Lifestyle and Health-Related Quality of Life Relationships Concerning Metabolic Disease Phenotypes on the Nutrimdea Online Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Physical Activity
2.3. Mediterranean Diet
2.4. Obesogenic Score (ObS)
2.5. Statistical Analyses
3. Results
4. Discussion
5. Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, J.E.; Stevens, G.A.; Mathers, C.D.; Bonita, R.; Rehm, J.; Kruk, M.E.; Riley, L.M.; Dain, K.; Kengne, A.P.; Chalkidou, K.; et al. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 19 June 2022).
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Alshammary, A.F.; Khan, I.A. Screening of Obese Offspring of First-Cousin Consanguineous Subjects for the Angiotensin-Converting Enzyme Gene with a 287-bp Alu Sequence. J. Obes. Metab. Syndr. 2021, 30, 63–71. [Google Scholar] [CrossRef]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Lonnie, M.; Wadolowska, L. Empirically derived dietary-lifestyle patterns and cardiometabolic health in young men: A review. Proc. Nutr. Soc. 2020, 79, 324–330. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 29 November 2022).
- Soriano, J.B.; Rojas-Rueda, D.; Alonso, J.; Antó, J.M.; Cardona, P.-J.; Fernández, E.; Garcia-Basteiro, A.L.; Benavides, F.G.; Glenn, S.D.; Krish, V.; et al. The burden of disease in Spain: Results from the Global Burden of Disease 2016. Med. Clin. 2018, 151, 171–190. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Miranda, N.; Cade, B.E.; Huang, T.; Redline, S.; Karlson, E.W.; Saxena, R. Interaction of obesity polygenic score with lifestyle risk factors in an electronic health record biobank. BMC Med. 2022, 20, 5. [Google Scholar] [CrossRef]
- Puchulu, F.M. Síndrome Metabólico. Revista Médica Separata 2008, 16, 1–28. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, P.H.; Jeejeebhoy, K.; Dhaliwal, R.; Royall, D.; Brauer, P.; Tremblay, A.; Klein, D.; Mutch, D.M. Lifestyle genomics and the metabolic syndrome: A review of genetic variants that influence response to diet and exercise interventions. Crit. Rev. Food Sci. Nutr. 2019, 59, 2028–2039. [Google Scholar] [CrossRef]
- Alshammary, A.F.; Alharbi, K.K.; Alshehri, N.J.; Vennu, V.; Ali Khan, I. Metabolic Syndrome and Coronary Artery Disease Risk: A Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 1773. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; MM Alberti, K.G.; Serrano Ríos, M. A new international diabetes federation worldwide definition of the metabolic syndrome: The rationale and the results. Rev. Esp. Cardiol. 2005, 58, 1371–1376. [Google Scholar]
- Graham, H.; White, P.C.L. Social determinants and lifestyles: Integrating environmental and public health perspectives. Public Health 2016, 141, 270–278. [Google Scholar] [CrossRef]
- Macia, L.; Galy, O.; Nanan, R.K.H. Editorial: Modern Lifestyle and Health: How Changes in the Environment Impacts Immune Function and Physiology. Front. Immunol. 2021, 12, 762166. [Google Scholar] [CrossRef]
- Owen, L.; Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef]
- Katz, D.L.; Meller, S. Can we say what diet is best for health? Annu. Rev. Public Health 2014, 35, 83–103. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef]
- Hu, F.B. Diet and lifestyle influences on risk of coronary heart disease. Curr. Atheroscler. Rep. 2009, 11, 257–263. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Martini, D. Health Benefits of Mediterranean Diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Alvarez, I.; Toledo, E.; Lecea, O.; Salas-Salvadó, J.; Corella, D.; Buil-Cosiales, P.; Zomeño, M.D.; Vioque, J.; Martinez, J.A.; Konieczna, J.; et al. Adherence to a priori dietary indexes and baseline prevalence of cardiovascular risk factors in the PREDIMED-Plus randomised trial. Eur. J. Nutr. 2020, 59, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- González-Gross, M.; Meléndez, A. Sedentarism, active lifestyle and sport: Impact on health and obesity prevention. Nutr. Hosp. 2013, 28 (Suppl. 5), 89–98. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef]
- Pareja-Galeano, H.; Garatachea, N.; Lucia, A. Exercise as a Polypill for Chronic Diseases. Prog. Mol. Biol. Transl. Sci. 2015, 135, 497–526. [Google Scholar] [CrossRef]
- Piepoli, M.F. Exercise Rehabilitation in Heart Disease: The real “polypill” for primary and secondary prevention. Monaldi Arch. Chest Dis. 2005, 64, 88–93. [Google Scholar] [CrossRef]
- Teixeira-Lemos, E.; Nunes, S.; Teixeira, F.; Reis, F. Regular physical exercise training assists in preventing type 2 diabetes development: Focus on its antioxidant and anti-inflammatory properties. Cardiovasc. Diabetol. 2011, 10, 12. [Google Scholar] [CrossRef]
- World Health Organization. Preamble to the Constitution of the World Health Organization; World Health Organization: Geneva, Switzerland, 1948. [Google Scholar]
- Huang, F.; Li, H. Factors influencing integrated wellbeing in older Chinese outpatients with chronic diseases. Aust. J. Prim. Health 2018, 24, 189–195. [Google Scholar] [CrossRef]
- Karimi, M.; Brazier, J. Health, Health-Related Quality of Life, and Quality of Life: What is the Difference? PharmacoEconomics 2016, 34, 645–649. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHOQOL: Measuring Quality of Life. Available online: https://www.who.int/tools/whoqol (accessed on 29 July 2022).
- Wiklund, I. The Nottingham Health Profile—A measure of health-related quality of life. Scand. J. Prim. Health Care 1990, 1, 15–18. [Google Scholar]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretation of SF-36 and SF-12 questionnaires in Spain: Physical and mental components. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Vilagut, G.; Garin, O.; Cunillera, O.; Tresserras, R.; Brugulat, P.; Mompart, A.; Medina, A.; Ferrer, M.; Alonso, J. Reference guidelines for the 12-Item Short-Form Health Survey version 2 based on the Catalan general population. Med. Clin. 2012, 139, 613–625. [Google Scholar] [CrossRef]
- de Cuevillas, B.; Álvarez-Álvarez, I.; Cuervo, M.; Fernández-Montero, A.; Navas-Carretero, S.; Martínez, J.A. Definition of nutritionally qualitative categorizing (proto)nutritypes and a pilot quantitative nutrimeter for mirroring nutritional well-being based on a quality of life health related questionnaire. Nutr. Hosp. 2019, 36, 862–874. [Google Scholar] [CrossRef]
- König, I.R.; Fuchs, O.; Hansen, G.; von Mutius, E.; Kopp, M.V. What is precision medicine? Eur. Respir. J. 2017, 50, 1700391. [Google Scholar] [CrossRef]
- Wantland, D.J.; Portillo, C.J.; Holzemer, W.L.; Slaughter, R.; McGhee, E.M. The Effectiveness of Web-Based vs. Non-Web-Based Interventions: A Meta-Analysis of Behavioral Change Outcomes. J. Med. Internet Res. 2004, 6, e40. [Google Scholar] [CrossRef]
- Rowen, D.; Carlton, J.; Elliott, J. PROM Validation Using Paper-Based or Online Surveys: Data Collection Methods Affect the Sociodemographic and Health Profile of the Sample. Value Health 2019, 22, 845–850. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Schröder, H.; Bawaked, R.A.; Ribas-Barba, L.; Izquierdo-Pulido, M.; Roman-Viñas, B.; Fíto, M.; Serra-Majem, L. Cumulative Effect of Obesogenic Behaviours on Adiposity in Spanish Children and Adolescents. Obes. Facts 2017, 10, 584–596. [Google Scholar] [CrossRef]
- Bibiloni, M.D.M.; Gallardo-Alfaro, L.; Gómez, S.F.; Wärnberg, J.; Osés-Recalde, M.; González-Gross, M.; Gusi, N.; Aznar, S.; Marín-Cascales, E.; González-Valeiro, M.; et al. Combined Body Mass Index and Waist-to-Height Ratio and Its Association with Lifestyle and Health Factors among Spanish Children: The PASOS Study. Nutrients 2022, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Gomez, A.; Ribot-Rodriguez, R.; San-Cristobal, R.; Martín-Hernández, R.; Mico, V.; Espinosa-Salinas, I.; Ramirez de Molina, A.; Martinez, J.A. HRQoL and nutritional well-being dissimilarities between two different online collection methods: Value for digital health implementation. Digit. Health 2022, 8, 20552076221138316. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Muñoz, S.; Corella, C.; Abarca-Sos, A.; Zaragoza, J. Validation of three short physical activity questionnaires with accelerometers among university students in Spain. J. Sports Med. Phys. Fit. 2017, 57, 1660–1668. [Google Scholar]
- Mantilla Toloza, S.; Gómez-Conesa, A. International Physical Activity Questionnaire. An adequate instrument in population physical activity monitoring. Rev. Iberoam. Fisioter. Kinesiol. 2007, 10, 48–52. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 2019, 366, l4570. [Google Scholar] [CrossRef]
- Sayón-Orea, C.; Santiago, S.; Bes-Rastrollo, M.; Martínez-González, M.A.; Pastor, M.R.; Moreno-Aliaga, M.J.; Tur, J.A.; Garcia, A.; Martínez, J.A. Determinants of Self-Rated Health Perception in a Sample of a Physically Active Population: PLENUFAR VI Study. Int. J. Environ. Res. Public Health 2018, 15, 2104. [Google Scholar] [CrossRef]
- Woodward, M. Cardiovascular Disease and the Female Disadvantage. Int. J. Environ. Res. Public Health 2019, 16, 1165. [Google Scholar] [CrossRef]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Lecube, A.; Sánchez, E.; Monereo, S.; Medina-Gómez, G.; Bellido, D.; García-Almeida, J.M.; Martínez de Icaya, P.; Malagón, M.M.; Goday, A.; Tinahones, F.J.; et al. Factors Accounting for Obesity and Its Perception among the Adult Spanish Population: Data from 1,000 Computer-Assisted Telephone Interviews. Obes. Facts 2020, 13, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ribot-Rodriguez, R.; Higuera-Gomez, A.; San-Cristobal, R.; Martín-Hernández, R.; Micó, V.; Espinosa-Salinas, I.; Ramírez de Molina, A.; Martínez, J.A. Cardiometabolic Health Status, Ethnicity and Health-Related Quality of Life (HRQoL) Disparities in an Adult Population: NutrIMDEA Observational Web-Based Study. Int. J. Environ. Res. Public Health 2022, 19, 2948. [Google Scholar] [CrossRef] [PubMed]
- Encuesta Europea de Salud en España (EESE); Instituto Nacional de Estadística (INE): Madrid, Spain, 2020.
- Fernández-Bergés, D.; Cabrera De León, A.; Sanz, H.; Elosua, R.; Guembe, M.J.; Alzamora, M.; Vega-Alonso, T.; Félix-Redondo, F.J.; Ortiz-Marrón, H.; Rigo, F.; et al. Metabolic syndrome in Spain: Prevalence and coronary risk associated with harmonized definition and who proposal. DARIOS study. Rev. Esp. Cardiol. 2012, 65, 241–248. [Google Scholar] [CrossRef]
- van Dongen, J.; Willemsen, G.; Chen, W.-M.; de Geus, E.J.C.; Boomsma, D.I. Heritability of metabolic syndrome traits in a large population-based sample. J. Lipid Res. 2013, 54, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Sieverding, M.; Arbogast, A.L.; Zintel, S.; von Wagner, C. Gender differences in self-reported family history of cancer: A review and secondary data analysis. Cancer Med. 2020, 9, 7772–7780. [Google Scholar] [CrossRef] [PubMed]
- Krakow, M.; Rising, C.J.; Trivedi, N.; Yoon, D.C.; Vanderpool, R.C. Prevalence and Correlates of Family Cancer History Knowledge and Communication Among US Adults. Prev. Chronic Dis. 2020, 17, E146. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Bullis, E.; Myers, R.; Zhou, C.J.; Cai, E.M.; Sharma, A.; Bhatia, S.; Orlando, L.A.; Haga, S.B. Awareness of family health history in a predominantly young adult population. PLoS ONE 2019, 14, e0224283. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad. Informe Anual del Sistema Nacional de Salud 2020–2021. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/sisInfSanSNS/tablasEstadisticas/InfAnualSNS2020_21/INFORME_ANUAL_2020_21.pdf (accessed on 17 August 2022).
- Mathus-Vliegen, E.M. Obesity and the elderly. J. Clin. Gastroenterol. 2012, 46, 533–544. [Google Scholar] [CrossRef]
- Serra, M.C.; Dondero, K.R.; Larkins, D.; Burns, A.; Addison, O. Healthy Lifestyle and Cognition: Interaction between Diet and Physical Activity. Curr. Nutr. Rep. 2020, 9, 64–74. [Google Scholar] [CrossRef]
- Cao, Z.-B.; Sasaki, A.; Oh, T.; Miyatake, N.; Tsushita, K.; Higuchi, M.; Sasaki, S.; Tabata, I. Association between dietary intake of micronutrients and cardiorespiratory fitness in Japanese men. J. Nutr. Sci. 2012, 1, e12. [Google Scholar] [CrossRef]
- Gilbert, L.; Gross, J.; Lanzi, S.; Quansah, D.Y.; Puder, J.; Horsch, A. How diet, physical activity and psychosocial well-being interact in women with gestational diabetes mellitus: An integrative review. BMC Pregnancy Childbirth 2019, 19, 60. [Google Scholar] [CrossRef]
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska, K.; Boraczyński, M.; Tang, Y.-Y.; Gronek, J.; Wochna, K.; Boraczyński, T.; Wieliński, D.; Gronek, P. Protective Effects of Exercise Become Especially Important for the Aging Immune System in The Covid-19 Era. Aging Dis. 2022, 13, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Ekblom-Bak, E.; Börjesson, M.; Bergman, F.; Bergström, G.; Dahlin-Almevall, A.; Drake, I.; Engström, G.; Engvall, J.E.; Gummesson, A.; Hagström, E.; et al. Accelerometer derived physical activity patterns in 27.890 middle-aged adults: The SCAPIS cohort study. Scand. J. Med. Sci. Sports 2022, 32, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Khunti, K.K.; Zaccardi, F.; Rowlands, A.V.; Yates, T.; Gillies, C.L.; Davies, M.J.; Dhalwani, N.N. Physical activity, multimorbidity, and life expectancy: A UK Biobank longitudinal study. BMC Med. 2019, 17, 108. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Yeh, T.-L.; Hsu, H.-Y.; Hsu, L.-Y.; Lee, C.-C.; Tseng, P.-J.; Chien, K.-L. Comparison of four healthy lifestyle scores for predicting cardiovascular events in a national cohort study. Sci. Rep. 2021, 11, 22146. [Google Scholar] [CrossRef]
- Chudasama, Y.V.; Khunti, K.; Gillies, C.L.; Dhalwani, N.N.; Davies, M.J.; Yates, T.; Zaccardi, F. Healthy lifestyle and life expectancy in people with multimorbidity in the UK Biobank: A longitudinal cohort study. PLoS Med. 2020, 17, e1003332. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef]
- Martínez-Vizcaíno, V.; Amaro-Gahete, F.J.; Fernández-Rodríguez, R.; Garrido-Miguel, M.; Cavero-Redondo, I.; Pozuelo-Carrascosa, D.P. Effectiveness of Fixed-Dose Combination Therapy (Polypill) Versus Exercise to Improve the Blood-Lipid Profile: A Network Meta-analysis. Sports Med. 2022, 52, 1161–1173. [Google Scholar] [CrossRef]
- Lecube, A.; Sánchez, E.; Andrés, A.; Saldaña, C.; Morales, M.J.; Calañas, A.; Miñambres, I.; Pellitero, S.; Cordido, F.; Bueno, M.; et al. Assessing Motivational Stages and Processes of Change for Weight Management Around Bariatric Surgery: A Multicenter Study. Obes. Surg. 2019, 29, 3348–3356. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, K.; Or, C.; Chen, J.; Yan, M.; Wang, H. An examination of the socio-demographic correlates of patient adherence to self-management behaviors and the mediating roles of health attitudes and self-efficacy among patients with coexisting type 2 diabetes and hypertension. BMC Public Health 2020, 20, 1227. [Google Scholar] [CrossRef] [PubMed]
- Barcones-Molero, M.F.; Sánchez-Villegas, A.; Martínez-González, M.A.; Bes-Rastrollo, M.; Martínez-Urbistondo, M.; Santabárbara, J.; Martínez, J.A. The influence of obesity and weight gain on quality of life according to the SF-36 for individuals of the dynamic follow-up cohort of the University of Navarra. Rev. Clin. Esp. 2018, 218, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.G.S.; Nogueras, D.; van Woerden, H.C.; Kiparoglou, V. The COVID-19 Pandemic: A Pandemic of Lockdown Loneliness and the Role of Digital Technology. J. Med. Internet Res. 2020, 22, e22287. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.; López, M.T.I.; Miguel, M.; Garcés-Rimón, M. Physical and Psychological Effects Related to Food Habits and Lifestyle Changes Derived from Covid-19 Home Confinement in the Spanish Population. Nutrients 2020, 12, 3445. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartín, A.; Sampedro-Nuñez, M.A.; Dávalos, A.; Marazuela, M. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef]
- Martínez-de-Quel, Ó.; Suárez-Iglesias, D.; López-Flores, M.; Pérez, C.A. Physical activity, dietary habits and sleep quality before and during COVID-19 lockdown: A longitudinal study. Appetite 2021, 158, 105019. [Google Scholar] [CrossRef]
- Caroppo, E.; Mazza, M.; Sannella, A.; Marano, G.; Avallone, C.; Claro, A.E.; Janiri, D.; Moccia, L.; Janiri, L.; Sani, G. Will Nothing Be the Same Again?: Changes in Lifestyle during COVID-19 Pandemic and Consequences on Mental Health. Int. J. Environ. Res. Public Health 2021, 18, 8433. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Leng, Y.; Peeters, G.M.; Kaup, A.R.; Allen, I.E.; Yaffe, K. Interventions involving a major dietary component improve cognitive function in cognitively healthy adults: A systematic review and meta-analysis. Nutr. Res. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Jacka, F.N.; Mykletun, A.; Berk, M. Moving towards a population health approach to the primary prevention of common mental disorders. BMC Med. 2012, 10, 149. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P., Jr.; Yaffe, K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 2005, 330, 1360. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, B.; Jeannot, E.; Razurel, C. Determinants of Health Behaviors After Gestational Diabetes Mellitus: A Prospective Cohort Study in Geneva. J. Midwifery Women’s Health 2016, 61, 571–577. [Google Scholar] [CrossRef]
- Ross, L.J.; Barnes, K.A.; Ball, L.E.; Mitchell, L.J.; Sladdin, I.; Lee, P.; Williams, L.T. Effectiveness of dietetic consultation for lowering blood lipid levels in the management of cardiovascular disease risk: A systematic review and meta-analysis of randomised controlled trials. Nutr. Diet. 2019, 76, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.J.; Ball, L.E.; Ross, L.J.; Barnes, K.A.; Williams, L.T. Effectiveness of Dietetic Consultations in Primary Health Care: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2017, 117, 1941–1962. [Google Scholar] [CrossRef]
- Ibarra, J.L.; Agas, J.M.; Lee, M.; Pan, J.L.; Buttenheim, A.M. Comparison of Online Survey Recruitment Platforms for Hard-to-Reach Pregnant Smoking Populations: Feasibility Study. JMIR Res. Protoc. 2018, 7, e101. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Foster, H.; O’Donovan, C.; Woolhead, C.; Marsaux, C. Validation of Web-based self-reported socio-demographic and anthropometric data collected in the Food4Me Study. Proc. Nutr. Soc. 2014, 73, E78. [Google Scholar] [CrossRef]
- Heponiemi, T.; Kaihlanen, A.-M.; Kouvonen, A.; Leemann, L.; Taipale, S.; Gluschkoff, K. The role of age and digital competence on the use of online health and social care services: A cross-sectional population-based survey. Digit. Health 2022, 8, 20552076221074485. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sánchez-Villegas, A.; De Irala, J.; Marti, A.; Martínez, J.A. Mediterranean Diet and Stroke: Objectives and Design of the SUN Project. Nutr. Neurosci. 2002, 5, 65–73. [Google Scholar] [CrossRef]
- Bao, Y.; Bertoia, M.L.; Lenart, E.B.; Stampfer, M.J.; Willett, W.C.; Speizer, F.E.; Chavarro, J.E. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am. J. Public Health 2016, 106, 1573–1581. [Google Scholar] [CrossRef]
- Khandpur, N.; Rossato, S.; Drouin-Chartier, J.-P.; Du, M.; Steele, E.M.; Sampson, L.; Monteiro, C.; Zhang, F.F.; Willett, W.; Fung, T.T.; et al. Categorising ultra-processed foods in large-scale cohort studies: Evidence from the Nurses’ Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. J. Nutr. Sci. 2021, 10, e77. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Berinsky, A.J.; Huber, G.A.; Lenz, G.S. Evaluating Online Labor Markets for Experimental Research: Amazon.com’s Mechanical Turk. Political Anal. 2012, 20, 351–368. [Google Scholar] [CrossRef]
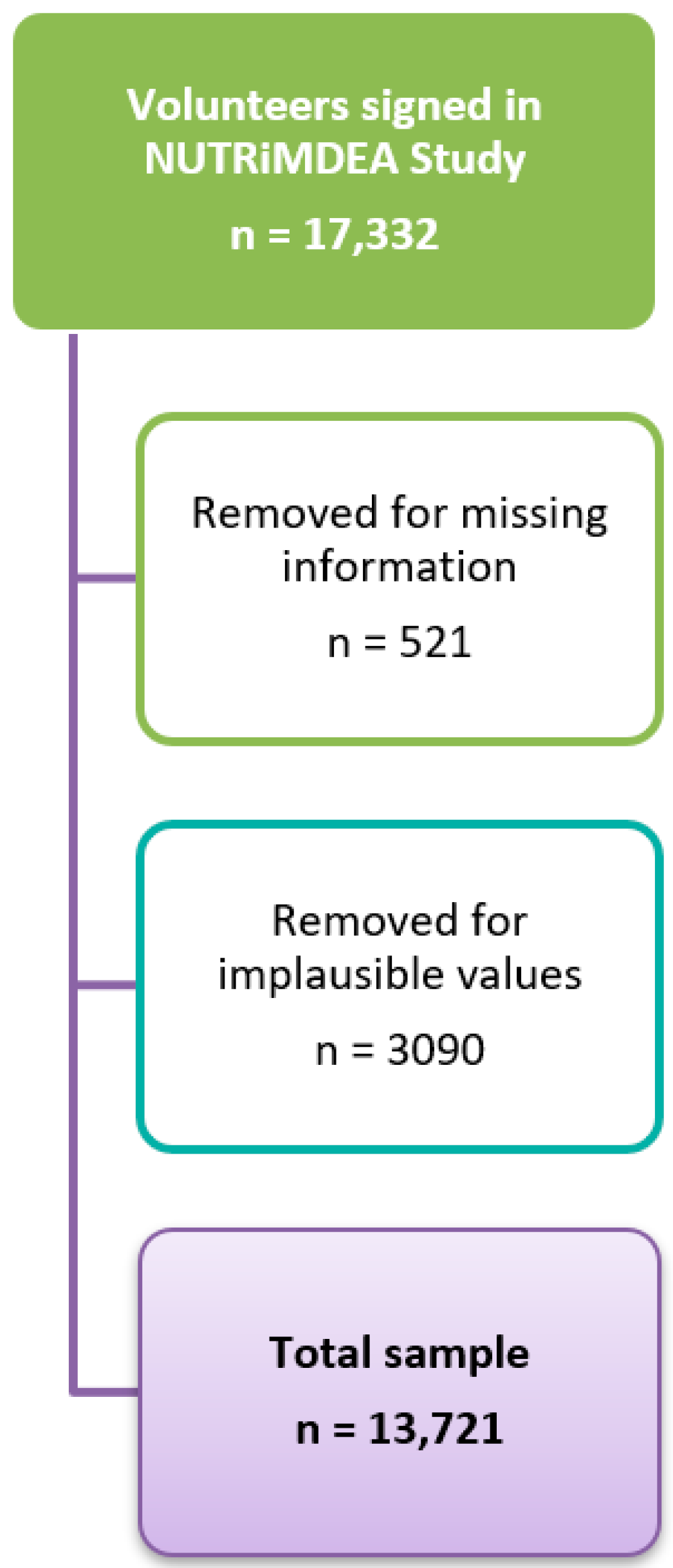
| Sex a | Age | ||||
|---|---|---|---|---|---|
| Overall | Female | Male | <40 Years | >40 Years | |
| n | 13,721 | 9031 | 4690 | 5335 | 8386 |
| Age, n (%) >40 years | 8386 (61.1) | 5361 (59.4) | 3025 (64.5) * | 0 (0.0) | 8386 (100.0) * |
| Sex, n (%) of female | 9031 (65.8) | 9031 (100.0) | 0 (0.0) * | 3670 (68.8) | 5361 (63.9) * |
| BMI b, mean (SD) | 24.07 (3.56) | 23.48 (3.62) | 25.22 (3.15) * | 23.18 (3.45) | 24.64 (3.52) * |
| Trousers size, mean (SD) | 40.47 (3.98) | 39.75 (3.43) | 41.86 (4.56) * | 39.14 (3.75) | 41.31 (3.89) * |
| Ethnicity, n (%) * | |||||
| Caucasian/European | 9063 (66.1) | 6204 (68.7) | 2859 (61.0) | 3457 (64.8) | 5606 (66.8) |
| Hispanic/Latino/a | 4145 (30.2) | 2483 (27.5) | 1662 (35.4) | 1579 (29.6) | 2566 (30.6) |
| Other c | 513 (3.7) | 344 (3.8) | 169 (3.6) | 299 (5.6) | 214 (2.6) |
| Education, n (%) * | |||||
| Compulsory/Professional Education | 3330 (24.3) | 1874 (20.8) | 1456 (31.0) | 1383 (25.9) | 1947 (23.2) |
| Higher education | 10,216 (74.5) | 7035 (77.9) | 3181 (67.8) | 3874 (72.6) | 6342 (75.6) |
| Other | 175 (1.3) | 122 (1.4) | 53 (1.1) | 78 (1.5) | 97 (1.2) |
| Work, n (%) * | |||||
| Employed | 10,202 (74.4) | 6744 (74.7) | 3458 (73.7) | 3676 (68.9) | 6526 (77.8) |
| Unemployed | 2565 (18.7) | 1673 (18.5) | 892 (19.0) | 848 (15.9) | 1717 (20.5) |
| Student | 954 (7.0) | 614 (6.8) | 340 (7.2) | 811 (15.2) | 143 (1.7) |
| Obesity, n (%) | 695 (5.1) | 415 (4.6) | 280 (6.0) * | 238 (4.5) | 457 (5.4) * |
| Diabetes, n (%) | 423 (3.1) | 201 (2.2) | 222 (4.7) * | 118 (2.2) | 305 (3.6) * |
| HBP d, n (%) | 1185 (8.6) | 545 (6.0) | 640 (13.6) * | 144 (2.7) | 1041 (12.4) * |
| Dyslipidemia, n (%) | 2067 (15.1) | 1192 (13.2) | 875 (18.7) * | 341 (6.4) | 1726 (20.6) * |
| Family obesity, n (%) | 2310 (16.8) | 1533 (17.0) | 777 (16.6) | 952 (17.8) | 1358 (16.2) * |
| Family diabetes, n (%) | 3924 (28.6) | 2622 (29.0) | 1302 (27.8) * | 1426 (26.7) | 2498 (29.8) * |
| Family HBP c, n (%) | 6387 (46.5) | 4489 (49.7) | 1898 (40.5) * | 2051 (38.4) | 4336 (51.7) * |
| Family dyslipidemia, n (%) | 5744 (41.9) | 4131 (45.7) | 1613 (34.4) * | 2129 (39.9) | 3615 (43.1) * |
| Depress, n (%) | 5256 (38.3) | 3760 (41.6) | 1496 (31.9) * | 2538 (47.6) | 2718 (32.4) * |
| Smoking habit, n (%) | 2497 (18.2) | 1501 (16.6) | 996 (21.2) * | 1040 (19.5) | 1457 (17.4) * |
| PCS12 e, mean (SD) | 53.67 (6.8) | 53.63 (7.0) | 53.76 (6.3) | 54.71 (6.4) | 53.11 (7.0) * |
| MCS12 f, mean (SD) | 43.59 (10.7) | 42.73 (10.9) | 45.34 (10.2) * | 41.05 (11.3) | 44.97 (10.2) * |
| Rewarded Survey, n (%) | 3942 (28.7) | 2170 (24.0) | 1772 (37.8) * | 1938 (36.3) | 2004 (23.9) * |
| Sex | Age | ||||
|---|---|---|---|---|---|
| Overall | Female | Male | <40 Years | >40 Years | |
| n | 13,721 | 9031 | 4690 | 5335 | 8386 |
| MDS14 a, mean (SD) | 7.48 (2.2) | 7.61 (2.1) | 7.24 (2.3) * | 7.02 (2.1) | 7.78 (2.1) * |
| Mean meals, n (%) >3 meals per day | 6396 (46.6) | 4625 (51.2) | 1771 (37.8) * | 2702 (50.7) | 3694 (44.1) * |
| Snacking habit, n (%) | 6652 (48.5) | 4482 (49.7) | 2170 (46.3) * | 3013 (56.5) | 3639 (43.4) * |
| Water, n (%) ≥7 glasses/day | 4450 (32.4) | 2961 (32.8) | 1489 (31.8) | 1956 (36.7) | 2494 (29.8) * |
| Added salt, n (%) sometimes or usually | 3310 (24.1) | 2066 (22.9) | 1244 (26.5) * | 1527 (28.6) | 1783 (21.3) * |
| Nap habit, n (%) | 4306 (31.4) | 2474 (27.4) | 1832 (39.1) * | 1479 (27.7) | 2827 (33.7) * |
| Sleep weekdays, n (%) ≥7 h/day | 8811 (64.2) | 5947 (65.9) | 2864 (61.1) * | 3798 (71.2) | 5013 (59.8) * |
| Sleep weekends, n (%) ≥7 h/day | 11,692 (85.2) | 7818 (86.6) | 3874 (82.6) * | 4656 (87.3) | 7036 (83.9) * |
| PA b light (min/week), median [IQR] | 225.0 [75.0, 270.0] | 225.0 [75.0, 270.0] | 180.0 [90.0, 270.0] | 180.0 [75.0, 270.0] | 225.0 [90.0, 270.0] * |
| PA moderate (min/week), median [IQR] | 45.0 [0.0, 135.0] | 30.0 [0.0, 90.0] | 45.0 [0.0, 135.0] * | 45.0 [0.0, 135.0] | 30.0 [0.0, 135.0] * |
| PA intense (min/week), median [IQR] | 90.0 [0.0, 180.0] | 45.0 [0.0, 135.0] | 90.0 [0.0, 225.0] * | 90.0 [0.0, 180.0] | 45.0 [0.0, 180.0] * |
| Total PA (METs-min/week), median [IQR] | 1687.5 [890.3, 2691.0] | 1525.5 [774.0, 2502.0] | 1917.0 [954.0, 3051.0] * | 1737.0 [891.0, 2722.5] | 1671.0 [879.0, 2682.0] * |
| Time sitting, n (%) * | |||||
| <4 h | 3472 (25.4) | 2195 (24.3) | 1277 (27.3) | 1360 (25.5) | 2112 (25.2) |
| 5–7 h | 4510 (32.9) | 2938 (32.6) | 1572 (33.6) | 1534 (28.8) | 2976 (35.5) |
| >8 h | 5714 (41.7) | 3886 (43.1) | 1828 (39.1) | 2430 (45.6) | 3284 (39.2) |
| Obesogenic Score, mean (SD) | 1.59 (0.91) | 1.59 (0.92) | 1.59 (0.90) | 1.67 (0.91) | 1.54 (0.91) * |
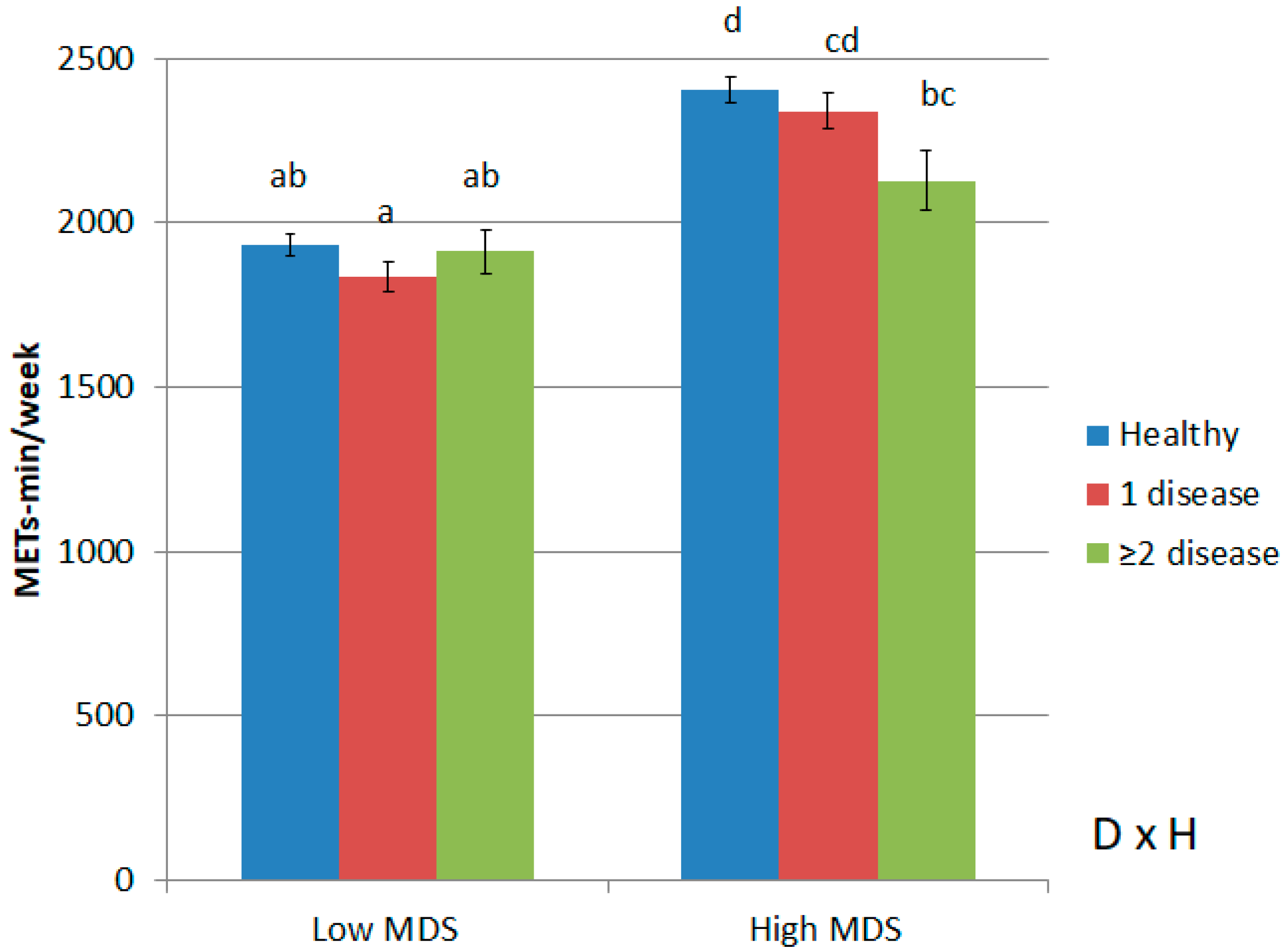
| Low PA, Low MDS14 | High PA, Low MDS14 | Low PA, High MDS14 | High PA, High MDS14 | p for PA | p for MDS14 | p of Interaction | |
|---|---|---|---|---|---|---|---|
| n | 4867 | 4049 | 1707 | 2797 | |||
| Weight, mean (SE) | 71.42 (0.18) c | 70.08 (0.19) b | 70.18 (0.28) b | 68.96 (0.23) a | 0.064 | <0.001 | 0.759 |
| Height, mean (SE) | 169.9 (0.11) a | 169.9 (0.12) a | 170.1 (0.17) ab | 170.4 (0.14) b | <0.001 | <0.001 | 0.220 |
| BMI, mean (SE) | 24.63 (0.06) c | 24.16 (0.06) b | 24.15 (0.09) b | 23.65 (0.07) a | <0.001 | <0.001 | 0.815 |
| Trousers size, mean (SE) | 41.08 (0.06) c | 40.61 (0.07) b | 41.05 (0.10) c | 40.36 (0.08) a | <0.001 | <0.001 | 0.112 |
| Obesity, n (%) | 304 (6.2) | 198 (4.9) | 73 (4.3) | 87 (3.1) | <0.001 | <0.001 | 0.787 |
| Diabetes, n (%) | 154 (3.2) | 143 (3.5) | 46 (2.7) | 54 (1.9) | 0.428 | 0.001 | 0.170 |
| HBP, n (%) | 423 (8.7) | 325 (8.0) | 159 (9.3) | 231 (8.3) | 0.229 | 0.412 | 0.868 |
| Dyslipidemia, n (%) | 754 (15.5) | 525 (13.0) | 307 (18.0) | 426 (15.2) | <0.001 | 0.059 | 0.387 |
| MDS14, mean (SE) | 6.20 (0.02) a | 6.49 (0.02) b | 9.46 (0.03) c | 9.62 (0.03) d | <0.001 | <0.001 | <0.01 |
| PA light (min/week), mean (SE) | 129.0 (2.57) a | 287.0 (2.69) c | 149.0 (3.99) b | 301.0 (3.22) d | <0.001 | <0.001 | 0.333 |
| PA moderate (min/week), mean (SE) | 36.2 (1.41) a | 116.2 (1.47) b | 42.1 (2.18) a | 122.8 (1.77) c | <0.001 | <0.01 | 0.827 |
| PA intense (min/week), mean (SE) | 37.9 (1.65) a | 179.8 (1.73) b | 44.2 (2.57) a | 191.9 (2.08) c | <0.001 | <0.001 | 0.114 |
| Total METs (METs-min/week), mean (SE) | 874.0 (13.9) a | 2852.0 (14. 6) c | 1012.0 (21.6) b | 3019.0 (17.5) d | <0.001 | <0.001 | 0.316 |
| PCS12, mean (SE) | 51.67 (0.11) a | 53.54 (0.12) c | 52.33 (0.17) b | 54.54 (0.14) d | <0.001 | <0.001 | 0.171 |
| MCS12, mean (SE) | 41.3 (0.18) a | 43.6 (0.19) b | 43.4 (0.28) b | 45.4 (0.23) c | <0.001 | <0.001 | 0.547 |
| Obesogenic Score, mean (SE) | 1.90 (0.02) d | 1.41 (0.02) b | 1.63 (0.02) c | 1.27 (0.02) a | <0.001 | <0.001 | <0.001 |
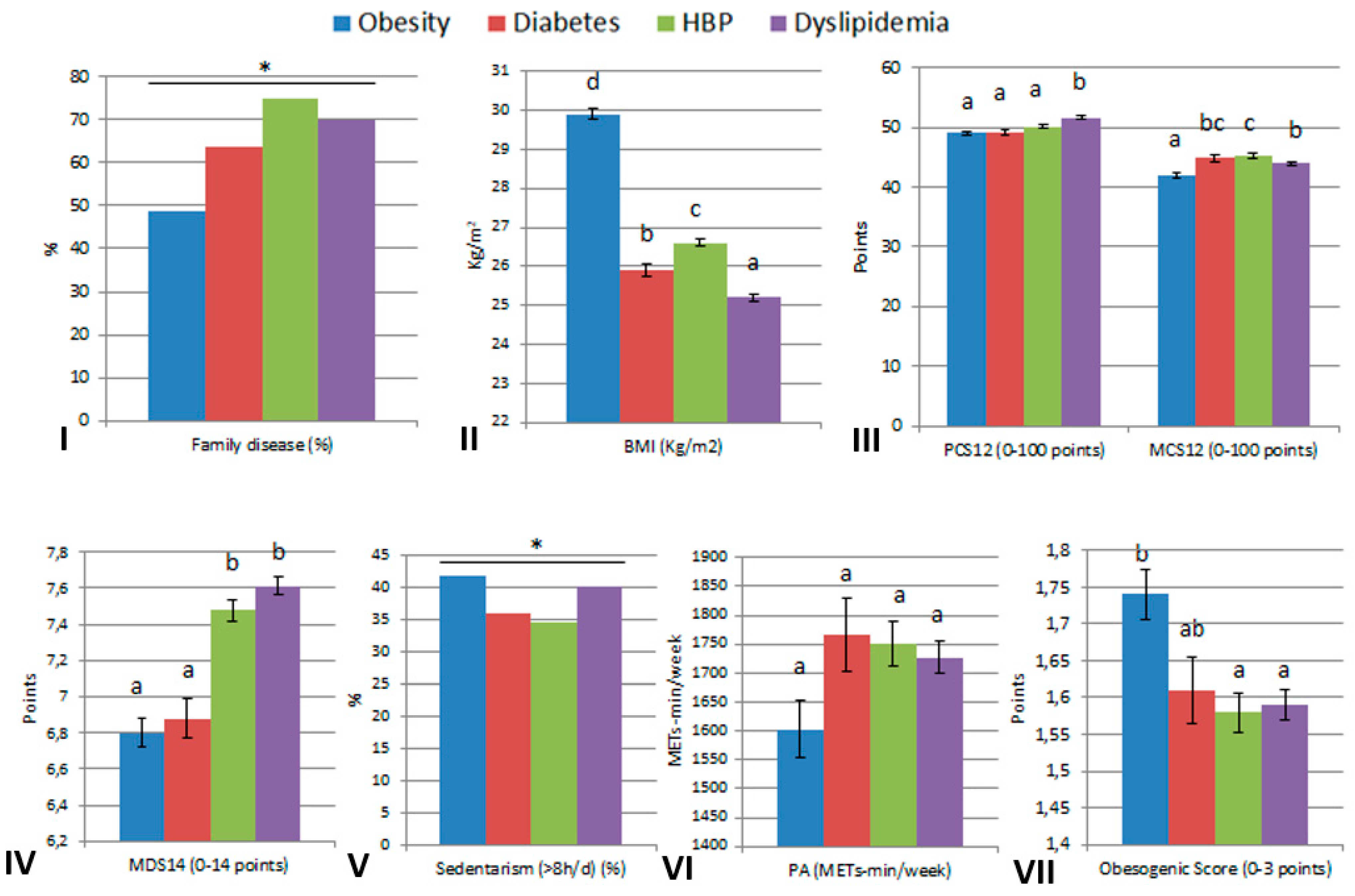
| Obesity | Diabetes | High Blood Pressure | Dyslipidemia | |||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | |
| n | 13,026 | 695 | 13,298 | 423 | 12,536 | 1185 | 11,654 | 2067 |
| Family disease, n (%) | 1971 (15.1) | 339 (48.8) * | 3654 (27.5) | 270 (63.8) * | 5500 (43.9) | 887 (74.9) * | 4297 (36.9) | 1447 (70.0) * |
| PCS12, mean (SD) | 53.9 (6.6) | 49.1 (8.7) * | 53.8 (6.7) | 49.3 (8.4) * | 54.0 (6.6) | 50.2 (8.1) * | 54.1 (6.5) | 51.7 (7.7) * |
| MCS12, mean (SD) | 43.7 (10.7) | 42.0 (11.4) * | 43.6 (10.7) | 44.9 (11.3) * | 43.4 (10.7) | 45.4 (10.6) * | 43.5 (10.7) | 44.0 (10.6) |
| MDS14, mean (SD) | 7.5 (2.1) | 6.8 (2.3) * | 7.5 (2.2) | 6.9 (2.3) * | 7.5 (2.2) | 7.5 (2.2) | 7.5 (2.2) | 7.6 (2.2) * |
| Sedentarism (>8 h) n (%) | 5424 (41.7) | 290 (41.8) | 5563 (41.9) | 151 (35.9) * | 5305 (42.4) | 409 (34.6) * | 4886 (42.0) | 828 (40.1) |
| PA (METs-min/w), median [IQR] | 1722.0 [891.0, 2691.0] | 1251.0 [477.0, 2394.0] * | 1705.5 [891.0, 2691.0] | 1557.0 [688.5, 2754.0] | 1708.5 [891.0, 2691.0] | 1551.0 [742.5, 2573.3] * | 1737.0 [891.0, 2722.5] | 1525.5 [804.0, 2457.0] * |
| ObS, mean (SD) | 1.58 (0.91) | 1.74 (0.92) * | 1.59 (0.91) | 1.61 (0.91) | 1.59 (0.91) | 1.58 (0.92) | 1.59 (0.91) | 1.59 (0.91) |
| BMI (Kg/m2), mean (SD) | 23.8 (3.4) | 29.2 (3.1) * | 24.0 (3.5) | 25.9 (3.7) * | 23.8 (3.5) | 26.6 (3.5) * | 23.9 (3.5) | 25.2 (3.5) * |
| Obesity (n = 695) | Diabetes (n = 423) | High Blood Pressure (n = 1185) | Dyslipidemia (n = 2067) | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate | p Value | Estimate | p Value | Estimate | p Value | Estimate | p Value | |
| Sex (female) | −0.220 | 0.069 | −0.656 | <0.001 | −1.071 | <0.001 | −0.591 | <0.001 |
| Age (>40 years) | 0.504 | <0.001 | 0.454 | <0.01 | 1.377 | <0.001 | 1.242 | <0.001 |
| Survey (RS) | 0.781 | <0.001 | 0.881 | <0.001 | 0.430 | <0.001 | −0.074 | 0.419 |
| Family disease * | 1.867 | <0.001 | 1.215 | <0.001 | 1.526 | <0.001 | 1.714 | <0.001 |
| PCS12 | −0.060 | <0.001 | −0.052 | <0.001 | −0.059 | <0.001 | −0.044 | <0.001 |
| MCS12 | −0.014 | <0.05 | −0.006 | 0.409 | −0.003 | 0.555 | −0.009 | <0.01 |
| MDS14 | −0.074 | <0.05 | −0.035 | 0.337 | 0.026 | 0.240 | −0.014 | 0.419 |
| Sedentarism (>8 h/d) | 0.162 | 0.168 | 0.0241 | 0.872 | −0.257 | <0.01 | −0.083 | 0.232 |
| METs-min/week | 0.000 | 0.934 | 0.000 | 0.872 | 0.000 | 0.348 | 0.000 | 0.059 |
| Smoke (Yes) | −0.080 | 0.5611 | 0.212 | 0.185 | 0.070 | 0.516 | 0.260 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuera-Gómez, A.; Ribot-Rodríguez, R.; Micó, V.; Cuevas-Sierra, A.; San Cristóbal, R.; Martínez, J.A. Lifestyle and Health-Related Quality of Life Relationships Concerning Metabolic Disease Phenotypes on the Nutrimdea Online Cohort. Int. J. Environ. Res. Public Health 2023, 20, 767. https://doi.org/10.3390/ijerph20010767
Higuera-Gómez A, Ribot-Rodríguez R, Micó V, Cuevas-Sierra A, San Cristóbal R, Martínez JA. Lifestyle and Health-Related Quality of Life Relationships Concerning Metabolic Disease Phenotypes on the Nutrimdea Online Cohort. International Journal of Environmental Research and Public Health. 2023; 20(1):767. https://doi.org/10.3390/ijerph20010767
Chicago/Turabian StyleHiguera-Gómez, Andrea, Rosa Ribot-Rodríguez, Victor Micó, Amanda Cuevas-Sierra, Rodrigo San Cristóbal, and Jose Alfredo Martínez. 2023. "Lifestyle and Health-Related Quality of Life Relationships Concerning Metabolic Disease Phenotypes on the Nutrimdea Online Cohort" International Journal of Environmental Research and Public Health 20, no. 1: 767. https://doi.org/10.3390/ijerph20010767
APA StyleHiguera-Gómez, A., Ribot-Rodríguez, R., Micó, V., Cuevas-Sierra, A., San Cristóbal, R., & Martínez, J. A. (2023). Lifestyle and Health-Related Quality of Life Relationships Concerning Metabolic Disease Phenotypes on the Nutrimdea Online Cohort. International Journal of Environmental Research and Public Health, 20(1), 767. https://doi.org/10.3390/ijerph20010767






