | 1.04 (1.04, 1.05) *** | 1.01 (1.00, 1.02) * | 1.02 (1.01, 1.03) *** |
| Variables | Age Adjusted OR (95%CI) | Fully Adjusted OR (95%CI) | ||
|---|---|---|---|---|
| Prefrail | Frail | Prefrail | Frail | |
| Quartiles of AT in January | ||||
| Q4 | Reference | Reference | Reference | Reference |
| Q3 | 1.05 (0.96, 1.14) | 1.18 (1.06, 1.31) ** | 1.02 (0.93, 1.12) | 1.08 (0.95, 1.22) |
| Q2 | 1.21 (1.11, 1.32) *** | 1.38 (1.23, 1.54) *** | 1.16 (1.06, 1.27) ** | 1.24 (1.09, 1.41) *** |
| Q1 | 1.20 (1.10, 1.31) *** | 1.50 (1.34, 1.67) *** | 1.08 (0.98, 1.19) | 1.16 (1.02, 1.31) * |
| 1-unit decrease of temperature change | 1.02 (1.01, 1.03) ** | 1.05 (1.04, 1.07) *** | 1.01 (0.99, 1.02) | 1.02 (1.00, 1.04) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Zhou, W.; Wang, W.; Fan, C.; Chen, W.; Ling, L. Associations between Frailty and Ambient Temperature in Winter: Findings from a Population-Based Study. Int. J. Environ. Res. Public Health 2023, 20, 513. https://doi.org/10.3390/ijerph20010513
Zhou F, Zhou W, Wang W, Fan C, Chen W, Ling L. Associations between Frailty and Ambient Temperature in Winter: Findings from a Population-Based Study. International Journal of Environmental Research and Public Health. 2023; 20(1):513. https://doi.org/10.3390/ijerph20010513
Chicago/Turabian StyleZhou, Fenfen, Wensu Zhou, Wenjuan Wang, Chaonan Fan, Wen Chen, and Li Ling. 2023. "Associations between Frailty and Ambient Temperature in Winter: Findings from a Population-Based Study" International Journal of Environmental Research and Public Health 20, no. 1: 513. https://doi.org/10.3390/ijerph20010513
APA StyleZhou, F., Zhou, W., Wang, W., Fan, C., Chen, W., & Ling, L. (2023). Associations between Frailty and Ambient Temperature in Winter: Findings from a Population-Based Study. International Journal of Environmental Research and Public Health, 20(1), 513. https://doi.org/10.3390/ijerph20010513






