New Frontiers in Autoimmune Diagnostics: A Systematic Review on Saliva Testing
Abstract
:1. Introduction
2. Materials and Methods
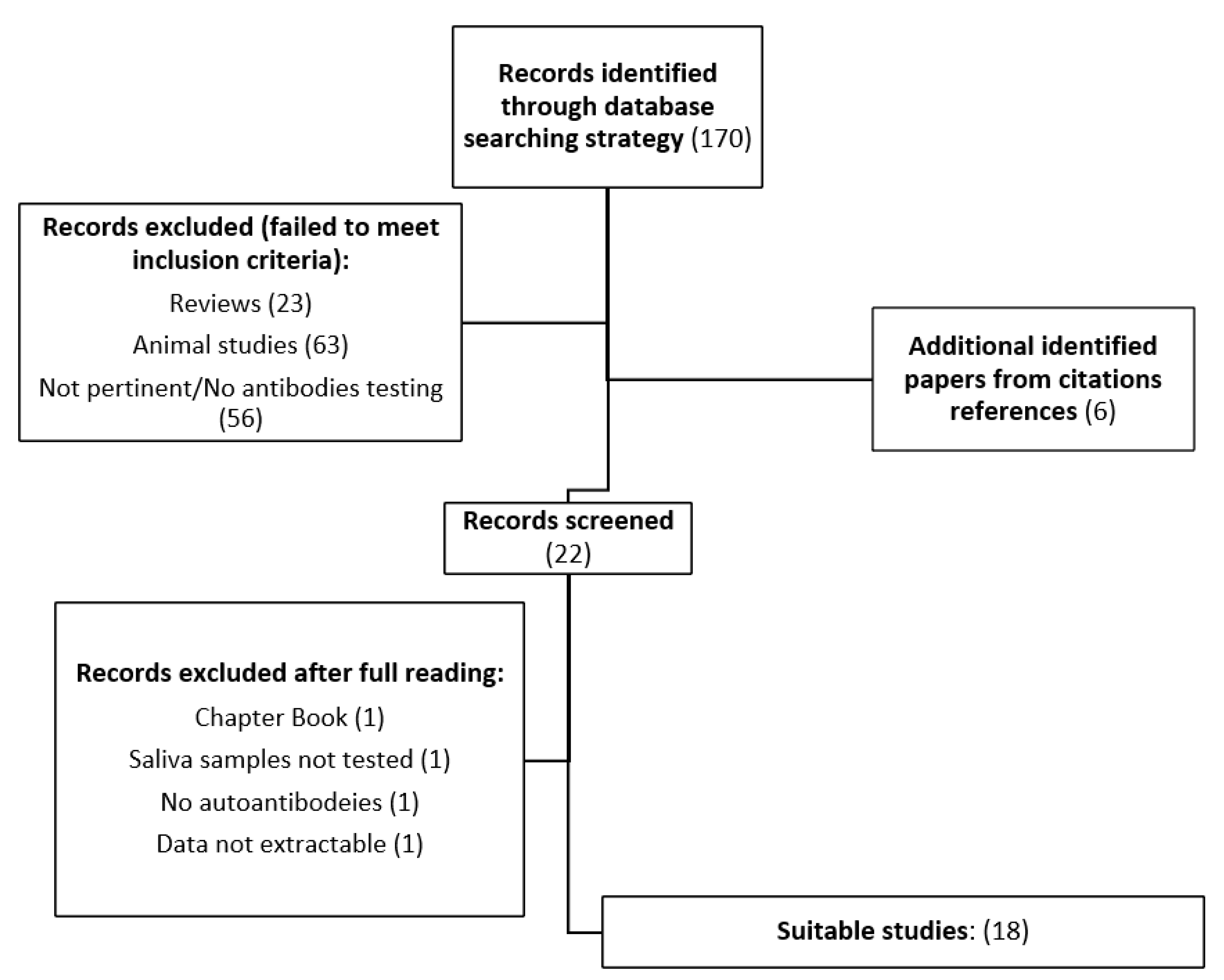
2.1. Systematic Review Process
- (a)
- Original works written exclusively in English;
- (b)
- Testing in humans;
- (c)
- At least 10 patients enrolled and tested;
- (d)
- Detailed description of the techniques used for autoantibody detection.
2.2. Data Extraction and Synthesis
3. Results
3.1. Methodologies Applied
3.2. Sample Collection and Processing
3.3. Diagnostic Categories
3.3.1. Rheumatologic Disorders
3.3.2. Dermatological Disorders
3.3.3. Gastrointestinal Disorders
3.3.4. Endocrinological Disorders
3.4. Saliva Testing as a Diagnostic Tool
3.5. Serum–Saliva Comparison
3.6. Association between Saliva Testing and Clinical Manifestations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfaffe, T.; Cooper-White, J.; Beyerlein, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future ap-plications. Clin. Chem. 2011, 57, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.P.; Parry, J.V. Detection of antibody to HIV in saliva: A brief review. Clin. Diagn. Virol. 1994, 2, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.B.; Mackett, K.; Ali, M.U.; Yamamura, D.; Balion, C. Performance of saliva compared with nasopharyngeal swab for diagnosis of COVID-19 by NAAT in cross-sectional studies: Systematic review and meta-analysis. Clin. Biochem. 2023, 117, 84–93. [Google Scholar] [CrossRef]
- Hema Shree, K.; Ramani, P.; Sherlin, H.; Sukumaran, G.; Jeyaraj, G.; Don, K.R.; Santhanam, A.; Ramasubramanian, A.; Sundar, R. Saliva as a Diagnostic Tool in Oral Squamous Cell Carcinoma—A Systematic Review with Meta Analysis. Pathol. Oncol. Res. 2019, 25, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Goud, E.V.; Kannan, R.; Rao, U.; Joshua, E.; Tavaraja, R.; Jain, Y. Identification of Helicobacter pylori in Saliva of Patients with and without Gastritis by Polymerase Chain Reaction. J. Pharm. Bioallied Sci. Wolters Kluwer Medknow Publ. 2019, 11, S523. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.R.; Tecco, S.; Gastaldi, G.; Polizzi, E.; D’Amicantonio, T.; Negri, S.; Gardini, I.; Schlusnus, K.; Gherlone, E.; Capparè, P.; et al. Point-of-care testing for hepatitis C virus infection at alternative and high-risk sites: An Italian pilot study in a dental clinic. New Microbiol. 2017, 40, 242–245. [Google Scholar] [PubMed]
- Smith, D.H.; Raslan, S.; Samuels, M.A.; Bs, T.I.; Buitron, I.; Deo, S.; Daunert, S.; Thomas, G.R.; Califano, J.; Franzmann, E.J. Current salivary biomarkers for detection of human papilloma virus-induced oropharyngeal squamous cell carcinoma. Head Neck 2021, 43, 3618–3630. [Google Scholar] [CrossRef]
- Bastin, P.; Maiter, D.; Gruson, D. Salivary cortisol testing: Preanalytic and analytic aspects. Ann. De Biol. Clin. 2018, 76, 393–405. [Google Scholar] [CrossRef]
- Sundberg, I.; Rasmusson, A.; Ramklint, M.; Just, D.; Ekselius, L.; Cunningham, J.L. Daytime melatonin levels in saliva are associated with inflammatory markers and anxiety disorders. Psychoneuroendocrinology 2020, 112, 104514. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, X.; Wang, D.; Li, Y.; Zong, Y.; Liu, Y.; Zhang, Y.; Yang, P.; Zuo, Y.; Yang, H.; et al. Quantification of 10 steroid hormones in human saliva from Chinese adult volunteers. J. Int. Med. Res. 2018, 46, 1414–1427. [Google Scholar] [CrossRef]
- Rodrigues, R.P.C.B.; Vieira, W.D.A.; Siqueira, W.L.; Blumenberg, C.; Bernardino, D.M.; Cardoso, S.V.; Flores-Mir, C.; Paranhos, L.R. Saliva as an alternative to blood in the determination of uremic state in adult patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, D.; Antonelli, R.; Setti, G.; Ardissino, D.; Pertinhez, T.; Gallo, M.; Niccoli, G.; Nicolini, F.; Georgaki, M.; Formica, F.; et al. Salivary biomarkers for diagnosis of acute myocardial infarction: A systematic review. Int. J. Cardiol. 2023, 371, 54–64. [Google Scholar]
- Wolgin, M.; Zobernig, M.; Dvornyk, V.; Braun, R.J.; Kielbassa, A.M. Systematic Review on Saliva Biomarkers in Patients Diagnosed with Morbus Alzheimer and Morbus Parkinson. Biomedicines 2022, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Naing, C.; Mak, J.W. Salivary glucose in monitoring glycaemia in patients with type 1 diabetes mellitus: A systematic review. J. Diabetes Metab. Disord. 2017, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomás, I. Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, T.; Zhu, P.; Sun, Z.; Li, S.; Li, F.; Zhang, Y.; Tan, K.; Lu, J.; Yuan, R.; et al. Quantitative Analysis of Salivary Oral Bacteria Associated with Severe Early Childhood Caries and Construction of Caries Assessment Model. Sci. Rep. 2020, 10, 6365. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Gupta, Y.K.; Singh, M.; Joshi, R.; Tiwari, P.; Kaleekal, T.; Tripathi, M. Correlation of saliva and serum free valproic acid concentrations in persons with epilepsy. Seizure 2015, 25, 187–190. [Google Scholar] [CrossRef]
- Scherer, J.N.; Fiorentin, T.R.; Sousa, T.R.V.; Limberger, R.P.; Pechansky, F. Oral Fluid Testing for Cocaine: Analytical Evaluation of Two Point-of-Collection Drug Screening Devices. J. Anal. Toxicol. 2017, 41, 392–398. [Google Scholar] [CrossRef]
- Malamud, D.; Rodriguez-Chavez, I.R. Saliva as a Diagnostic Fluid. Dent. Clin. N. Am. 2011, 55, 159–178. [Google Scholar] [CrossRef]
- Verstraete, A.G. Oral fluid testing for driving under the influence of drugs: History, recent progress and remaining challenges. Forensic Sci. Int. 2005, 150, 143–150. [Google Scholar] [CrossRef]
- Reichardt, E.M.; Baldwin, D.; Osselton, M.D. Effects of Oral Fluid Contamination on Two Oral Fluid Testing Systems. J. Anal. Toxicol. 2013, 37, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 372, 105906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Du, Y.; Wu, Q.; Li, H.; Nguyen, T.; Gidley, G.; Duran, V.; Goldman, D.; Petri, M.; Mohan, C. Salivary anti-nuclear antibody (ANA) mirrors serum ANA in systemic lupus erythematosus. Thromb. Haemost. 2022, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Bentow, C.; Radin, M.; Barinotti, A.; Cecchi, I.; Foddai, S.; Roccatello, D.; Mahler, M. Detection of Autoantibodies in Saliva as New Avenue for the Diagnosis and Management of Autoimmune Patients. Diagnostics 2022, 12, 2026. [Google Scholar] [CrossRef] [PubMed]
- Ching, K.H.; Burbelo, P.D.; Gonzalez-Begne, M.; Roberts, M.E.P.; Coca, A.; Sanz, I.; Iadarola, M.J. Salivary anti-Ro60 and anti-Ro52 an-tibody profiles to diagnose sjögren’s syndrome. J. Dent. Res. 2011, 90, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.; Ferré, E.; Chaturvedi, A.; Chiorini, J.; Alevizos, I.; Lionakis, M.; Warner, B. Profiling Autoantibodies against Salivary Proteins in Sicca Conditions. J. Dent. Res. 2019, 98, 772–778. [Google Scholar] [CrossRef]
- Demoruelle, M.K.; Wang, H.; Davis, R.L.; Visser, A.; Hoang, J.; Norris, J.M.; Holers, V.M.; Deane, K.D.; Darrah, E. Anti-peptidylarginine deiminase-4 antibodies at mucosal sites can activate peptidylarginine deiminase-4 enzyme activity in rheumatoid arthritis. Thromb. Haemost. 2021, 23, 163. [Google Scholar] [CrossRef]
- Roos Ljungberg, K.; Börjesson, E.; Martinsson, K.; Wetterö, J.; Kastbom, A.; Svärd, A. Presence of salivary IgA anti-citrullinated protein antibodies associate with higher disease activity in patients with rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 274. [Google Scholar] [CrossRef]
- Svärd, A.; Renvert, S.; Berglund, J.S.; Persson, R.G.; Söderlin, M. Antibodies to citrullinated peptides in serum and saliva in patients with rheumatoid arthritis and their association to periodontitis. Ann. Rheum. Dis. 2020, 38, 699–704. [Google Scholar]
- Svärd, A.; Roos Ljungberg, K.; Brink, M.; Martinsson, K.; Sjöwall, C.; Dahlqvist, S.R.; Kastbom, A. Secretory antibodies to citrul-linated peptides in plasma and saliva from rheumatoid arthritis patients and their unaffected first-degree relatives. Clin. Exp. Immunol. 2020, 199, 143–149. [Google Scholar] [CrossRef]
- Koopaie, M.; Mortazavi, H.; Khatami, A.; Khodashenas, Z. Salivary and serum anti-desmoglein 1 and 3 ELISA and indirect im-munofluorescence in pemphigus vulgaris: Correlations with serum elisa, indirect immunofluorescence and disease severity. Acta Dermatovenerol. Croat. 2018, 26, 91–99. [Google Scholar]
- Esmaili, N.; Mortazavi, H.; Kamyab-Hesari, K.; Aghazadeh, N.; Daneshpazhooh, M.; Khani, S.; Chams-Davatchi, C. Diagnostic ac-curacy of BP180 NC16a and BP230-C3 ELISA in serum and saliva of patients with bullous pemphigoid. Clin. Exp. Dermatol. 2015, 40, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Hallaji, Z.; Mortazavi, H.; Lajevardi, V.; Tamizifar, B.; Amirzargar, A.; Daneshpazhooh, M.; Chams-Davatchi, C. Serum and salivary desmoglein 1 and 3 enzyme-linked immunosorbent assay in pemphigus vulgaris: Correlation with phenotype and severity. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 275–280. [Google Scholar] [CrossRef]
- Todd, A.L.; Ng, W.-Y.; Lee, Y.S.; Loke, K.Y.; Thai, A.C. Evidence of autoantibodies to glutamic acid decarboxylase in oral fluid of type 1 diabetic patients. Diabetes Res. Clin. Pract. 2002, 57, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, A.K.; Belazi, M.A.; Drakoulakos, D. Glutamic acid decarboxylase autoantibodies in saliva of children with type 1 diabetes. Diabetes Res. Clin. Pract. 1997, 38, 169–172. [Google Scholar] [CrossRef]
- Tiberti, C.; Shashaj, B.; Verrienti, A.; Vecci, E.G.; Lucantoni, F.; Masotti, D.; Morano, S.; Sulli, N.; Dotta, F. GAD and IA-2 autoantibody detection in type 1 diabetic patient saliva. Clin. Immunol. 2009, 131, 271–276. [Google Scholar] [CrossRef]
- Lu, C.; Hou, X.; Li, M.; Wang, L.; Zeng, P.; Jia, H.; Chen, J.; Wei, Y.; He, H.; Liu, X.; et al. Detection of AMA-M2 in human saliva: Potentials in diagnosis and monitoring of primary biliary cholangitis. Sci. Rep. 2017, 7, 796. [Google Scholar] [CrossRef]
- Palmer, J.M.; Doshi, M.; Kirby, J.A.; Yeaman, S.J.; Bassendine, M.F.; Jones, D.E.J. Secretory autoantibodies in primary biliary cirrhosis (PBC). Clin. Exp. Immunol. 2000, 122, 423–428. [Google Scholar] [CrossRef]
- Ikuno, N.; Mackay, I.R.; Jois, J.; Omagari, K.; Rowley, M.J. Antimitochondrial autoantibodies in saliva and sera from patients with primary biliary cirrhosis. J. Gastroenterol. Hepatol. 2001, 16, 1390–1394. [Google Scholar] [CrossRef]
- Ajdani, M.; Mortazavi, N.; Besharat, S.; Mohammadi, S.; Amiriani, T.; Sohrabi, A.; Norouzi, A.; Edris, G. Serum and salivary tissue transglutaminase IGA (tTG-IGA) level in celiac patients. BMC Gastroenterol. 2022, 22, 375. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Dawes, C. Circadian rhythms in human salivary flow rate and composition. J. Physiol. 1972, 220, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.; Koh, D.; Mok, B.; Lim, L.P.; Yang, Y.; Chia, S.E. Stressful life events of dental students and salivary immunoglobulin A. Int. J. Immunopathol. Pharmacol. 2004, 17, 49–56. [Google Scholar] [CrossRef]
- Challacombe, S.J.; Percival, R.S.; Marsh, P.D. Age-related changes in immunoglobulin isotypes in whole and parotid saliva and serum in healthy individuals. Oral Microbiol. Immunol. 1995, 10, 202–207. [Google Scholar] [CrossRef] [PubMed]
| Author, Year of Publication | Study Design | Population Numerosity | Tested Antibodies and Isotypes | Methodology | |
|---|---|---|---|---|---|
| CTDs | Zhang, 2021 [23] | C-S | 70 SLE, 10 HC | ANA (IgG/M/A) | IIF, commercial ELISA |
| CTDs | Sciascia, 2022 [24] | O | 48 patients (21 SLE, 10 PAPS, 2 SSc, 2 SjS, 8 UCTD, 1 thyroiditis, 4 RA) | dsDNA, RNP, Sm, Ro52, Ro60, SS-B, CENP, Scl-70, Ribo-P, and Jo-1, DFS70 (IgG) | PMAT |
| Sicca syndrome | Ching, 2011 [25] | C-C | 27 SjS, 27 HC | Ro52 and Ro60 (NS) | LIPS |
| Sicca syndrome | Burbelo, 2019 [26] | C-S | 83 (20 HC, 23 ICIS, 20 SjS, 20 APECED) | Ro52, Ro60, La, LPO, BPIFA1, and BPIFA2 (NS) | LIPS |
| RA | Demoruelle, 2021 [27] | C-S | 37 RA, 25 HC, and 46 subjects “at-risk” for RA | anti-PAD4, anti-PAD3/4, and APCA (IgG, IgA) | Immunoprecipitation, commercial ELISA |
| RA | Ljungberg, 2020 [28] | C-S | 196 RA, 101 HC | ACPA (IgG, IgA, IgA1, IgA2, and serum SC) | Modified commercial ELISA |
| RA | Svärd, 2020 [29] | C-S | 132 RA | ACPA (IgA, IgG) | Commercial ELISA |
| RA | Svärd, 2019 [30] | C-S | 25 RA, 21 FDRs, 70 SLE, 11 HC | Secretory ACPA, Salivary IgA ACPA | Modified commercial ELISA |
| PV | Koopaie, 2018 [31] | C-S | 50 PV | Anti-Dsg1-3 (IgG) | IIF, commercial ELISA |
| PB | Esmaili, 2014 [32] | C-C | 50 BP, 50 HC | BP230 and BP180 (IgG) | Commercial ELISA |
| PV | Hallaj, 2010 [33] | C-S | 50 PV | Anti-Dsg1-3 (IgG) | Commercial ELISA |
| DM | Todd, 2002 [34] | O | 31 T1DM | GADA (IgG) | RIPA |
| DM | Markopoulos, 1997 [35] | C-S | 30 T1DM, 80 HC | GADA (NS) | Commercial ELISA |
| DM | Tiberti, 2009 [36] | O | 70 T1DM, 24 FDR, 76 HC | GADA, IA, and 2A (IgG) | RIPA |
| PBC | Lu, 2017 [37] | C-S | 49 PBC, 60 HC, 42 OLP | AMA-M2 (IgG) | Commercial ELISA |
| PBC | Palmer, 2000 [38] | C-S | 44 PBC, 28 HC, 11 PBC patients post liver transplantation | PDC (IgA) | WB, in-house ELISA |
| PBC | Ikuno, 2001 [39] | C-S | 12 PBC, 11 HC | AMA directed versus 2-OAD enzymes (IgG, IgA, and IgM) | WB and EIA |
| Celiac disease | Ajdani, 2022 [40] | C-C | 39 celiac disease, 39 HC | Salivary and serum tTg (IgA) | ELISA (not specified whether in-house or commercial kits were used) |
| Saliva Testing Performance * | Saliva–Serum Correlation or Significant Association | Correlation between Saliva Antibody Testing and Clinical Manifestations | |
|---|---|---|---|
| Zhang [23] | ANA IF intensities were significantly higher in SLE patients than in healthy controls (p < 0.01) | R = 0.33, p = 0.0058 | IgM-ANA correlated with PGA (R = 0.24, p < 0.05), dsDNA (R = 0.35, p < 0.01), SLEDAI (R = 0.3, p < 0.05) serum C3 (R = −0.35, p < 0.01), and C4 (R = −0.26, p < 0.05); IgG-ANA correlated with ESR (R = 0.39, p < 0.01), SLEDAI (R = 0.25, p < 0.05), dsDNA (R = 0.29, p < 0.05), and serum C3 (R = 0.29, p < 0.05); IgA-ANA correlated with dsDNA (R = 0.24, p < 0.05) and ESR (R = 0.33, p < 0.05). |
| Sciascia [24] | N/A | R = 0.73 (95% CI: 0.68–0.76, p < 0.0001). The results obtained with saliva were usedto generate an ROC curve using the serum results as a binary classifier: AUC = 0.97 | N/A |
| Ching [25] | A Mann–Whitney U test showed a marked difference in autoantibodytiters between SjS and control groups (p < 0.0001). Ro60: 70% sensitivity (95% CI: 50%–86%), 96% specificity (95% CI: 81%–100%); Ro52: 67% sensitivity (CI 46–83%) 100% specificity (CI 87–100%) | Anti-Ro60 and Ro52titers did not correlate quantitatively with serum titers (R = 0.23, R = 0.2). | N/A |
| Burbelo [26] | N/A | N/A | N/A |
| Demoruelle [27] | Statistically significant difference in saliva anti-CCP prevalence between RA patients and controls (p < 0.01) | N/A | N/A |
| Ljungberg [28] | Difference between saliva IgA2 levels of RA patients and controls (p < 0.05) | Saliva IgA ACPA moderately correlated with serum levels of IgA (r = 0.455) and IgA1 (r = 0.434) and weakly with IgA2 (r = 0.277), SC ACPA (r = 0.29), and IgG ACPA (R = 0.342) (all at p < 0.001) | Saliva IgA ACPA-positive samples had significantly higher ESR (p = 0.031), DAS28 (p = 0.04), tender joint count (TJC) (p = 0.039), HAQ (p = 0.006), and PGA (p = 0.03) than negative samples. In linear regression analysis, salivary ACPA levels were associated with DAS28 (p = 0.016). Salivary IgA ACPA was associated with DAS28 after adjusting for disease duration and treatment (p = 0.021). |
| Svärd 2020 [29] | N/A | Low correlation between IgG and IgA ACPA serum/saliva results (R = 0.235, p = 0.014; R = 0.208, p = 0.030) | N/A |
| Svärd 2019 [30] | Salivary IgA ACPA was not statistically more prevalent in RA patients compared to HC or FDR (p > 0.05) | N/A | N/A |
| Koopaie [31] | N/A | ELISA: Dsg1 (R = 0.369, p = 0.008); Dsg3 (R = 0.463, p = 0.001). IIF: (R = 0.409, p = 0.003) | ELISA anti-Dsg1-3 showed a moderate correlation with the total PDAI score (R = 0.336, p = 0.017; R = 0.510, p < 0.001), Dsg3 showed a moderate correlation with mucosal PDAI (R = 0.513, p < 0.001), and Dsg1 showed a moderate correlation with body–head–neck PDAI 8 (R = 0.477–0.492, p < 0.001). |
| Esmaili [32] | N/A | BP180(R = 0.9, p < 0.001); BP230 (R = 0.5, p < 0.001)’ BP180 sensitivity: serum 88%, SALIVA 87%; specificity, 96% vs. 96%. BP230 serum/saliva sensitivity: 48% vs. 77%; specificity, 96% vs. 62%. | A statistically significant correlation was found between saliva BP180 values and severity of skin disease (R = 0.54, p = 0.01); BP230 levels correlated with mucosal involvement (p = 0.03). |
| Hallaji [33] | N/A | 1, Dsg1 p < 0.001, Dsg3 p = 0.001Dsg1 sensitivity (serum/saliva): 72/70%; Dsg3 sensitivity (serum/saliva): 94/94% | Anti-Dsg1 titers were significantly higher in the saliva of patients with mucocutaneous phenotype (p = 0.021). Salivary anti-Dsg1 antibodies correlated with severity of mucosal lesions (R = 0.496, p < 0.001). |
| Todd [34] | N/A | (R = 0.67, p < 0.001; R = 0.85, p < 0.001 when only seropositive GADA subjects were considered) | N/A |
| Markopulos [35] | GADA was found both in the serum and saliva of all diabetic children, allowing the distinction of patients and controls using both serum and saliva testing (p < 0.0001) | N/A | N/A |
| Tiberti [36] | Salivary GADA and IA2 autoantibodies were present in only one control (1.3%, 1/76) | GADA: (R = 0.749, p < 0.0001); IA-2A: (R = 0.689, p < 0.0001). GADA correctly identified 91.1% of T1DM patients (PPV, 91.7%; NPV, 80%) | N/A |
| Lu [37] | AUC 0.88 (95% CI: 0.65–0.93). Threshold value of 0.61 RU/mL, 81.82% sensitivity, and 80% specificity | R = 0.63, p < 0.001 | IL6 and INFγ saliva values were significantly increased in the PBC group (p<0.001). |
| Palmer [38] | A significant difference was detected between the saliva of PBC patients and HC controls, both for IgA and anti-SC titers (p < 0.0001; p < 0.001) | N/A | No correlation between anti-PDC IgA and severity of dry mouth and fatigue (R = -0.17, p = NS; R = 0.18, p = NS) |
| Ikuno [39] | All controls were negative for the tested antibodies | Good correspondence between serum and saliva for all Ig isotypes: 12 (serum) vs. 9 (saliva); perfect correspondence between IgG isotypes: 9 (serum positivities) vs. 9 (saliva positivities) | N/A |
| Ajdani [40] | Salivary levels of tTG were greater among patients (p < 0.001) | compared to serum gold standard. Saliva results showed an AUC of 0.9309, a sensitivity of 98.15%, a specificity of 80%, and a diagnostic accuracy of 91.67% | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foddai, S.G.; Radin, M.; Barinotti, A.; Cecchi, I.; Rubini, E.; Arbrile, M.; Mantello, E.; Menegatti, E.; Roccatello, D.; Sciascia, S. New Frontiers in Autoimmune Diagnostics: A Systematic Review on Saliva Testing. Int. J. Environ. Res. Public Health 2023, 20, 5782. https://doi.org/10.3390/ijerph20105782
Foddai SG, Radin M, Barinotti A, Cecchi I, Rubini E, Arbrile M, Mantello E, Menegatti E, Roccatello D, Sciascia S. New Frontiers in Autoimmune Diagnostics: A Systematic Review on Saliva Testing. International Journal of Environmental Research and Public Health. 2023; 20(10):5782. https://doi.org/10.3390/ijerph20105782
Chicago/Turabian StyleFoddai, Silvia Grazietta, Massimo Radin, Alice Barinotti, Irene Cecchi, Elena Rubini, Marta Arbrile, Ester Mantello, Elisa Menegatti, Dario Roccatello, and Savino Sciascia. 2023. "New Frontiers in Autoimmune Diagnostics: A Systematic Review on Saliva Testing" International Journal of Environmental Research and Public Health 20, no. 10: 5782. https://doi.org/10.3390/ijerph20105782







