Determination of Neonicotinoid Insecticides in Environmental Water by the Enrichment of MIL-53 Mixed Matrix Membrane Coupled with High Performance Liquid Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Synthesis of MIL-53-PVDF MMM
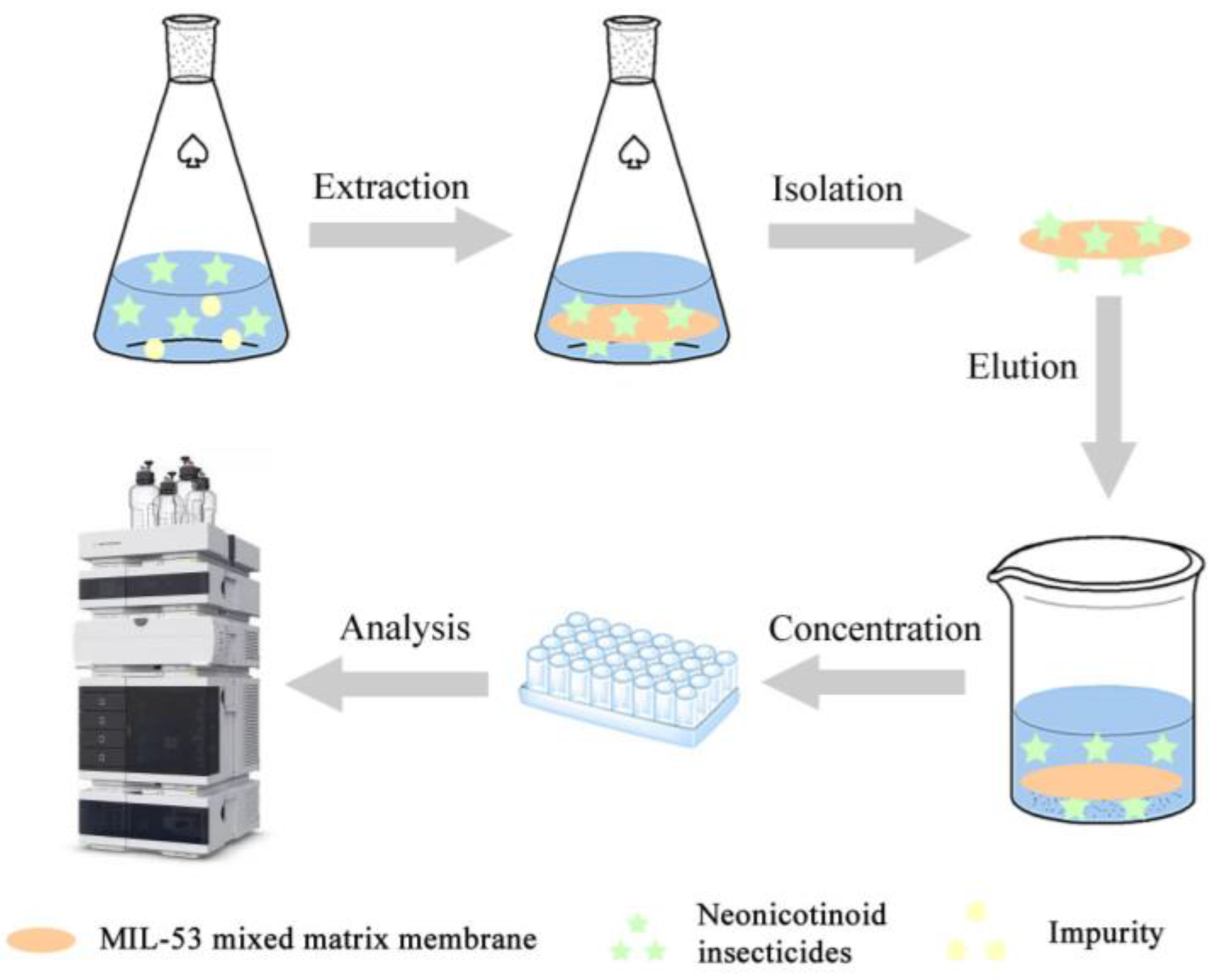
2.4. DME Procedure
3. Results and Discussion
3.1. Choice of Membrane Material
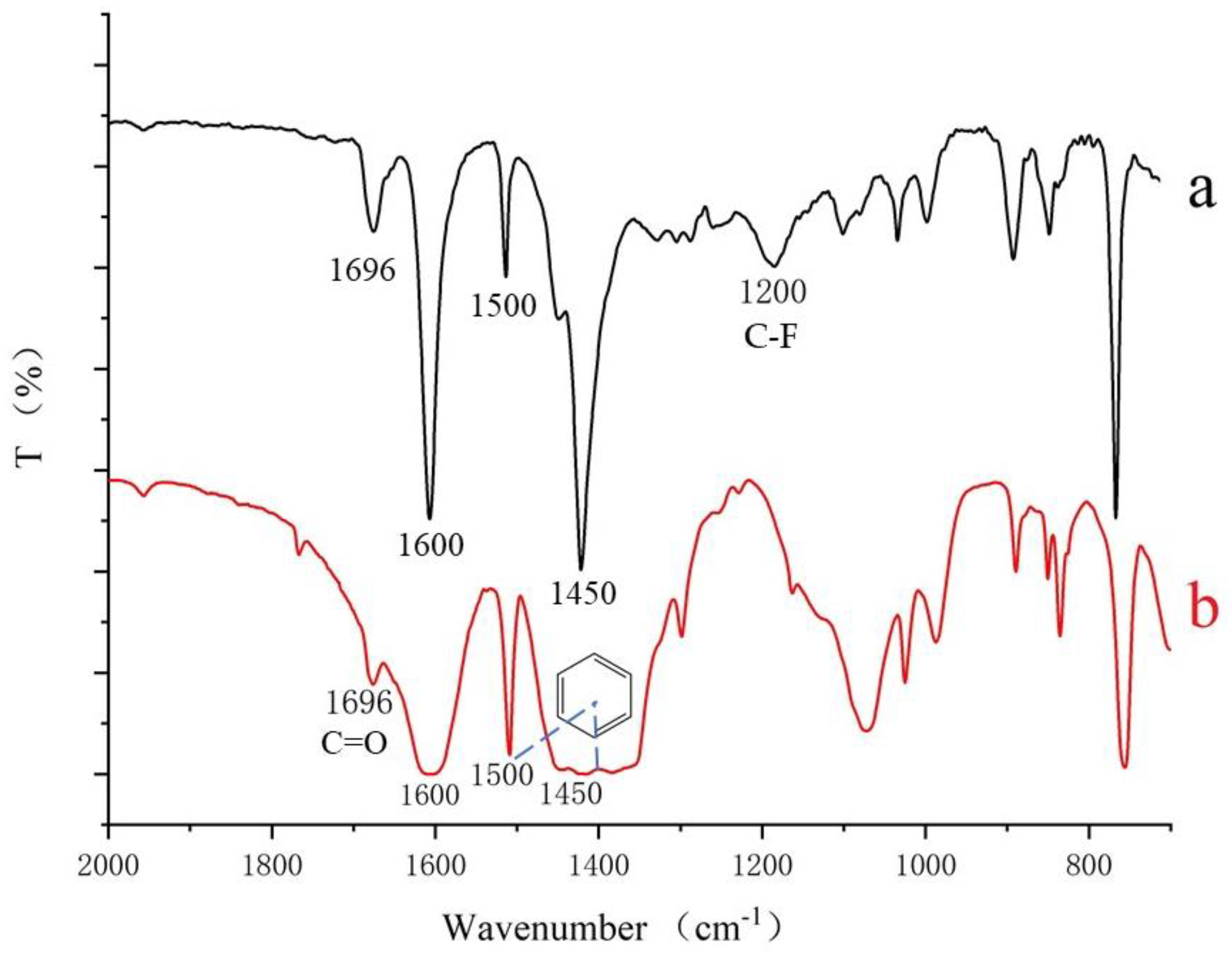
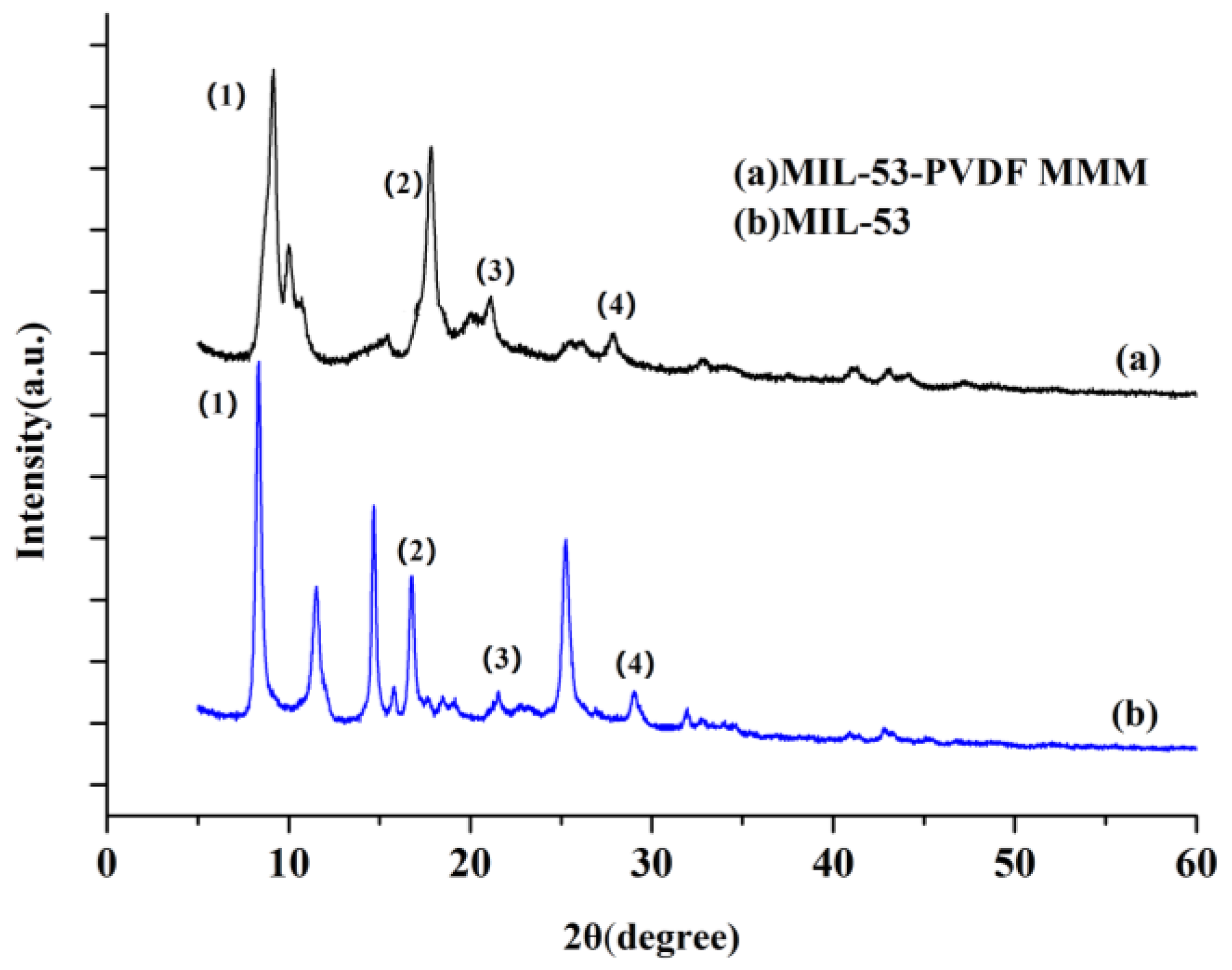
3.2. Characterization of MIL-53 MMM
3.3. Optimization of DME Conditions
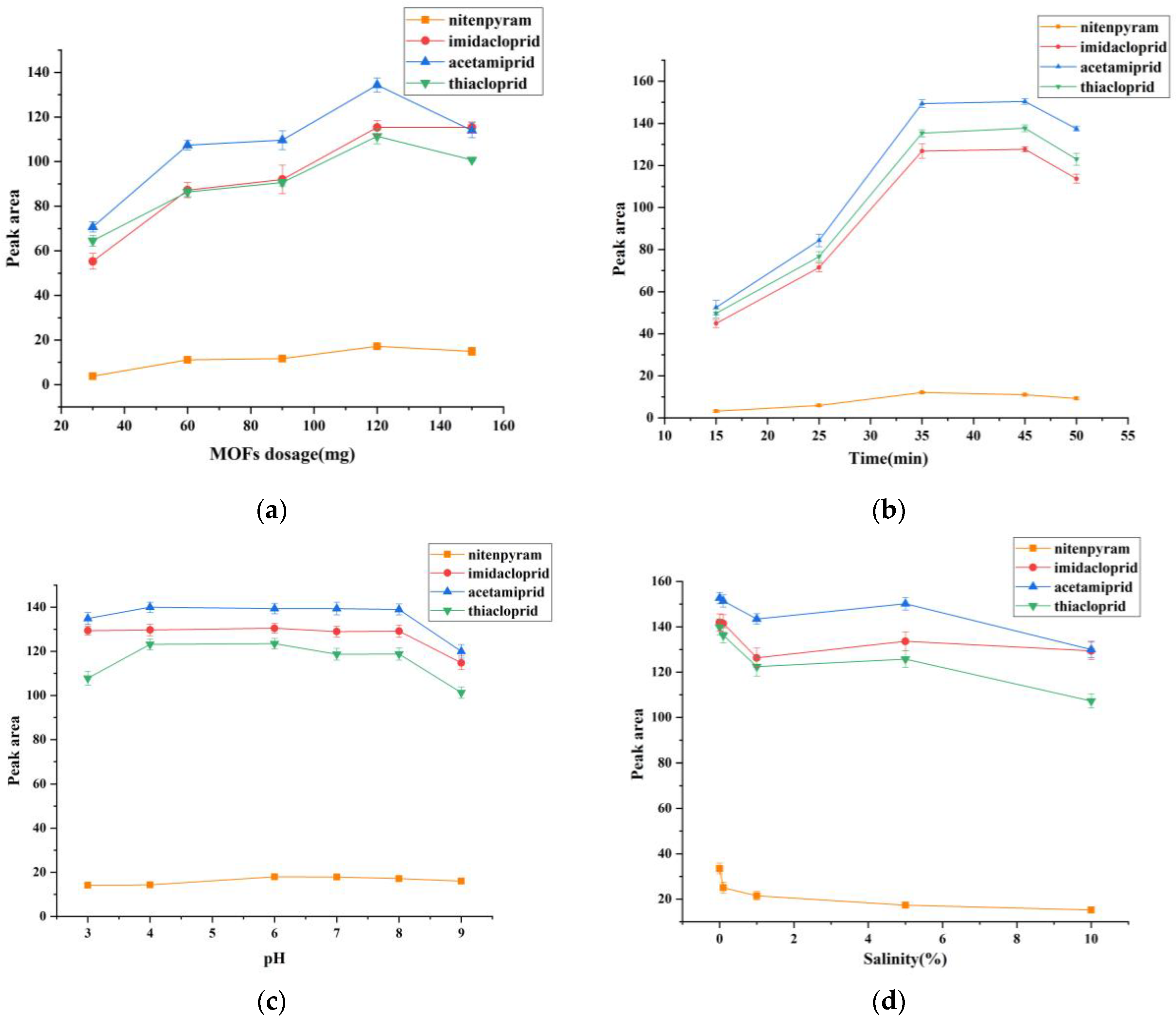
3.3.1. Effect of the Dosage of MOFs
3.3.2. Effect of Extraction Time
3.3.3. Effect of Sample Solution pH
3.3.4. Effect of Salt Concentration
3.3.5. Effect of Desorption Condition
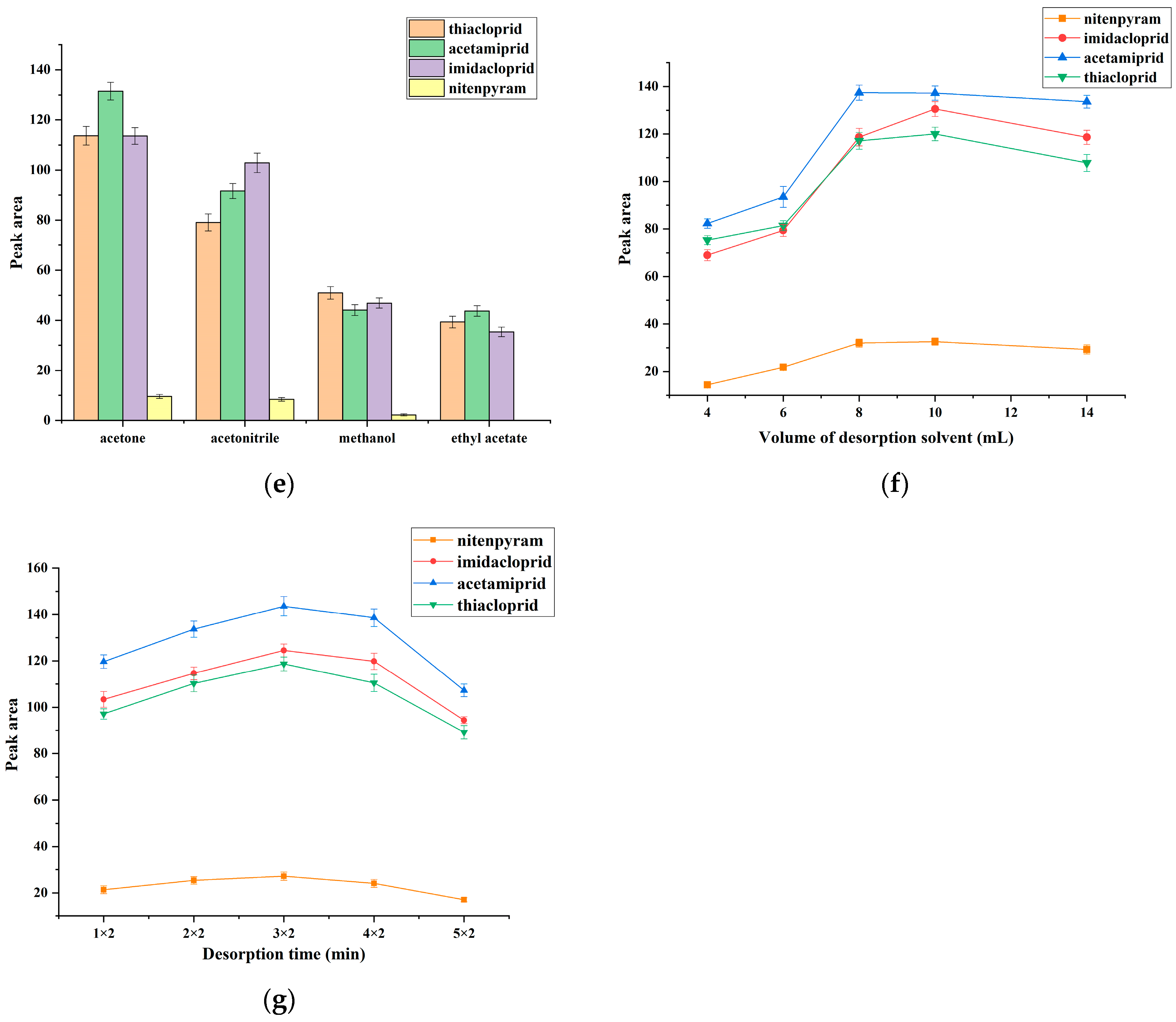
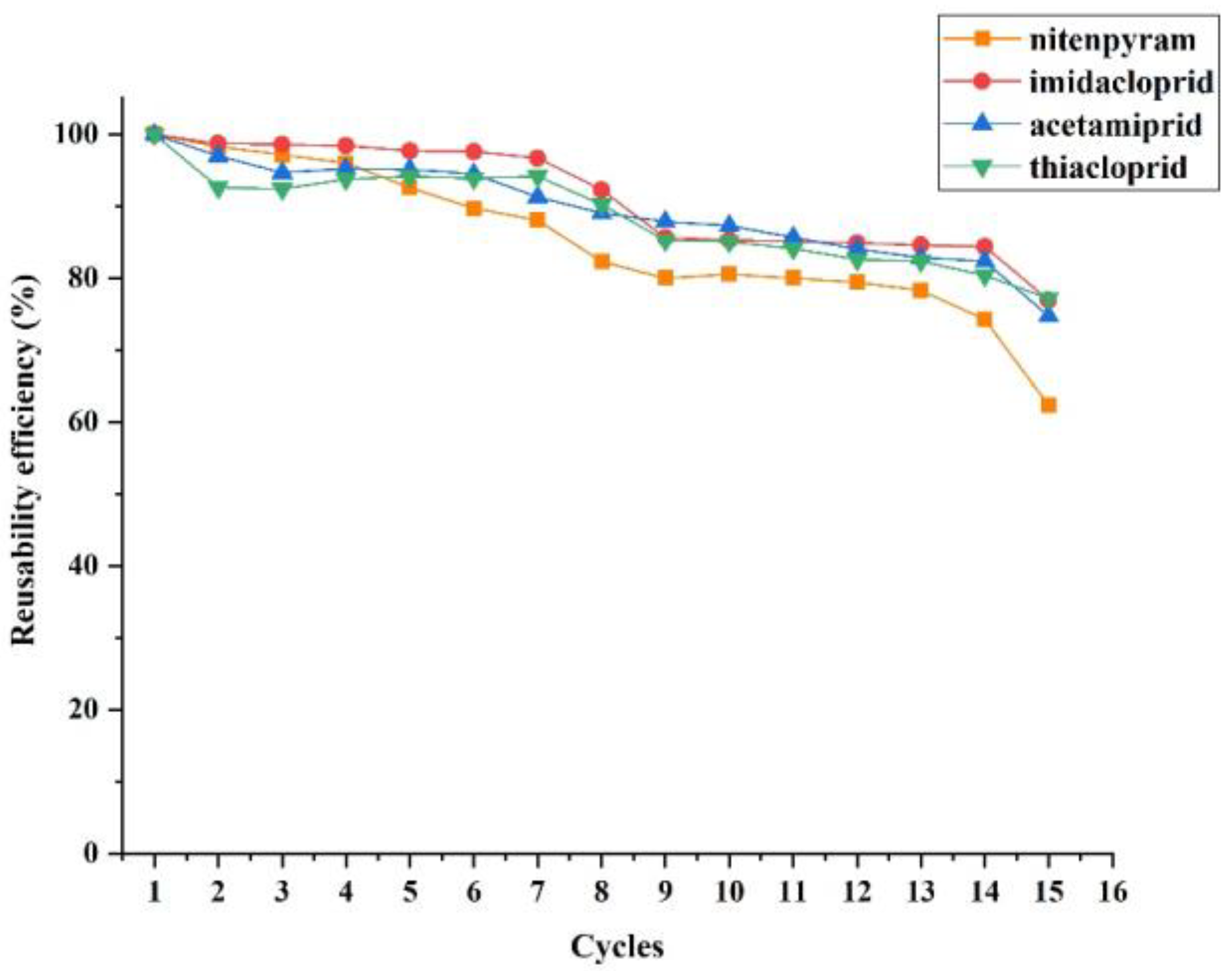
3.4. Regeneration of MIL-53 MMM for DME
3.5. Analytical Performance of the DME-HPLC Method
3.6. Application of the DME-HPLC Method to Real Water Samples
3.7. Method Performance Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goulson, D. Review: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Valverde, S.; Ibáñez, M.; Bernal, J.L.; Nozal, M.J.; Hernández, F.; Bernal, J. Development and validation of ultrahigh performance-liquid chromatography-tandem mass spectrometry based methods for the determination of neonicotinoid insecticides in honey. Food Chem. 2018, 266, 215–222. [Google Scholar] [CrossRef]
- Yin, K.; Deng, Y.; Liu, C.; He, Q.; Wei, Y.; Chen, S.; Liu, T.; Luo, S. Kinetics, pathways and toxicity evaluation of neonicotinoid insecticides degradation via UV/chlorine process. Chem. Eng. J. 2018, 346, 298–306. [Google Scholar] [CrossRef]
- Thuyet, D.Q.; Jorgenson, B.C.; Wissel-Tyson, C.; Watanabe, H.; Young, T.M. Wash off of imidacloprid and fipronil from turf and concrete surfaces using simulated rainfall. Sci. Total Environ. 2012, 414, 515–524. [Google Scholar] [CrossRef]
- DeLorenzo, M.E.; Thompson, B.; Cooper, E.; Moore, J.; Fulton, M.H. A long-term monitoring study of chlorophyll, microbial contaminants, and pesticides in a coastal residential stormwater pond and its adjacent tidal creek. Environ. Monit. Assess. 2012, 184, 343–359. [Google Scholar] [CrossRef]
- Wang, Z.; Brooks, B.W.; Zeng, E.Y.; You, J. Comparative mammalian hazards of neonicotinoid insecticides among exposure durations. Environ. Int. 2019, 125, 9–24. [Google Scholar] [CrossRef]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of neonicotinoid pesticide exposure on human health: A systematic review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [Green Version]
- Selahle, S.K.; Mpupa, A.; Nqombolo, A.; Nomngongo, P.N. A nanostructured o-hydroxyazobenzene porous organic polymer as an effective sorbent for the extraction and preconcentration of selected hormones and insecticides in river water. Microchem. J. 2022, 181, 107791. [Google Scholar] [CrossRef]
- Selahle, S.K.; Mpupa, A.; Nomngongo, P.N. Combination of zeolitic imidazolate framework-67 and magnetic porous porphyrin organic polymer for preconcentration of neonicotinoid insecticides in river water. J. Chromatogr. A 2022, 1661, 462685. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Zhu, X.; Hua, R.; Wu, X.; Xue, J. Combination of polyurethane and polymethyl methacrylate thin films as a microextraction sorbent for rapid adsorption and sensitive determination of neonicotinoid insecticides in fruit juice and tea by ultrahigh performance liquid chromatography with tandem mass spectrometry. J. Chromatogr. A 2021, 1659, 462646. [Google Scholar] [CrossRef]
- Zhou, Q.; Ding, Y.; Xiao, J. Sensitive determination of thiamethoxam, imidacloprid and acetamiprid in environmental water samples with solid-phase extraction packed with multiwalled carbon nanotubes prior to high-performance liquid chromatography. Anal. Bioanal. Chem. 2006, 385, 1520–1525. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Z.; Wang, S.; Hong, S.; Li, H.; Zhang, C.; Shao, Y.; She, Y.; Jin, F.; Jin, M.; et al. Metal-organic framework UiO-66 for rapid dispersive solid phase extraction of neonicotinoid insecticides in water samples. J. Chromatogr. B 2018, 1077–1078, 92–97. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Z.; Wang, S.; Hong, S.; Li, H.; Zhang, C.; Shao, Y.; She, Y.; Wang, J.; Jin, F.; et al. One-pot synthesis of magnetic zeolitic imidazolate framework/grapheme oxide composites for the extraction of neonicotinoid insecticides from environmental water samples. J. Sep. Sci. 2017, 40, 4747–4756. [Google Scholar] [CrossRef]
- Vichapong, J.; Burakham, R.; Srijaranai, S. Vortex-assisted surfactant- enhanced-emulsification liquid-liquid microextraction with solidification of floating organic droplet combined with HPLC for the determination of neonicotinoid pesticides. Talanta 2013, 117, 221–228. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, Q.; Zhang, G. Metal-organic framework composite membranes: Synthesis and separation applications. Chem. Eng. Sci. 2015, 135, 232–257. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Gandara, F.; Furukawa, H.; Lee, S.; Yaghi, O.M. High methane storage capacity in aluminum metal–organic frameworks. J. Am. Chem. Soc. 2014, 136, 5271–5274. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.; Xu, Q. Metal-organic frameworks as platforms for catalytic applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Rice, A.M.; Martin, C.R.; Shustova, N.B. Photochemistry and photophysics of MOFs: Steps towards MOF-based sensing enhancements. Chem. Soc. Rev. 2018, 47, 4710–4728. [Google Scholar] [CrossRef]
- Ma, M.; Lu, X.; Guo, Y.; Wang, L.; Liang, X. Combination of metal-organic frameworks (MOFs) and covalent organic frameworks (COFs): Recent advances in synthesis and analytical applications of MOF/COF composites. TrAC Trends Anal. Chem. 2022, 157, 116741. [Google Scholar] [CrossRef]
- Bautista, P.R.; Fernández, I.P.; Pasán, J.; Pino, V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings?—A review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Hao, L.; Liu, X.; Wang, J.; Wang, C.; Wu, Q.; Wang, Z. Use of ZIF-8-derived nanoporous carbon as the adsorbent for the solid phase extraction of carbamate pesticides prior to high-performance liquid chromatographic analysis. Talanta 2015, 142, 104–109. [Google Scholar] [CrossRef]
- Lirio, S.; Liu, W.; Lan, L.; Lin, C.; Huang, H. Aluminum based metal-organic framework-polymer monolith in solid-phase microextraction of penicillins in river water and milk samples. J. Chromatogr. A 2016, 1428, 236–245. [Google Scholar] [CrossRef]
- Lin, S.; Gan, N.; Qiao, L.; Zhang, J.; Cao, Y.; Chen, Y. Magnetic metal-organic frameworks coated stir bar sorptive extraction coupled with GC-MS for determination of polychlorinated biphenyls in fish samples. Talanta 2015, 144, 1139–1145. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Z.; Hou, L.; Lu, W.; Yang, Q.; Li, J.; Chen, L. Metal organic frameworks (MOFs) for magnetic solid-phase extraction of pyrazole/pyrrole pesticides in environmental water samples followed by HPLC-DAD determination. Talanta 2016, 161, 686–692. [Google Scholar] [CrossRef]
- Ma, J.; Wu, G.; Li, S.; Tan, W.; Wang, X.; Li, J.; Chen, L. Magnetic solid-phase extraction of heterocyclic pesticides in environmental water samples using metal-organic frameworks coupled to high performance liquid chromatography determination. J. Chromatogr. A 2018, 1553, 57–66. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Lv, K.; Chen, X. S-scheme photocatalyst TaON/Bi2WO6 nanofibers with oxygen vacancies for efficient abatement of antibiotics and Cr(VI): Intermediate eco-toxicity analysis and mechanistic insights. Chin. J. Catal. 2022, 43, 2652–2664. [Google Scholar] [CrossRef]
- Wang, C.; Yan, R.; Cai, M.; Li, S. A novel organic/inorganic S-scheme heterostructure of TCPP/Bi12O17Cl2 for boosting photodegradation of tetracycline hydrochloride: Kinetic, degradation mechanism, and toxic assessment. Appl. Surf. Sci. 2023, 610, 155346. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Wang, C.; Lin, W.; Li, S. Novel Cd0.5Zn0.5S/Bi2MoO6 S-scheme heterojunction for boosting the photodegradation of antibiotic enrofloxacin: Degradation pathway, mechanism and toxicity assessment. Sep. Purif. Technol. 2023, 304, 122401. [Google Scholar] [CrossRef]
- Denny, M.S.; Cohen, S.M. In Situ Modification of Metal-Organic Frameworks in Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2015, 54, 9029–9032. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal-organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Kang, Z.; Fan, L.; Sun, D. Recent advances and challenges of metal-organic framework membranes for gas separation. J. Mater. Chem. A 2017, 5, 10073–10091. [Google Scholar] [CrossRef]
- Bachman, J.E.; Smith, Z.P.; Li, T.; Xu, T.; Long, J.R. Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal-organic framework nanocrystals. Nat. Mater. 2016, 15, 845–849. [Google Scholar] [CrossRef]
- Shahid, S.; Baron, G.V.; Denayer, J.F.M.; Martens, J.A.; Wee, L.H.; Vankelecom, I.F.J. Hierarchical ZIF-8 composite membranes: Enhancing gas separation performance by exploiting molecular dynamics in hierarchical hybrid materials. J. Membr. Sci. 2021, 620, 118943. [Google Scholar] [CrossRef]
- Sompornpailin, D.; Ratanatawanate, C.; Sattayanon, C.; Namuangruk, S.; Punyapalakul, P. Selective adsorption mechanisms of pharmaceuticals on benzene-1,4-dicarboxylic acid-based MOFs: Effects of a flexible framework, adsorptive interactions and the DFT study. Sci. Total Environ. 2020, 720, 137449. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, Y.; Qiao, J.; Zhang, J.; McDonald, A.; Li, G.; Li, F. The removal of bisphenol A from aqueous solutions by MIL-53(Al) and mesostructured MIL-53(Al). J. Colloid Interface Sci. 2013, 405, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hamon, L.; Serre, C.; Devic, T.; Loiseau, T.; Millange, F.; Férey, G.; Weireld, G. Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal−Organic Frameworks at Room Temperature. J. Am. Chem. Soc. 2009, 131, 8775–8777. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zang, H.; Wang, X.; Cheng, J.; Zhao, R.; Cheng, C.; Lu, X. Metal-organic framework MIL-53(Al) as a solid-phase microextraction adsorbent for the determination of 16 polycyclic aromatic hydrocarbons in water samples by gas chromatography-tandem mass spectrometry. Analyst 2012, 137, 5411. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Guo, C.; Yu, H.; Shen, J.; Gao, C.; Sotto, A.; Bruggen, B. Fabrication of a MIL-53(Al) Nanocomposite Membrane and Potential Application in Desalination of Dye Solutions. Ind. Eng. Chem. Res. 2016, 55, 12099–12110. [Google Scholar] [CrossRef]
- Seoane, B.; Sorribas, S.; Mayoral, Á.; Téllez, C.; Coronas, J. Real-time monitoring of breathing of MIL-53(Al) by environmental SEM. Microporous Mesoporous Mater. 2015, 203, 17–23. [Google Scholar] [CrossRef]
- Gao, G.; Li, S.; Li, S.; Wang, Y.; Zhao, P.; Zhang, X.; Hou, X. A combination of computational−experimental study on metal-organic frameworks MIL-53(Al) as sorbent for simultaneous determination of estrogens and glucocorticoids in water and urine samples by dispersive micro-solid-phase extraction coupled to UPLC-MS/MS. Talanta 2018, 180, 358–367. [Google Scholar] [CrossRef]
- Hao, L.; Wang, C.; Wu, Q.; Li, Z.; Zang, X.; Wang, Z. Metal-organic framework derived magnetic nanoporous carbon: A novel adsorbent for magnetic solid-phase extraction. Anal. Chem. 2014, 24, 12199–12205. [Google Scholar] [CrossRef]
- Liu, L.; Hao, Y.; Zhou, X.; Wang, C.; Wu, Q.; Wang, Z. Magnetic porous carbon based solid-phase extraction coupled with high performance liquid chromatography for the determination of neonicotinoid insecticides in environmental water and peanut milk samples. Anal. Methods 2015, 7, 2762–2769. [Google Scholar] [CrossRef]



| Analyte | Regression Equation a | Correlation Coefficient (r2) | Linear Range (µg L−1) | LOD (µg L−1) | LOQ (µg L−1) |
|---|---|---|---|---|---|
| Nitenpyram | y = 1.764x − 0.6893 | 0.9937 | 0.20–15.00 | 0.064 | 0.190 |
| Imidacloprid | y = 12.801x − 1.0903 | 0.9957 | 0.04–15.00 | 0.013 | 0.038 |
| Acetamiprid | y = 14.542x + 0.0519 | 0.9960 | 0.05–15.00 | 0.017 | 0.050 |
| Thiacloprid | y = 12.638x − 2.8553 | 0.9901 | 0.04–15.00 | 0.014 | 0.041 |
| Insecticides | Spiked (µg L−1) | Intra-Day (n = 6) | Inter-Day (n = 6) | ||
|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| Nitenpyram | 0.50 | 112.17 | 12.04 | 111.03 | 12.55 |
| 5.00 | 104.19 | 10.04 | 87.97 | 9.02 | |
| 10.00 | 84.52 | 5.06 | 81.12 | 4.44 | |
| Imidacloprid | 0.50 | 111.25 | 3.07 | 119.68 | 13.12 |
| 5.00 | 104.27 | 3.95 | 105.48 | 6.37 | |
| 10.00 | 96.84 | 3.72 | 90.88 | 3.43 | |
| Acetamiprid | 0.50 | 92.53 | 8.53 | 80.43 | 12.65 |
| 5.00 | 85.02 | 4.19 | 90.08 | 4.92 | |
| 10.00 | 92.37 | 3.65 | 80.83 | 7.04 | |
| Thiacloprid | 0.50 | 106.11 | 12.78 | 96.14 | 11.97 |
| 5.00 | 95.40 | 6.97 | 88.28 | 6.39 | |
| 10.00 | 92.86 | 3.62 | 78.72 | 9.82 | |
| Insecticides | Spiked (μg L−1) | Tap Water | Surface Water | Sea Water | |||
|---|---|---|---|---|---|---|---|
| Found (μg L−1) | Recovery (% ± RSD, n = 3) | Found (μg L−1) | Recovery (% ± RSD, n = 3) | Found (μg L−1) | Recovery (% ± RSD, n = 3) | ||
| Nitenpyram | 0.00 | ND | ND | ND | |||
| 0.50 | 0.43 | 86.09 ± 12.91 | 0.57 | 114.06 ± 11.96 | 0.58 | 115.57 ± 10.93 | |
| 5.00 | 3.96 | 79.24 ± 5.10 | 5.34 | 106.83 ± 6.31 | 5.54 | 110.99 ± 6.12 | |
| 10.00 | 12.1 | 72.50 ± 11.58 | 9.03 | 90.26 ± 4.93 | 8.80 | 88.00 ± 13.25 | |
| Imidacloprid | 0.00 | ND | ND | ND | |||
| 0.50 | 0.40 | 79.53 ± 12.66 | 0.56 | 112.33 ± 7.9 | 0.57 | 114.94 ± 8.03 | |
| 5.00 | 3.88 | 77.64 ± 3.59 | 4.41 | 88.10 ± 3.83 | 5.47 | 109.45 ± 2.28 | |
| 10.00 | 7.38 | 73.81 ± 10.59 | 7.51 | 75.14 ± 5.79 | 10.29 | 102.88 ± 3.46 | |
| Acetamiprid | 0.00 | ND | ND | ND | |||
| 0.50 | 0.51 | 101.06 ± 10.31 | 0.45 | 90.52 ± 11.11 | 0.38 | 75.85 ± 7.26 | |
| 5.00 | 3.96 | 79.14 ± 3.30 | 4.01 | 80.25 ± 7.36 | 5.69 | 113.85 ± 3.33 | |
| 10.00 | 7.42 | 74.23 ± 4.62 | 8.11 | 81.11 ± 3.59 | 9.50 | 94.95 ± 8.70 | |
| Thiacloprid | 0.00 | ND | ND | ND | |||
| 0.50 | 0.59 | 117.98 ± 13.76 | 0.59 | 117.98 ± 7.16 | 0.57 | 113.76 ± 9.33 | |
| 5.00 | 3.76 | 75.26 ± 5.98 | 4.20 | 83.96 ± 5.77 | 5.79 | 115.82 ± 4.62 | |
| 10.00 | 7.66 | 76.56 ± 1.08 | 8.22 | 82.18 ± 5.64 | 9.90 | 98.95 ± 4.13 | |
| Insecticides | Pretreatment Technique | Adsorbents | Detection Techniques | LOD (μg L−1) | LOQ (μg L−1) | Reusability | Refs. |
|---|---|---|---|---|---|---|---|
| Imidacloprid, acetamiprid, thiacloprid, thiamethoxam | Magnetic solid phase extraction | Magnetic nanoporous carbon | HPLC-UV | 0.01–0.06 | NA | NA | [47] |
| Imidacloprid, acetamiprid, thiamethoxam thiacloprid | Magnetic solid phase extraction | Magnetic porous carbon | HPLC-UV | 0.1–0.2 | NA | NA | [48] |
| Dinotefuran, thiamethoxam, clothianidin, imidacloprid, acetamiprid, thiacloprid | Magnetic solid phase extraction | Magnetic zeolitic imidazolate framework/grapheme oxide | HPLC-MS/MS | 0.06–1.0 | 0.2–3.0 | NA | [15] |
| Dinotefuran, thiamethoxam, clothianidin, imidacloprid, acetamiprid and thiacloprid | Dispersive solid phase extraction | UiO-66 | HPLC-MS/MS | 0.02–0.4 | 0.05–1 | NA | [14] |
| Idacloprid, acetamiprid, thiacloprid, nitenpyram | Dispersion membrane extraction | MIL-53(Al) MMM | HPLC-DAD | 0.01–0.06 | 0.038–0.19 | 13 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Ma, J.; Wei, C.; Li, S.; Li, J.; Wang, X.; Chen, L. Determination of Neonicotinoid Insecticides in Environmental Water by the Enrichment of MIL-53 Mixed Matrix Membrane Coupled with High Performance Liquid Chromatography. Int. J. Environ. Res. Public Health 2023, 20, 715. https://doi.org/10.3390/ijerph20010715
Wu G, Ma J, Wei C, Li S, Li J, Wang X, Chen L. Determination of Neonicotinoid Insecticides in Environmental Water by the Enrichment of MIL-53 Mixed Matrix Membrane Coupled with High Performance Liquid Chromatography. International Journal of Environmental Research and Public Health. 2023; 20(1):715. https://doi.org/10.3390/ijerph20010715
Chicago/Turabian StyleWu, Gege, Jiping Ma, Chenxi Wei, Shuang Li, Jinhua Li, Xiaoyan Wang, and Lingxin Chen. 2023. "Determination of Neonicotinoid Insecticides in Environmental Water by the Enrichment of MIL-53 Mixed Matrix Membrane Coupled with High Performance Liquid Chromatography" International Journal of Environmental Research and Public Health 20, no. 1: 715. https://doi.org/10.3390/ijerph20010715








