Factors Associated with the Prevalence and Severity of Menstrual-Related Symptoms: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Primary Dysmenorrhea
3.3.1. Characteristics of Reporting of PD
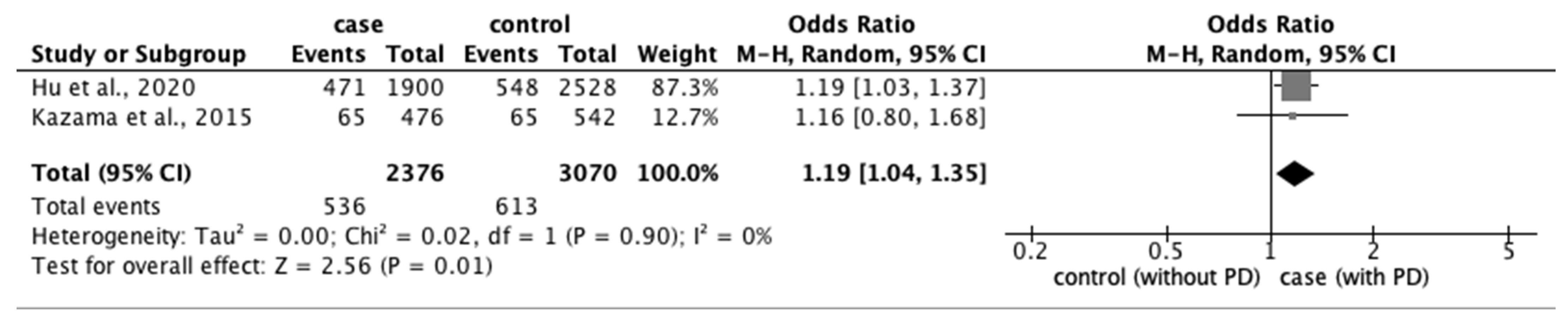
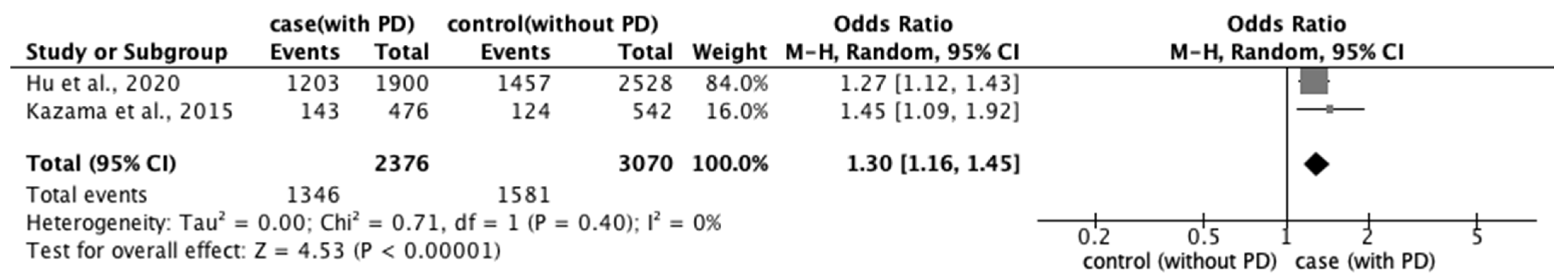
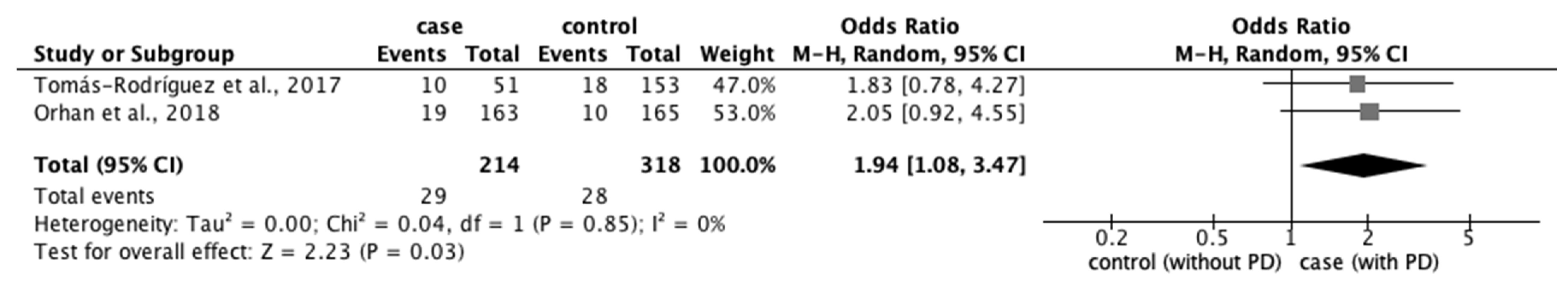
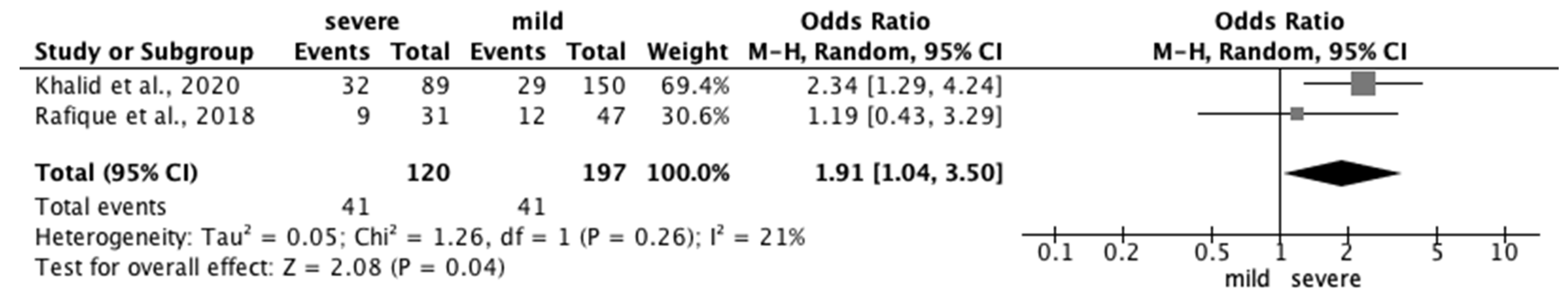
3.3.2. Risk Factors for PD
3.4. Premenstrual Syndrome
3.4.1. Characteristics of Reporting of PMS
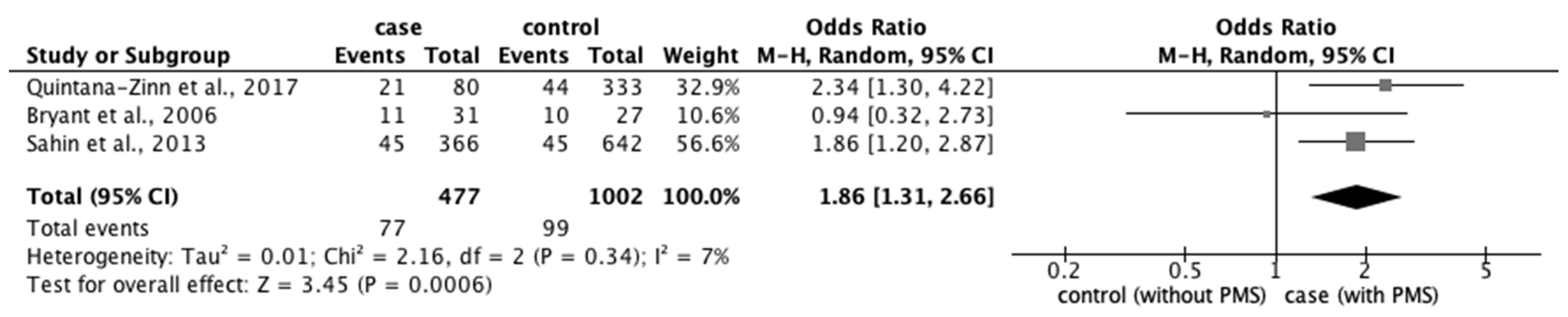
3.4.2. Risk Factors for PMS
4. Discussion
4.1. Principal Findings
4.2. Strengths and Limitations
4.3. Future Direction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farage, M.A.; Neill, S.; MacLean, A.B. Physiological changes associated with the menstrual cycle: A review. Obstet. Gynecol. Surv. 2009, 64, 58–72. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Soliman, N.A.; Soliman, R.; El Kholy, M. Dysmenorrhea in adolescents and young adults: A review in different country. Acta Biomed. 2016, 87, 233–246. [Google Scholar]
- Bernardi, M.; Lazzeri, L.; Perelli, F.; Reis, F.M.; Petraglia, F. Dysmenorrhea and related disorders. F1000Research 2017, 6, 1645. [Google Scholar] [CrossRef]
- Direkvand-Moghadam, A.; Sayehmiri, K.; Delpisheh, A.; Sattar, K. Epidemiology of Premenstrual Syndrome (PMS)—A systematic review and meta-analysis study. J. Clin. Diagn. Res. 2014, 8, 106–109. [Google Scholar]
- Faramarzi, M.; Salmalian, H. Association of psychologic and nonpsychologic factors with primary dysmenorrhea. Iran. Red Crescent Med. J. 2014, 16, e16307. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, L.; Chen, L.; Kaminga, A.C.; Xu, H. Prevalence and risk factors associated with primary dysmenorrhea among Chinese female university students: Across–sectional study. J. Pediatr. Adolesc. Gynecol. 2020, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- The Joaanna Briggs Institute. The Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument. Available online: https://jbi.global/critical-appraisal-tools (accessed on 23 December 2021).
- Abadi Bavil, D.; Dolatian, M.; Mahmoodi, Z.; Akbarzadeh Baghban, A. A comparison of physical activity and nutrition in young women with and without primary dysmenorrhea. F1000Research 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.N.; Laloglu, E.; Ozkaya, A.L.; Yilmaz, E.P.T. Serum heme oxygenase–1 levels in patients with primary dysmenorrhea. Arch. Gynecol. Obstet. 2017, 295, 929–934. [Google Scholar] [CrossRef]
- Azoulay, M.; Reuveni, I.; Dan, R.; Goelman, G.; Segman, R.; Kalla, C.; Bonne, O.; Canetti, L. Childhood trauma and premenstrual symptoms: The role of emotion regulation. Child Abus. Negl. 2020, 108, 104637. [Google Scholar] [CrossRef]
- Bahrami, A.; Bahrami–Taghanaki, H.; Khorasanchi, Z.; Timar, A.; Jaberi, N.; Azaryan, E.; Tayefi, M.; Ferns, G.A.; Sadeghnia, H.R.; Ghayour–Mobarhan, M. Menstrual problems in adolescence: Relationship to serum vitamins A and E, and systemic inflammation. Arch. Gynecol. Obstet. 2020, 301, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Musone, R.; Menditto, A.; Di Prisco, L.; Cassese, E.; D'Ajello, M.; Ambrosio, D.; Cardone, A. Influence of menstrual factors and dietary habits on menstrual pain in adolescence age. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 91, 143–148. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Chocano-Bedoya, P.O.; Zagarins, S.E.; Micka, A.E.; Ronnenberg, A.G. Dietary vitamin D intake, 25-hydroxyvitamin D3 levels and premenstrual syndrome in a college-aged population. J. Steroid Biochem. Mol. Biol. 2010, 121, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, V.; Magnay, J.L.; O'Brien, P.M.S.; Chapman, G.; Fryer, A.A.; Ismail, K.M. Serotonin receptor 1A C (-1019) G polymorphism associated with premenstrual dysphoric disorder. Obstet. Gynecol. 2007, 110, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Liu, H.; Pang, Y.; Liu, P.; Liu, Y.; Wang, G.; Liao, H.; Tang, L.; Chen, W.; Mo, X. Hippocampal fractional amplitude of low-frequency fluctuation and functional connectivity changes in premenstrual syndrome. J. Magn. Reson. Imaging 2018, 47, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Gollenberg, A.L.; Hediger, M.L.; Mumford, S.L.; Whitcomb, B.W.; Hovey, K.M.; Wactawski-Wende, J.; Schisterman, E.F. Perceived stress and severity of perimenstrual symptoms: The BioCycle Study. J. Womens Health 2010, 19, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Isgin-Atici, K.; Buyuktuncer, Z.; Akgül, S.; Kanbur, N. Adolescents with premenstrual syndrome: Not only what you eat but also how you eat matters! J. Pediatr. Endocrinol. Metab. 2018, 31, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.C.; El-Sohemy, A. Association between Vitamin D Status and Premenstrual Symptoms. J. Acad. Nutr. Diet. 2019, 119, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kazama, M.; Maruyama, K.; Nakamura, K. Prevalence of dysmenorrhea and its correlating lifestyle factors in Japanese female junior high school students. Tohoku J. Exp. Med. 2015, 236, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kordi, M.; Mohamadirizi, S.; Shakeri, M.T. The relationship between occupational stress and dysmenorrhea in midwives employed at public and private hospitals and health care centers in Iran (Mashhad) in the years 2010 and 2011. Iran. J. Nurs. Midwifery Res. 2013, 18, 316–322. [Google Scholar]
- Liao, H.; Pang, Y.; Liu, P.; Liu, H.; Duan, G.; Liu, Y.; Tang, L.; Tao, J.; Wen, D.; Li, S. Abnormal spontaneous brain activity in women with premenstrual syndrome revealed by regional homogeneity. Front. Hum. Neurosci. 2017, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Mu, J.; Xu, Q.; Chen, T.; Dun, W.; Yang, J.; Tian, J.; Hu, L.; Zhang, M. Altered white matter microarchitecture in the cingulum bundle in women with primary dysmenorrhea: A tract-based analysis study. Hum. Brain Mapp. 2017, 38, 4430–4443. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, Y.; Wang, G.; Li, R.; Wei, Y.; Fan, Y.; Yu, Y.; Deng, D.; Qin, W. Changes of functional connectivity of the anterior cingulate cortex in women with primary dysmenorrhea. Brain Imaging Behav. 2018, 12, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wei, Y.; Fan, Y.; Li, R.; Liu, Y.; Wang, G.; Wei, Y.; Pang, Y.; Deng, D.; Qin, W. Altered brain structure in women with premenstrual syndrome. J. Affect. Disord. 2018, 229, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wei, Y.; Fan, Y.; Liao, H.; Wang, G.; Li, R.; Duan, G.; Deng, D.; Qin, W. Cortical and subcortical changes in patients with premenstrual syndrome. J. Affect. Disord. 2018, 235, 191–197. [Google Scholar] [CrossRef]
- Mustaniemi, S.; Sipola-Leppänen, M.; Hovi, P.; Halbreich, U.; Vääräsmäki, M.; Räikkönen, K.; Pesonen, A.K.; Heinonen, K.; Järvenpää, A.L.; Eriksson, J.G. Premenstrual symptoms in young adults born preterm at very low birth weight-from the Helsinki Study of very low birth weight adults. BMC Women’s Health 2011, 11, 25. [Google Scholar] [CrossRef][Green Version]
- Najafi, N.; Khalkhali, H.; Moghaddam Tabrizi, F.; Zarrin, R. Major dietary patterns in relation to menstrual pain: A nested case control study. BMC Women’s Health 2018, 18, 69. [Google Scholar] [CrossRef]
- Nicolau, Z.F.M.; Bezerra, A.G.; Polesel, D.N.; Andersen, M.L.; Bittencourt, L.; Tufik, S.; Hachul, H. Premenstrual syndrome and sleep disturbances: Results from the Sao Paulo Epidemiologic Sleep Study. Psychiatry Res. 2018, 264, 427–431. [Google Scholar] [CrossRef]
- Nisar, N.; Zehra, N.; Haider, G.; Munir, A.A.; Sohoo, N.A. Frequency, intensity and impact of premenstrual syndrome in medical students. J. Coll. Physicians Surg. Pak. 2008, 18, 481–484. [Google Scholar]
- Perkonigg, A.; Yonkers, K.A.; Pfister, H.; Lieb, R.; Wittchen, H.U. Risk factors for premenstrual dysphoric disorder in a community sample of young women: The role of traumatic events and posttraumatic stress disorder. J. Clin. Psychiatry 2004, 65, 1314–1322. [Google Scholar] [CrossRef]
- Quintana–Zinn, F.A.; Whitcomb, B.W.; Ronnenberg, A.G.; Bigelow, C.; Houghton, S.C.; Bertone–Johnson, E.R. Premenstrual symptom patterns and behavioral risk factors in young women: A cross–sectional study. J. Womens Health 2017, 26, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Roomruangwong, C.; Sirivichayakul, S.; Carvalho, A.F.; Maes, M. The uterine–chemokine–brain axis: Menstrual cycle–associated symptoms (MCAS) are in part mediated by CCL2, CCL5, CCL11, CXCL8 and CXCL10. J. Affect. Disord. 2020, 269, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Rodríguez, M.I.; Palazón-Bru, A.; Martínez-St John, D.R.; Navarro-Cremades, F.; Toledo-Marhuenda, J.V.; Gil-Guillén, V.F. Factors associated with increased pain in primary dysmenorrhea: Analysis using a multivariate ordered logistic regression model. J. Pediatr. Adolesc. Gynecol. 2017, 30, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Turhan, N.; Çelik, H.; Duvan, C.İ.; Onaran, Y.; Aydın, M.; Armutcu, F. Investigation of oxidative balance in patients with dysmenorrhea by multiple serum markers. J. Turk. Ger. Gynecol. Assoc. 2012, 13, 233. [Google Scholar] [CrossRef]
- Woods, N.F.; Lentz, M.J.; Mitchell, E.S.; Kogan, H. Arousal and stress response across the menstrual cycle in women with three perimenstrual symptom patterns. Res. Nurs. Health 1994, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, K.; Shiina, M.; Takeda, T. Lifestyle factors associated with premenstrual syndrome: Across–sectional study of Japanese high school students. J. Pediatr. Adolesc. Gynecol. 2019, 32, 590–595. [Google Scholar] [CrossRef]
- Zurawiecka, M.; Wronka, I. Association of primary dysmenorrhea with anthropometrical and socio-economic factors in Polish university students. J. Obstet. Gynaecol. Res 2018, 44, 1259–1267. [Google Scholar] [CrossRef]
- Ader, D.N.; South-Paul, J.; Adera, T.; Deuster, P.A. Cyclical mastalgia: Prevalence and associated health and behavioral factors. J. Psychosom. Obstet. Gynaecol. 2001, 22, 71–76. [Google Scholar] [CrossRef]
- Ansong, E.; Arhin, S.K.; Cai, Y.; Xu, X.; Wu, X. Menstrual characteristics, disorders and associated risk factors among female international students in Zhejiang Province, China: A cross-sectional survey. BMC Womens Health 2019, 19, 35. [Google Scholar] [CrossRef]
- Baker, F.C.; Kahan, T.L.; Trinder, J.; Colrain, I.M. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep 2007, 30, 1283–1291. [Google Scholar] [CrossRef]
- Beck, L.E.; Gevirtz, R.; Mortola, J.F. The predictive role of psychosocial stress on symptom severity in premenstrual syndrome. Psychosom. Med. 1990, 25, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.; Truesdale, K.P.; Dye, L. Modest changes in dietary intake across the menstrual cycle: Implications for food intake research. Br. J. Nutr. 2006, 96, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Carman, K.B.; Arslantas, D.; Unsal, A.; Atay, E.; Ocal, E.E.; Demirtas, Z.; Saglan, R.; Dinleyici, M.; Yarar, C. Menstruation-related headache in adolescents: Point prevalence and associated factors. Pediatr. Int. 2018, 60, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Chen, C.H. Related factors and consequences of menstrual distress in adolescent girls with dysmenorrhea. Kaohsiung J. Med. Sci. 2005, 21, 121–127. [Google Scholar] [CrossRef]
- Cleckner-Smith, C.S.; Doughty, A.S.; Grossman, J.A. Premenstrual symptoms. Prevalence and severity in an adolescent sample. J. Adolesc. Health 1998, 22, 403–408. [Google Scholar] [CrossRef]
- Deng, D.; Pang, Y.; Duan, G.; Liu, H.; Liao, H.; Liu, P.; Liu, Y.; Li, S.; Chen, W.; Wen, D.; et al. Larger volume and different functional connectivity of the amygdala in women with premenstrual syndrome. Eur. Radiol. 2018, 28, 1900–1908. [Google Scholar] [CrossRef]
- Dmitrovic, R.; Peter, B.; Cvitkovic-Kuzmic, A.; Strelec, M.; Kereshi, T. Severity of symptoms in primary dysmenorrhea—A Doppler study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 107, 191–194. [Google Scholar] [CrossRef]
- Engin–Ustün, Y.; Ustün, Y.; Gürdal, H.; Güngör, M. Serotonergic activity and the Beck Depression Inventory in the premenstrual syndrome. J. Reprod. Med. 2005, 50, 327–331. [Google Scholar]
- Farahmand, M.; Ramezani Tehrani, F.; Khalili, D.; Amin, G.; Negarandeh, R. Factors associated with the severity of premenstrual syndrome among Iranian college students. J. Obstet. Gynaecol. Res. 2017, 43, 1726–1731. [Google Scholar] [CrossRef]
- Gurguis, G.N.; Yonkers, K.A.; Blakeley, J.E.; Phan, S.P.; Williams, A.; Rush, A.J. Adrenergic receptors in premenstrual dysphoric disorder. II. Neutrophil beta2-adrenergic receptors: Gs protein coupling, phase of menstrual cycle and prediction of luteal phase symptom severity. Psychiatry Res. 1998, 79, 31–42. [Google Scholar] [CrossRef]
- Hallman, J.; Oreland, L.; Edman, G.; Schalling, D. Thrombocyte monoamine oxidase activity and personality traits in women with severe premenstrual syndrome. Acta Psychiatr. Scand. 1987, 76, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Park, M. A longitudinal study of risk factors for the occurrence, duration and severity of menstrual cramps in a cohort of college women. Br. J. Obstet. Gynaecol. 1996, 103, 1134–1142. [Google Scholar] [CrossRef]
- Hashim, M.S.; Obaideen, A.A.; Jahrami, H.A.; Radwan, H.; Hamad, H.J.; Owais, A.A.; Alardah, L.G.; Qiblawi, S.; Al-Yateem, N.; Faris, M.A.E. Premenstrual syndrome is associated with dietary and lifestyle behaviors among university students: A cross-sectional study from Sharjah, UAE. Nutrients 2019, 11, 1939. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Huang, Y.; Zhou, R. Premenstrual syndrome is associated with altered cortisol awakening response. Stress 2019, 22, 640–646. [Google Scholar] [CrossRef] [PubMed]
- İşik, H.; Ergöl, Ş.; Aynioğlu, Ö.; Şahbaz, A.; Kuzu, A.; Uzun, M. Premenstrual syndrome and life quality in Turkish health science students. Turk. J. Med. Sci. 2016, 46, 695–701. [Google Scholar] [CrossRef]
- Kamat, S.V.; Nimbalkar, A.; Phatak, A.G.; Nimbalkar, S.M. Premenstrual syndrome in Anand District, Gujarat: A cross-sectional survey. J. Fam. Med. Prim. Care 2019, 8, 640–647. [Google Scholar]
- Khalid, M.; Jamali, T.; Ghani, U.; Shahid, T.; Ahmed, T.; Nasir, T. Severity and relation of primary dysmenorrhea and body mass index in undergraduate students of Karachi: A cross sectional survey. J. Pak. Med. Assoc. 2020, 70, 1299–1304. [Google Scholar]
- Kural, M.; Noor, N.N.; Pandit, D.; Joshi, T.; Patil, A. Menstrual characteristics and prevalence of dysmenorrhea in college going girls. J. Fam. Med. Prim. Care 2015, 4, 426–431. [Google Scholar]
- Liu, P.; Wang, G.; Liu, Y.; Yu, Q.; Yang, F.; Jin, L.; Sun, J.; Yang, X.; Qin, W.; Calhoun, V.D. White matter microstructure alterations in primary dysmenorrhea assessed by diffusion tensor imaging. Sci. Rep. 2016, 6, 25836. [Google Scholar] [CrossRef]
- Liu, P.; Wei, Y.; Liao, H.; Fan, Y.; Li, R.; Feng, N.; Duan, G.; Deng, D.; Qin, W. Thalamocortical dysconnectivity in premenstrual syndrome. Brain Imaging Behav. 2019, 13, 717–724. [Google Scholar] [CrossRef]
- Marván, M.L.; Díaz-Erosa, M.; Montesinos, A. Premenstrual symptoms in Mexican women with different educational levels. J. Psychol. 1998, 132, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Egawa, M.; Kimura, T.; Hayashi, T. A potential relation between premenstrual symptoms and subjective perception of health and stress among college students: A cross-sectional study. BioPsychoSocial Med. 2019, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Monday, I.; Anthony, P.; Olunu, E.; Otohinoyi, D.; Abiodun, S.; Owolabi, A.; Mobolaji, B.; Fakoya, A.O.J. Prevalence and correlation between diet and dysmenorrhea among high school and college students in Saint Vincent and Grenadines. Open Access Maced. J. Med. Sci. 2019, 7, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Njoku, U.C.; Amadi, P.U.; Agomuo, E.N.; Bhebhe, M. The relationship between pain and vascular function biomarkers in dysmenorrheal university students. Chonnam Med. J. 2020, 56, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Orhan, C.; Çelenay, Ş.T.; Demirtürk, F.; Özgül, S.; Üzelpasacı, E.; Akbayrak, T. Effects of menstrual pain on the academic performance and participation in sports and social activities in Turkish university students with primary dysmenorrhea: A case control study. J. Obstet. Gynaecol. Res. 2018, 44, 2101–2109. [Google Scholar] [CrossRef]
- Pinar, G.; Colak, M.; Oksuz, E. Premenstrual Syndrome in Turkish college students and its effects on life quality. Sex. Reprod. Healthc. 2011, 2, 21–27. [Google Scholar] [CrossRef]
- Rafique, N.; Al-Sheikh, M.H. Prevalence of primary dysmenorrhea and its relationship with body mass index. J. Obstet. Gynaecol. Res. 2018, 44, 1773–1778. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Matsumoto, A.K.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Moreira, E.G.; Sirivichayakul, S.; Carvalho, A.; Barbosa, D.S.; Maes, M. The role of immune and oxidative pathways in menstrual cycle associated depressive, physio-somatic, breast and anxiety symptoms: Modulation by sex hormones. J. Psychosom. Res. 2020, 135, 110158. [Google Scholar] [CrossRef]
- Sahin, S.; Ozdemir, K.; Unsal, A. Evaluation of premenstrual syndrome and quality of life in university students. J. Pak. Med. Assoc. 2014, 64, 915–922. [Google Scholar]
- Seippel, L.; Bäckström, T. Luteal-phase estradiol relates to symptom severity in patients with premenstrual syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 1988–1992. [Google Scholar] [CrossRef]
- Şentürk, Ş. Relation between uterine morphology and severity of primary dysmenorrhea. Turk. J. Obstet. Gynecol. 2020, 17, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tadakawa, M.; Takeda, T.; Monma, Y.; Koga, S.; Yaegashi, N. The prevalence and risk factors of school absenteeism due to premenstrual disorders in Japanese high school students—A school-based cross-sectional study. BioPsychoSocial Med. 2016, 10, 13. [Google Scholar] [CrossRef]
- Takeda, T.; Tadakawa, M.; Koga, S.; Nagase, S.; Yaegashi, N. Premenstrual symptoms and posttraumatic stress disorder in Japanese high school students 9 months after the great East-Japan earthquake. Tohoku J. Exp. Med. 2013, 230, 151–154. [Google Scholar] [CrossRef]
- Tangchai, K.; Titapant, V.; Boriboonhirunsarn, D. Dysmenorrhea in Thai adolescents: Prevalence, impact and knowledge of treatment. J. Med. Assoc. Thail. 2004, 87, S69–S73. [Google Scholar]
- Tu, C.H.; Niddam, D.M.; Chao, H.T.; Chen, L.F.; Chen, Y.S.; Wu, Y.T.; Yeh, T.C.; Lirng, J.F.; Hsieh, J.C. Brain morphological changes associated with cyclic menstrual pain. Pain 2010, 150, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Seippel, L.; Purdy, R.H.; Bãckström, T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: Study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J. Clin. Endocrinol. Metab. 1996, 81, 1076–1082. [Google Scholar]
- Woods, N.F.; Lentz, M.J.; Mitchell, E.S.; Heitkemper, M.; Shaver, J.; Henker, R. Perceived stress, physiologic stress arousal, and premenstrual symptoms: Group differences and intra-individual patterns. Res. Nurs. Health 1998, 21, 511–523. [Google Scholar] [CrossRef]
- Woods, N.F.; Lentz, M.J.; Mitchell, E.S.; Shaver, J.; Heitkemper, M. Luteal phase ovarian steroids, stress arousal, premenses perceived stress, and premenstrual symptoms. Res. Nurs. Health 1998, 21, 129–142. [Google Scholar] [CrossRef]
- Wronka, I.; Teul, I.; Marchewka, J. The influence of age at menarche on the prevalence of disorders of the menstrual cycle among healthy university students. Ann. Acad. Med. Stetin. 2013, 59, 94–98. [Google Scholar] [PubMed]
- Yokose, H.; Suzuki, M.; Konno, M.; Takahashi, S.; Ishihara, K.; Doi, Y.; Uchiyama, M. Factors associated with premenstrual dysphoric disorder in Japanese female college students. J. Pediatr. Adolesc. Gynecol. 2015, 19, 310–321. [Google Scholar]
- Zeynali, M.; Haghighian, H.K. Is there a relationship between serum vitamin D with dysmenorrhea pain in young women? J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Zukri, S.M.; Naing, L.; Hamzah, T.N.T. Primary dysmenorrhea among medical and dental university students in Kelantan: Prevalence and associated factors. Int. Med. J. 2009, 16, 93–99. [Google Scholar]
- Abdul-Razzak, K.K.; Ayoub, N.M.; Abu-Taleb, A.A.; Obeidat, B.A. Influence of dietary intake of dairy products on dysmenorrhea. J. Obstet. Gynaecol. Res. 2010, 36, 377–383. [Google Scholar] [CrossRef]
- Adams, M.; McCrone, S. SRD5A1 genotype frequency differences in women with mild versus severe premenstrual symptoms. Issues Ment. Health Nurs. 2012, 33, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, D. Prevalence and factors affecting dysmenorrhea in female university students: Effect on general comfort level. Pain Manag. Nurs. 2015, 16, 534–543. [Google Scholar] [CrossRef]
- Al-Matouq, S.; Al-Mutairi, H.; Al-Mutairi, O.; Abdulaziz, F.; Al-Basri, D.; Al-Enzi, M.; Al-Taiar, A. Dysmenorrhea among high-school students and its associated factors in Kuwait. BMC Pediatr. 2019, 19, 80. [Google Scholar] [CrossRef]
- Alam, F.; Nayyar, S.; Richie, W.; Archibong, A.; Nayyar, T. Beta-arrestin1 levels in mononuclear leukocytes support depression scores for women with premenstrual dysphoric disorder. Int. J. Environ. Res. Public Health 2015, 13, 43. [Google Scholar] [CrossRef]
- Albsoul-Younes, A.; Alefishat, E.; Farha, R.A.; Tashman, L.; Hijjih, E.; AlKhatib, R. Premenstrual syndrome and premenstrual dysphoric disorders among Jordanian women. Perspect. Psychiatr. Care 2018, 54, 348–353. [Google Scholar] [CrossRef]
- Bakhshani, N.M.; Mousavi, M.N.; Khodabandeh, G. Prevalence and severity of premenstrual symptoms among Iranian female university students. J. Pak. Med. Assoc. 2009, 59, 205–208. [Google Scholar]
- Barcikowska, Z.; Wójcik-Bilkiewicz, K.; Sobierajska-Rek, A.; Grzybowska, M.E.; Wąż, P.; Zorena, K. Dysmenorrhea and associated factors among Polish women: A cross-sectional study. Pain Res. Manag. 2020, 2020, 6161536. [Google Scholar] [CrossRef]
- Buchpiguel, C.; Alavi, A.; Crawford, D.; Freeman, E.; Newberg, A. Changes in cerebral blood flow associated with premenstrual syndrome: A preliminary study. J. Psychosom. Obstet. Gynaecol. 2000, 21, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Çoban, Ö.G.; Karakaya, D.; Önder, A.; İşleyen, Z.; Adanır, A.S. Association of premenstrual dysphoric disorder and eating behaviors among nursing students: A cross-sectional study. J. Pediatr. Adolesc. Gynecol. 2021, 34, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Craner, J.R.; Sigmon, S.T.; Martinson, A.A.; McGillicuddy, M.L. Premenstrual disorders and rumination. J. Clin. Psychol. 2014, 70, 32–47. [Google Scholar] [CrossRef]
- Czajkowska, M.; Drosdzol-Cop, A.; Naworska, B.; Galazka, I.; Gogola, C.; Rutkowska, M.; Skrzypulec-Plinta, V. The impact of competitive sports on menstrual cycle and menstrual disorders, including premenstrual syndrome, premenstrual dysphoric disorder and hormonal imbalances. Ginekol. Pol. 2020, 91, 503–512. [Google Scholar] [CrossRef]
- Danborno, A.M.; Nwankwo, M.; Kure, J.; Eluwa, C. Prevalence of premenstrual syndrome and changes in blood pressure with menstrual cycle among university students. Niger. J. Physiol. Sci. 2018, 33, 117–124. [Google Scholar] [PubMed]
- Farrokh-Eslamlou, H.; Oshnouei, S.; Heshmatian, B.; Akbari, E. Premenstrual syndrome and quality of life in Iranian medical students. Sex. Reprod. Healthc. 2015, 6, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Flug, D.; Largo, R.H.; Prader, A. Symptoms related to menstruation in adolescent Swiss girls: A longitudinal study. Ann. Hum. Biol. 1985, 12, 161–168. [Google Scholar] [CrossRef]
- Fujiwara, T.; Sato, N.; Awaji, H.; Sakamoto, H.; Nakata, R. Skipping breakfast adversely affects menstrual disorders in young college students. Int. J. Food Sci. Nutr. 2009, 60, 23–31. [Google Scholar] [CrossRef]
- Goker, A.; Artunc-Ulkumen, B.; Aktenk, F.; Ikiz, N. Premenstrual syndrome in Turkish medical students and their quality of life. J. Obstet. Gynaecol. 2015, 35, 275–278. [Google Scholar] [CrossRef]
- Gupta, M.; Dua, D.; Kaur, H.; Grover, S. Prevalence of premenstrual dysphoric disorder among school-going adolescent girls. Ind. Psychiatry J. 2019, 28, 198–202. [Google Scholar]
- Hammarbäck, S.; Damber, J.E.; Bäckström, T. Relationship between symptom severity and hormone changes in women with premenstrual syndrome. J. Clin. Endocrinol. Metab. 1989, 68, 125–130. [Google Scholar] [CrossRef]
- Hata, K.; Nozu, A.; Kawatani, M.; Nawata, K. Association between dietary intake and dysmenorrhea among female college studentes. Clin. Gynecol. Obstet. 2016, 70, 1079–1083. [Google Scholar]
- Issa, B.A.; Yussuf, A.D.; Olatinwo, A.W.; Ighodalo, M. Premenstrual dysphoric disorder among medical students of a Nigerian university. Ann. Afr. Med. 2010, 9, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Jahanfar, S.; Lye, M.S.; Krishnarajah, I.S. The heritability of premenstrual syndrome. Twin Res. Hum. Genet. 2011, 14, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.; Rossignol, A.M.; Bonnlander, H. Evidence against multiple premenstrual syndromes: Results of a multivariate profile analysis of premenstrual symptomatology. J. Psychosom. Res. 1993, 37, 257–263. [Google Scholar] [CrossRef]
- Katwal, P.C.; Karki, N.R.; Sharma, P.; Tamrakar, S.R. Dysmenorrhea and Stress among the Nepalese Medical Students. Kathmandu Univ. Med. J. (KUMJ) 2016, 14, 318–321. [Google Scholar] [PubMed]
- Koci, A.; Strickland, O. Relationship of adolescent physical and sexual abuse to perimenstrual symptoms (PMS) in adulthood. Issues Ment. Health Nurs. 2007, 28, 75–87. [Google Scholar] [CrossRef]
- Koshikawa, N.; Tatsunuma, T.; Furuya, K.; Seki, K. Prostaglandins and premenstrual syndrome. Prostaglandins Leukot. Essent. Fat. Acids 1992, 45, 33–36. [Google Scholar] [CrossRef]
- Lin, I.M.; Tsai, Y.C.; Peper, E.; Yen, C.F. Depressive mood and frontal alpha asymmetry during the luteal phase in premenstrual dysphoric disorder. J. Obstet. Gynaecol. Res. 2013, 39, 998–1006. [Google Scholar] [CrossRef]
- Lovick, T.A.; Guapo, V.G.; Anselmo-Franci, J.A.; Loureiro, C.M.; Faleiros, M.C.M.; Del Ben, C.M.; Brandão, M.L. A specific profile of luteal phase progesterone is associated with the development of premenstrual symptoms. Psychoneuroendocrinology 2017, 75, 83–90. [Google Scholar] [CrossRef]
- Lustyk, M.K.; Douglas, H.A.; Shilling, E.A.; Woods, N.F. Hemodynamic and psychological responses to laboratory stressors in women: Assessing the roles of menstrual cycle phase, premenstrual symptomatology, and sleep characteristics. Int. J. Psychophysiol. 2012, 86, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lustyk, M.K.; Widman, L.; Becker Lde, L. Abuse history and premenstrual symptomatology: Assessing the mediating role of perceived stress. Women Health 2007, 46, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Lustyk, M.K.; Widman, L.; Paschane, A.; Ecker, E. Stress, quality of life and physical activity in women with varying degrees of premenstrual symptomatology. Women Health 2004, 39, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Magnay, J.L.; El-Shourbagy, M.; Fryer, A.A.; O'Brien, S.; Ismail, K.M. Analysis of the serotonin transporter promoter rs25531 polymorphism in premenstrual dysphoric disorder. Am. J. Obstet. Gynecol. 2010, 203, 181. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, M.G.; Braiden, V.; Livesey, J.H. Symptom cyclicity in women with the premenstrual syndrome: An 8-year follow-up study. J. Psychosom. Res. 1992, 36, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, M.G.; Livesey, J.H.; Wells, J.E.; Braiden, V. Physical symptom cyclicity in women with and without the premenstrual syndrome. J. Psychosom. Res. 1990, 34, 203–213. [Google Scholar] [CrossRef]
- Nigam, S.; Benedetto, C.; Zonca, M.; Leo-Rossberg, I.; Lübbert, H.; Hammerstein, J. Increased concentrations of eicosanoids and platelet-activating factor in menstrual blood from women with primary dysmenorrhea. Eicosanoids 1991, 4, 137–141. [Google Scholar]
- Nillni, Y.I.; Berenz, E.C.; Pineles, S.L.; Coffey, S.F.; Zvolensky, M.J. Anxiety sensitivity as a moderator of the association between premenstrual symptoms and posttraumatic stress disorder symptom severity. Psychol. Trauma Theory Res. Pract. Policy 2014, 6, 167–175. [Google Scholar] [CrossRef][Green Version]
- Olson, B.R.; Forman, M.R.; Lanza, E.; McAdam, P.A.; Beecher, G.; Kimzey, L.M.; Campbell, W.S.; Raymond, E.G.; Brentzel, S.L.; Güttsches-Ebeling, B. Relation between sodium balance and menstrual cycle symptoms in normal women. Ann. Intern. Med. 1996, 125, 564–567. [Google Scholar] [CrossRef]
- Oral, E.; Ozcan, H.; Kirkan, T.S.; Askin, S.; Gulec, M.; Aydin, N. Luteal serum BDNF and HSP70 levels in women with premenstrual dysphoric disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 685–693. [Google Scholar] [CrossRef]
- Ozgocer, T.; Ucar, C.; Yildiz, S. Cortisol awakening response is blunted and pain perception is increased during menses in cyclic women. Psychoneuroendocrinology 2017, 77, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.; Al-Sowielem, L.S. Prevalence and predictors of premenstrual syndrome among college-aged women in Saudi Arabia. Ann. Saudi Med. 2003, 23, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, A.M. Caffeine-containing beverages and premenstrual syndrome in young women. Am. J. Public Health 1985, 75, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, A.M.; Bonnlander, H. Prevalence and severity of the premenstrual syndrome. Effects of foods and beverages that are sweet or high in sugar content. J. Reprod. Med. 1991, 36, 131–136. [Google Scholar]
- Sadler, C.; Smith, H.; Hammond, J.; Bayly, R.; Borland, S.; Panay, N.; Crook, D.; Inskip, H. Lifestyle factors, hormonal contraception, and premenstrual symptoms: The United Kingdom Southampton Women's Survey. J. Womens Health 2010, 19, 391–396. [Google Scholar] [CrossRef]
- Sahin, M.E.; Sahin, E.; Madendag, Y.; Madendag, I.C.; Tayyar, A.T.; Özdemir, F.; Acmaz, G.; Muderris, I.I. The effect of anterior uterocervical angle on primary dysmenorrhea and disease severity. Pain Res. Manag. 2018, 2018, 9819402. [Google Scholar] [CrossRef]
- Sen, E.; Ozdemir, O.; Ozdemir, S.; Atalay, C.R. The relationship between serum ischemia-modified albumin levels and uterine artery doppler parameters in patients with primary dysmenorrhea. Rev. Bras. Ginecol. Obs. 2020, 42, 630–633. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Shrestha, S.; Dangol, D.; Aryal, B.B.; Shrestha, S.; Sapkota, B.; Rai, S. Premenstrual syndrome in students of a teaching hospital. J. Nepal Health Res. Counc. 2019, 17, 253–257. [Google Scholar] [CrossRef]
- Simpson, L.O.; Shand, B.I.; Nyhof, R.B. Factors influencing the cyclical symptoms relating to the menstrual cycle. N. Zealand Med. J. 1988, 101, 225–228. [Google Scholar]
- Takeda, T.; Shiina, M. Effect of an educational program on adolescent premenstrual syndrome: Lessons from the Great East Japan Earthquake. Adolesc. Health Med. 2018, 9, 95–101. [Google Scholar] [CrossRef]
- Takeda, T.; Tadakawa, M.; Koga, S.; Nagase, S.; Yaegashi, N. Relationship between dysmenorrhea and posttraumatic stress disorder in Japanese high school students 9 months after the Great East Japan Earthquake. J. Pediatr. Adolesc. Gynecol. 2013, 26, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Telek, T.; Gonda, X.; Lazary, J.; Benko, A.; Pap, D.; Vargha, A.; Bagdy, G. The possible protective role of personality dimensions against premenstrual syndrome. Psychiatry Res. 2010, 179, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.L.; Gick, M.L. Medical care-seeking for menstrual symptoms. J. Psychosom. Res. 2000, 49, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.J.; Baker, S. Coping resources of women with premenstrual syndrome. Arch. Psychiatr. Nurs. 1992, 6, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liang, Y.; Wang, Q.; Zhao, Y.; Zhou, R. Emotion dysregulation of women with premenstrual syndrome. Sci. Rep. 2016, 6, 38501. [Google Scholar] [CrossRef]
- Yen, J.Y.; Lin, H.C.; Lin, P.C.; Liu, T.L.; Long, C.Y.; Ko, C.H. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int. J. Environ. Res. Public Health 2019, 16, 4352. [Google Scholar] [CrossRef]
- Lindh, I.; Ellström, A.A.; Milsom, I. The effect of combined oral contraceptives and age on dysmenorrhoea: An epidemiological study. Hum. Reprod. 2012, 27, 676–682. [Google Scholar] [CrossRef]
- Warren, M.P.; Goodman, L.R. Exercise-induced endocrine pathologies. J. Endocrinol. Investig. 2003, 26, 873–878. [Google Scholar] [CrossRef]
- Manore, M.M.; Kam, L.C.; Loucks, A.B. The female athlete triad: Components, nutrition issues, and health consequences. J. Sport. Sci. 2007, 25, S61–S71. [Google Scholar] [CrossRef]
- Ju, H.; Jones, M.; Mishra, G.D. A U-shaped relationship between body mass index and dysmenorrhea: A longitudinal study. PLoS ONE 2015, 10, e0134187. [Google Scholar] [CrossRef]
- Pasquali, R.; Pelusi, C.; Genghini, S.; Cacciari, M.; Gambineri, A. Obesity and reproductive disorders in women. Hum. Reprod. Update 2003, 9, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Duan, J.; Wang, P.; Liu, P.; Guo, J.; Shang, E.; Qian, D.; Tang, Y.; Tang, Z. Metabolomic study of biochemical changes in the plasma and urine of primary dysmenorrhea patients using UPLC-MS coupled with a pattern recognition approach. J. Proteome Res. 2013, 12, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Nohara, M.; Momoeda, M.; Kubota, T.; Nakabayashi, M. Menstrual cycle and menstrual pain problems and related risk factors among Japanese female workers. Ind. Health 2011, 49, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Sooki, Z.; Shariati, M.; Chaman, R.; Khosravi, A.; Effatpanah, M.; Keramat, A. The Role of mother in informing girls about puberty: A meta-analysis study. Nurs. Midwifery Stud. 2016, 5, e30360. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Torpy, D.J.; Gold, P.W. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: Clinical implications. Ann. Intern. Med. 1998, 129, 229–240. [Google Scholar] [CrossRef]
- Crowley, S.J.; Acebo, C.; Carskadon, M.A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007, 8, 602–612. [Google Scholar] [CrossRef]
- Rojansky, N.; Brzezinski, A.; Schenker, J.G. Seasonality in human reproduction: An update. Hum. Reprod. 1992, 7, 735–745. [Google Scholar] [CrossRef]
- Esquifino, A.I.; Villanúa, M.A.; Agrasal, C. Effect of neonatal melatonin administration on sexual development in the rat. J. Steroid Biochem. 1987, 27, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Villanúa, M.A.; Agrasal, C.; Esquifino, A.I. Neonatal melatonin administration advances rat vaginal opening and disrupts estrous cyclicity and estrogen-dependent regulatory mechanisms of luteinizing hormone and prolactin. J. Pineal Res. 1989, 7, 165–174. [Google Scholar]
- Dehnavi, Z.M.; Jafarnejad, F.; Kamali, Z. The Effect of aerobic exercise on primary dysmenorrhea: A clinical trial study. J. Educ. Health Promot. 2018, 7, 3. [Google Scholar] [CrossRef]
- Pascoe, M.C.; Thompson, D.R.; Ski, C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology 2017, 86, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Momma, R.; Nakata, Y.; Sawai, A.; Takeda, M.; Natsui, H.; Mukai, N.; Watanabe, K. Comparisons of the prevalence, severity, and risk factors of dysmenorrhea between Japanese female athletes and non-athletes in universities. Int. J. Environ. Res. Public Health 2021, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Buscicchio, G.; Piemontese, M.; Gentilucci, L.; Ferretti, F.; Tranquilli, A.L. The effects of maternal caffeine and chocolate intake on fetal heart rate. J. Matern.-Fetal Neonatal Med. 2012, 25, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Hashim, R.T.; Alkhalifah, S.S.; Alsalman, A.A.; Alfaris, D.M.; Alhussaini, M.A.; Qasim, R.S.; Shaik, S.A. Prevalence of primary dysmenorrhea and its effect on the quality of life amongst female medical students at King Saud University, Riyadh, Saudi Arabia. A cross-sectional study. Saudi Med. J. 2020, 41, 283–289. [Google Scholar] [CrossRef]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine's vascular mechanisms of action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef]
- Sawant, O.B.; Ramadoss, J.; Hankins, G.D.; Wu, G.; Washburn, S.E. Effects of L-glutamine supplementation on maternal and fetal hemodynamics in gestating ewes exposed to alcohol. Amino Acids 2014, 46, 1981–1996. [Google Scholar] [CrossRef]
- Nagata, C.; Hirokawa, K.; Shimizu, N.; Shimizu, H. Associations of menstrual pain with intakes of soy, fat and dietary fiber in Japanese women. Eur. J. Clin. Nutr. 2005, 59, 88–92. [Google Scholar] [CrossRef][Green Version]
- Fujiwara, T.; Nakata, R. Skipping breakfast is associated with reproductive dysfunction in post-adolescent female college students. Appetite 2010, 55, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Kaimura, M.; Ueda, K. Menstruation in young women: Associated symptoms, related factors, and effects on QOL. J. Jpn. Soc. Psychosom. Obstet. Gynecol. 2013, 18, 412–421. (In Japanese) [Google Scholar]








| Databases | Search Strategies (January 2021) |
|---|---|
| PubMed | ((“premenstrual syndrome” [All Fields]) OR (“premenstrual dysphoric disorder” [All Fields]) OR (“functional dysmenorrhea” [All Fields]) OR (“primary dysmenorrhea” [All Fields]) OR (“menstrual pain” [All Fields]) OR (“menstrual symptoms” [All Fields])) AND ((“risk factors” [All Fields]) OR (“severity” [All Fields]) OR (“exacerbation” [All Fields]) OR (“limitation” [All Fields]) OR (“activity of daily life” [All Fields]) OR (“limiting factors” [All Fields])) |
| Ichushi | (“premenstrual syndrome” OR “premenstrual dysphoric disorder” OR “functional dysmenorrhea” OR “primary dysmenorrhea” OR “menstrual pain” OR “menstrual symptoms”) AND (“risk factors” OR “severity” OR “exacerbation” OR “limitation” OR “activity of daily life” OR “limiting factors”) |
| First Author (Year) | Before Menstruation | First Day of Menstruation | on or after the Second Day of Menstruation |
|---|---|---|---|
| Hu et al. (2020) [6] | 24.5 | 60.8 | 14.7 |
| Balbi et al. (2000) [13] | 70.0 | N/A | |
| Chen et al. (2005) [45] | N/A | 49.8 | N/A |
| Kural et al. (2015) [59] | 23.5 | 61.5 | 14.2 |
| Monday et al. (2019) [64] | N/A | 31.6 | N/A |
| Orhan et al. (2018) [66] | 35.0 | 65.0 | N/A |
| Rafique et al. (2018) [68] | 44.5 | 40.1 | 15.5 |
| First Author (Year) | Mean ± SD | 1 Day | 2 Days | 3 Days | 4 Days | Over 5 Days |
|---|---|---|---|---|---|---|
| Hu et al. (2020) [6] | N/A | N/A | 95.3 | 4.7 | N/A | N/A |
| Balbi et al. (2000) [13] | N/A | N/A | Most frequent | N/A | N/A | N/A |
| Liu et al. (2017) [23] | 1.9 ± 0.38 (days) | N/A | N/A | N/A | N/A | N/A |
| Carman et al. (2018) [44] | 4.37 ± 5.55 a (hours) | N/A | N/A | N/A | N/A | N/A |
| Harlow et al. (1996) [53] | 1.7 b (days) | N/A | N/A | N/A | N/A | N/A |
| Kural et al. (2015) [59] | N/A | 37.0 | 39.8 | 15.4 | 5.4 | 2.3 |
| Orhan et al. (2018) [66] | 2.0 [1.0–3.0] c (days) | N/A | N/A | N/A | N/A | N/A |
| Rafique et al. (2018) [68] | N/A | 25.2 | 55.5 | N/A | N/A | N/A |
| First Author (Year) | Daily Life | Work or School | Sports Activities | Social Activities | Less Concentration | Felt More Rested | Avoidance of Responsibility |
|---|---|---|---|---|---|---|---|
| Chen et al. (2005) [45] | 92.4 | 25.3 | N/A | N/A | N/A | N/A | N/A |
| Orhan et al. (2018) [66] | N/A | 4.4 | 4.5 | 4.5 | N/A | N/A | N/A |
| Rafique et al. (2018) [68] | 54.5 | N/A | 25.2 | N/A | N/A | N/A | N/A |
| Tangchai et al. (2004) [75] | N/A | 21.1 | 37.3 | 18.2 | 63.6 | N/A | N/A |
| Zukri et al. (2009) [83] | 88.2 | 31.1 | N/A | 81.2 | N/A | 99.9 | 92.1 |
| First Author (year) | Analgesics | Warming | Rest | Exercise | Medical Treatment | Herbal Medicine | Meditating |
|---|---|---|---|---|---|---|---|
| Kordi et al. (2013) [21] | 67.0 | N/A | N/A | N/A | N/A | N/A | N/A |
| Chen et al. (2005) [45] | 39.9 | N/A | N/A | N/A | N/A | N/A | N/A |
| Rafique et al. (2018) [68] | 63.7 | 18.3 | N/A | N/A | N/A | N/A | N/A |
| Tangchai et al. (2004) [75] | 32.5 | 34.0 | 92.0 | 6.8 | 7.1 | 12.7 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsuhashi, R.; Sawai, A.; Kiyohara, K.; Shiraki, H.; Nakata, Y. Factors Associated with the Prevalence and Severity of Menstrual-Related Symptoms: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 569. https://doi.org/10.3390/ijerph20010569
Mitsuhashi R, Sawai A, Kiyohara K, Shiraki H, Nakata Y. Factors Associated with the Prevalence and Severity of Menstrual-Related Symptoms: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(1):569. https://doi.org/10.3390/ijerph20010569
Chicago/Turabian StyleMitsuhashi, Risa, Akemi Sawai, Kosuke Kiyohara, Hitoshi Shiraki, and Yoshio Nakata. 2023. "Factors Associated with the Prevalence and Severity of Menstrual-Related Symptoms: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 1: 569. https://doi.org/10.3390/ijerph20010569
APA StyleMitsuhashi, R., Sawai, A., Kiyohara, K., Shiraki, H., & Nakata, Y. (2023). Factors Associated with the Prevalence and Severity of Menstrual-Related Symptoms: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(1), 569. https://doi.org/10.3390/ijerph20010569







