Continuous Vital Signs Monitoring with a Wireless Device on a General Ward: A Survey to Explore Nurses’ Experiences in a Post-Implementation Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. The Device and Implementation
2.3. Data Collection
2.4. Data Analysis
2.5. Data Presentation
3. Results
3.1. Theme 1: Timely Signalling and Early Action
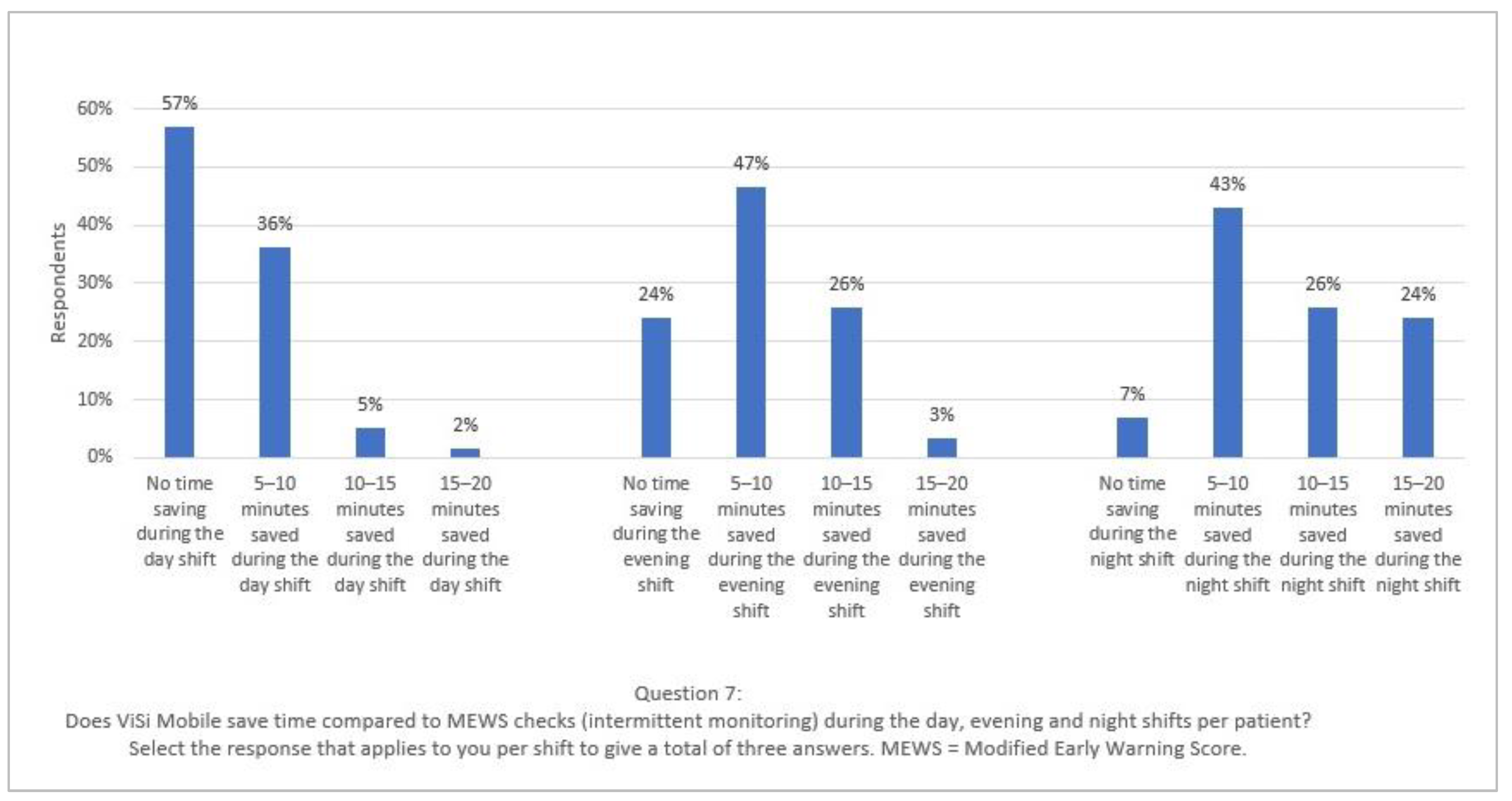
3.2. Theme 2: Time Savings and Time Consumption
In the day and evening shift, it does give you some gain, but usually only 5–10 min because it almost never happens that all three to six are functioning. In the evening shift, maybe sometimes a little more time gain, because you have more patients there and especially when they all function. On the night shift, the morning round, it certainly saves a lot of time. But also only when they are functioning; otherwise, you still have to calibrate them or manually measure the controls. (Respondent 18)
3.3. Theme 3: Patient Comfort and Satisfaction
3.4. Theme 4: Preconditions
3.4.1. Device Design and Technical Concerns
3.4.2. Vital Signs’ Reliability
3.4.3. Internet Connectivity
3.4.4. Nurses’ Knowledge and Training
3.4.5. Logistics
4. Discussion
Limitations
5. Conclusions
Relevance to Clinical Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Checklist for Reporting of Survey Studies (CROSS) [31]
| Section/Topic | Item | Item Description | Reported on Page # |
|---|---|---|---|
| Title and abstract | |||
| Title and abstract | 1a | State the word “survey” along with a commonly used term in title or abstract to introduce the study’s design. | #1 |
| 1b | Provide an informative summary in the abstract, covering background, objectives, methods, findings/results, interpretation/discussion, and conclusions. | #1 | |
| Introduction | |||
| Background | 2 | Provide a background about the rationale of study, what has been previously done, and why this survey is needed. | #1–3 |
| Purpose/aim | 3 | Identify specific purposes, aims, goals, or objectives of the study. | #2–3 |
| Methods | |||
| Study design | 4 | Specify the study design in the methods section with a commonly used term (e.g., cross-sectional or longitudinal). | #3 |
| Data collection methods | 5a | Describe the questionnaire (e.g., number of sections, number of questions, number and names of instruments used). | #4–5 + Appendix C |
| 5b | Describe all questionnaire instruments that were used in the survey to measure particular concepts. Report target population, reported validity and reliability information, scoring/classification procedure, and reference links (if any). | N/A | |
| 5c | Provide information on pretesting of the questionnaire, if performed (in the article or in an online supplement). Report the method of pretesting, number of times questionnaire was pre-tested, number and demographics of participants used for pretesting, and the level of similarity of demographics between pre-testing participants and sample population. | #4–5 | |
| 5d | Questionnaire if possible, should be fully provided (in the article, or as appendices or as an online supplement). | Appendix C | |
| Sample characteristics | 6a | Describe the study population (i.e., background, locations, eligibility criteria for participant inclusion in survey, exclusion criteria). | #3 |
| 6b | Describe the sampling techniques used (e.g., single stage or multistage sampling, simple random sampling, stratified sampling, cluster sampling, convenience sampling). Specify the locations of sample participants whenever clustered sampling was applied. | #3 | |
| 6c | Provide information on sample size, along with details of sample size calculation. | #3–4 + N/A | |
| 6d | Describe how representative the sample is of the study population (or target population if possible), particularly for population-based surveys. | N/A | |
| Survey administration | 7a | Provide information on modes of questionnaire administration, including the type and number of contacts, the location where the survey was conducted (e.g., outpatient room or by use of online tools, such as SurveyMonkey). | #4–5 |
| 7b | Provide information of survey’s time frame, such as periods of recruitment, exposure, and follow-up days. | #4–5 | |
| 7c | Provide information on the entry process: | ||
| N/A | ||
| #5 | ||
| Study preparation | 8 | Describe any preparation process before conducting the survey (e.g., interviewers’ training process, advertising the survey). | #4–5 |
| Ethical considerations | 9a | Provide information on ethical approval for the survey if obtained, including informed consent, institutional review board [IRB] approval, Helsinki declaration, and good clinical practice [GCP] declaration (as appropriate). | #3 |
| 9b | Provide information about survey anonymity and confidentiality and describe what mechanisms were used to protect unauthorized access. | #3 | |
| Statistical analysis | 10a | Describe statistical methods and analytical approach. Report the statistical software that was used for data analysis. | #5 |
| 10b | Report any modification of variables used in the analysis, along with reference (if available). | N/A | |
| 10c | Report details about how missing data was handled. Include rate of missing items, missing data mechanism (i.e., missing completely at random [MCAR], missing at random [MAR] or missing not at random [MNAR]) and methods used to deal with missing data (e.g., multiple imputation). | #5 | |
| 10d | State how non-response error was addressed. | N/A | |
| 10e | For longitudinal surveys, state how loss to follow-up was addressed. | N/A | |
| 10f | Indicate whether any methods such as weighting of items or propensity scores have been used to adjust for non-representativeness of the sample. | N/A | |
| 10g | Describe any sensitivity analysis conducted. | N/A | |
| Results | |||
| Respondent characteristics | 11a | Report numbers of individuals at each stage of the study. Consider using a flow diagram, if possible. | #5 (Table 1) |
| 11b | Provide reasons for non-participation at each stage, if possible. | N/A | |
| 11c | Report response rate, present the definition of response rate or the formula used to calculate response rate. | #5 | |
| 11d | Provide information to define how unique visitors are determined. Report number of unique visitors along with relevant proportions (e.g., view proportion, participation proportion, completion proportion). | N/A | |
| Descriptive results | 12 | Provide characteristics of study participants, as well as information on potential confounders and assessed outcomes. | #5–6 |
| Main findings | 13a | Give unadjusted estimates and, if applicable, confounder-adjusted estimates along with 95% confidence intervals and p-values. | N/A |
| 13b | For multivariable analysis, provide information on the model building process, model fit statistics, and model assumptions (as appropriate). | N/A | |
| 13c | Provide details about any sensitivity analysis performed. If there are considerable amount of missing data, report sensitivity analyses comparing the results of complete cases with that of the imputed dataset (if possible). | N/A | |
| Discussion | |||
| Limitations | 14 | Discuss the limitations of the study, considering sources of potential biases and imprecisions, such as non-representativeness of sample, study design, important uncontrolled confounders. | #12 |
| Interpretations | 15 | Give a cautious overall interpretation of results, based on potential biases and imprecisions and suggest areas for future research. | #10–12 |
| Generalizability | 16 | Discuss the external validity of the results. | #12 |
| Other sections | |||
| Role of funding source | 17 | State whether any funding organization has had any roles in the survey’s design, implementation, and analysis. | #13 |
| Conflict of interest | 18 | Declare any potential conflict of interest. | #13 |
| Acknowledgements | 19 | Provide names of organizations/persons that are acknowledged along with their contribution to the research. | #13 |
Appendix B. Working with ViSi Mobile
Appendix C. The Survey
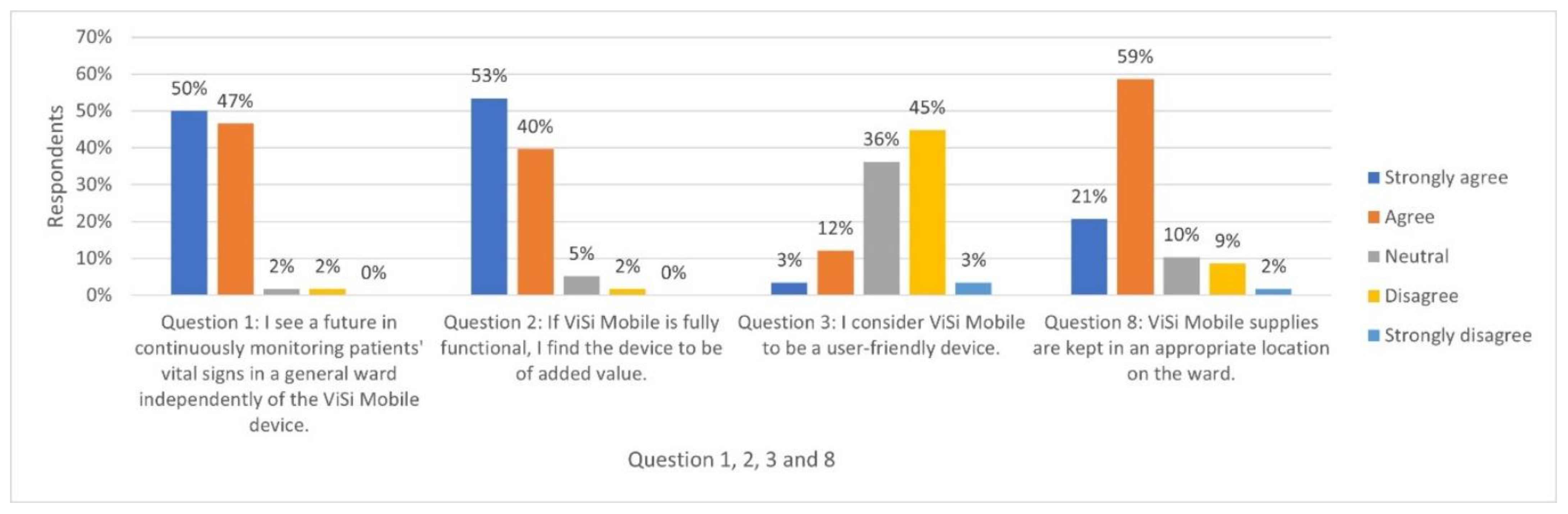
- I see a future in continuously monitoring patients’ vital signs in a general ward independently of the ViSi Mobile device *.
- Explain why you do or do not see a future in continuously monitoring patients’ vital signs.
- If ViSi Mobile is fully functional, I find the device to be of added value *.
- Explain this answer.
- I consider ViSi Mobile to be a user-friendly device *.
- If you find ViSi Mobile a less user-friendly device, explain why.
- If ViSi Mobile is fully functional, the main benefits are (give a maximum of three benefits).
- If ViSi Mobile does not function properly, it is often due to (give a maximum of three answers).
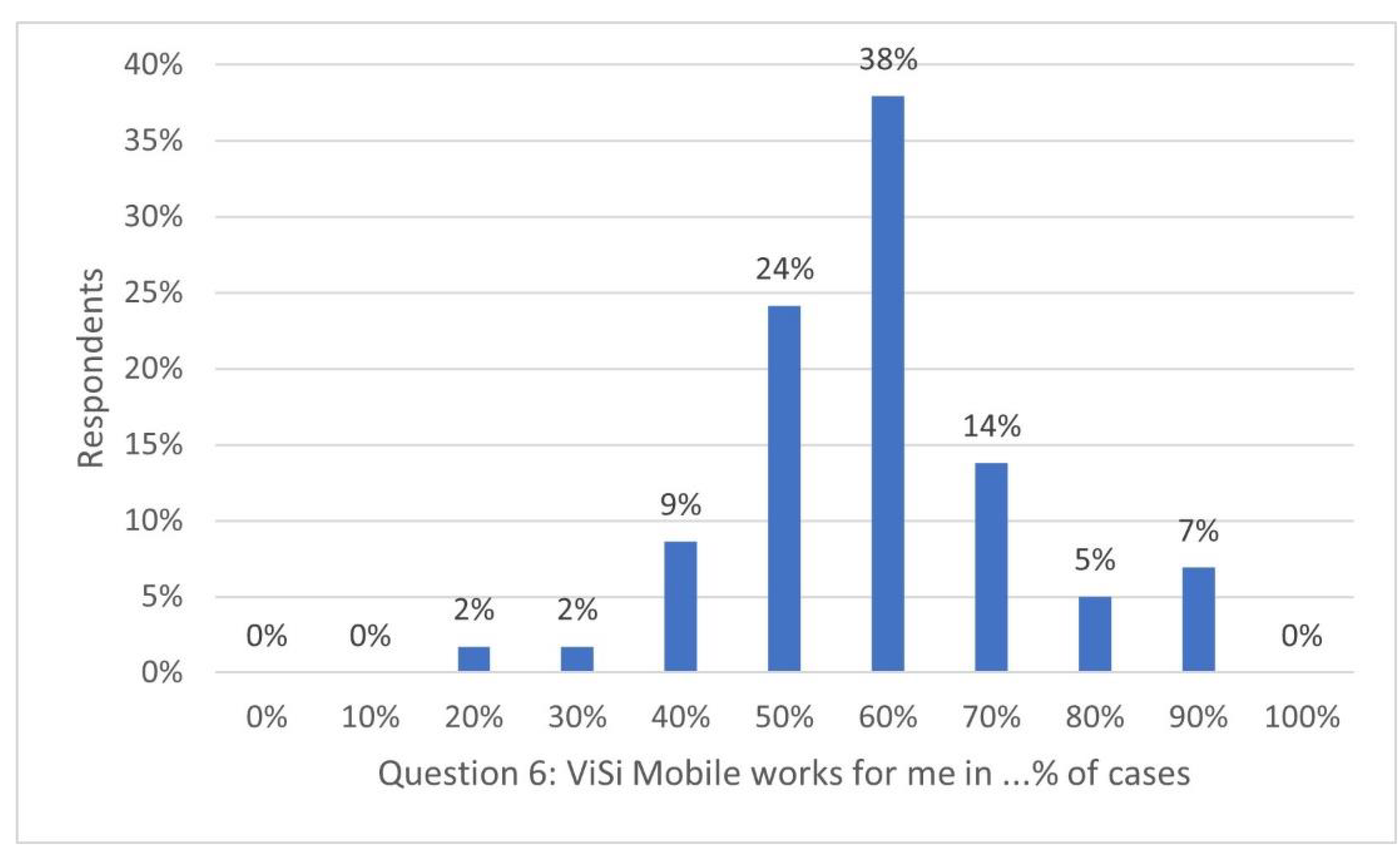
- ViSi Mobile functions for me in …% of cases (please give an estimation between 0% and 100%).
- Give an explanation, if possible.
- Does ViSi Mobile save time compared to MEWS checks (intermittent monitoring) during the day, evening and night shifts per patient? Select the response that applies to you per shift to give a total of three answers. MEWS = Modified Early Warning Score.
- -
- No time saving during the day/evening/night shift
- -
- 5–10 min saved during the day/evening/night shift
- -
- 10–15 min saved during the day/evening/night shift
- -
- 15–20 min saved during the day/evening/night shift
- If the answer is no, indicate how much more time you spend on average per patient, per shift, compared to your previous situation.
- ViSi Mobile supplies are kept in an appropriate location on the ward *.
- Indicate what you would like to see change on the ward concerning stock and location.
- On what topics would you like to receive further training?
- -
- Software knowledge of the dashboard in the nurses’ office
- -
- ViSi Mobile software knowledge (wrist monitor)
- -
- What to do when ViSi Mobile does not work (e.g., error messages, inability to calibrate, etc.)
- -
- Installing ViSi Mobile
- -
- Understanding how ViSi Mobile works
- -
- ViSi Mobile troubleshooting
- -
- Understanding rhythm assessment (irregular or regular heartbeat) and ViSi Mobile’s function regarding an irregular pulse
- -
- None, I am aware of everything
- How would you like to receive this continuing education?
- What do you consider the main areas of improvement that should be prioritised regarding ViSi Mobile (give a maximum of three answers)?
Appendix D. Figures for Closed Questions 6 and 9

Appendix E. Relevance to Clinical Practice
Appendix F. Impact Statement
- Nurses are positive towards using remote wireless technology to monitor vital signs continuously because it enables them to detect and react to clinical deterioration in patients early. It also enhances patient safety compared to intermittent monitoring.
- Wireless technology for continuously monitoring vital signs affects nursing care. It consumes time during day shifts and saves time during evening and night shifts.
- Contrary to expectations, nurses do not mention less contact with patients. These findings challenge thinking about if and how wireless technology for continuously monitoring of vital signs optimises fundamental nursing care delivery, which could influence nurse-sensitive outcomes and ‘care left undone’.
References
- Zhang, W.; Barriball, K.L.; While, A.E. Nurses’ attitudes towards medical devices in healthcare delivery: A systematic review. J. Clin. Nurs. 2014, 23, 2725–2739. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Morrell, M.; Prgomet, M.; Turner, R.M.; Nicholson, M.; Hillman, K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: A systematic review and meta-analysis. Int. J. Clin. Pract. 2016, 70, 806–824. [Google Scholar] [CrossRef]
- Prgomet, M.; Cardona-Morrell, M.; Nicholson, M.; Lake, R.; Long, J.; Westbrook, J.; Braithwaite, J.; Hillman, K. Vital signs monitoring on general wards: Clinical staff perceptions of current practices and the planned introduction of continuous monitoring technology. Int. J. Qual. Health Care 2016, 28, 515–521. [Google Scholar] [CrossRef]
- Subbe, C.P.; Duller, B.; Bellomo, R. Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit. Care 2017, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Downey, C.; Randell, R.; Brown, J.; Jayne, D.G. Continuous Versus Intermittent Vital Signs Monitoring Using a Wearable, Wireless Patch in Patients Admitted to Surgical Wards: Pilot Cluster Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e10802. [Google Scholar] [CrossRef]
- Eddahchouri, Y.; Peelen, R.V.; Koeneman, M.; Touw, H.R.W.; van Goor, H.; Bredie, S.J.H. Effect of continuous wireless vital sign monitoring on unplanned ICU admissions and rapid response team calls: A before-and-after study. Br. J. Anaesth. 2022, 128, 857–863. [Google Scholar] [CrossRef]
- Kitson, A.; Carr, D.; Conroy, T.; Feo, R.; Gronkjaer, M.; Huisman-de Waal, G.; Jackson, D.; Jeffs, L.; Merkley, J.; Muntlin Athlin, A.; et al. Speaking Up for Fundamental Care: The ILC Aalborg Statement. BMJ Open 2019, 9, e033077. [Google Scholar] [CrossRef] [PubMed]
- Considine, J.; Currey, J. Ensuring a proactive, evidence-based, patient safety approach to patient assessment. J. Clin. Nurs. 2015, 24, 300–307. [Google Scholar] [CrossRef]
- Downey, C.L.; Brown, J.M.; Jayne, D.G.; Randell, R. Patient attitudes towards remote continuous vital signs monitoring on general surgery wards: An interview study. Int. J. Med. Inform. 2018, 114, 52–56. [Google Scholar] [CrossRef]
- Douw, G.; Huisman-de Waal, G.; van Zanten, A.R.H.; van der Hoeven, J.G.; Schoonhoven, L. Surgical ward nurses’ responses to worry: An observational descriptive study. Int. J. Nurs. Stud. 2018, 85, 90–95. [Google Scholar] [CrossRef]
- Weenk, M.; Bredie, S.J.; Koeneman, M.; Hesselink, G.; van Goor, H.; van de Belt, T.H. Continuous Monitoring of Vital Signs in the General Ward Using Wearable Devices: Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e15471. [Google Scholar] [CrossRef]
- Douw, G. General Discussion. Just Worry, Exploring Triggers Used by Nurses to Identify Surgical Patients at Risk for Clinical Deterioration. Ph.D. Thesis, Radboud Universiteit Nijmegen, Nijmegen, The Netherlands, October 2018. [Google Scholar]
- Schoville, R.R.; Titler, M.G. Guiding healthcare technology implementation: A new integrated technology implementation model. Comput. Inform. Nurs. 2015, 33, 99–107. [Google Scholar] [CrossRef]
- Holden, R.J.; Karsh, B.-T. The technology acceptance model: Its past and its future in health care. J. Biomed. Inform. 2010, 43, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.R.; Kaushal, R.; Cleary, P.D.; Jenter, C.A.; Volk, L.A.; Orav, E.J.; Burdick, E.; Poon, E.G.; Bates, D.W. Physicians and electronic health records: A statewide survey. Arch. Intern. Med. 2007, 167, 507–512. [Google Scholar] [CrossRef]
- Downey, C.L.; Chapman, S.; Randell, R.; Brown, J.M.; Jayne, D.G. The impact of continuous versus intermittent vital signs monitoring in hospitals: A systematic review and narrative synthesis. Int. J. Nurs. Stud. 2018, 84, 19–27. [Google Scholar] [CrossRef]
- Davis, F.D.; Bagozzi, R.P.; Warshaw, P.R. User Acceptance of Computer Technology: A Comparison of Two Theoretical Models. Manag. Sci. 1989, 35, 982–1003. [Google Scholar] [CrossRef]
- Cadmus, E.; Brigley, P.; Pearson, M. Safe patient handling: Is your facility ready for a culture change? Nurs. Manag. 2011, 42, 12–15. [Google Scholar] [CrossRef]
- Stellpflug, C.; Pierson, L.; Roloff, D.; Mosman, E.; Gross, T.; Marsh, S.; Willis, V.; Gabrielson, D. Continuous Physiological Monitoring Improves Patient Outcomes. Am. J. Nurs. 2021, 121, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Jeskey, M.; Card, E.; Nelson, D.; Mercaldo, N.D.; Sanders, N.; Higgins, M.S.; Shi, Y.; Michaels, D.; Miller, A. Nurse adoption of continuous patient monitoring on acute post-surgical units: Managing technology implementation. J. Nurs. Manag. 2011, 19, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.H.; McGregor, A.H. Body-worn sensor design: What do patients and clinicians want? Ann. Biomed. Eng. 2011, 39, 2299–2312. [Google Scholar] [CrossRef]
- Jeffs, E.; Vollam, S.; Young, J.D.; Horsington, L.; Lynch, B.; Watkinson, P.J. Wearable monitors for patients following discharge from an intensive care unit: Practical lessons learnt from an observational study. J. Adv. Nurs. 2016, 72, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- de Veer, A.J.; Fleuren, M.A.; Bekkema, N.; Francke, A.L. Successful implementation of new technologies in nursing care: A questionnaire survey of nurse-users. BMC Med. Inform. Decis. Mak. 2011, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Watkins, T.; Whisman, L.; Booker, P. Nursing assessment of continuous vital sign surveillance to improve patient safety on the medical/surgical unit. J. Clin. Nurs. 2016, 25, 278–281. [Google Scholar] [CrossRef]
- Rouleau, G.; Gagnon, M.P.; Côté, J.; Payne-Gagnon, J.; Hudson, E.; Dubois, C.A. Impact of Information and Communication Technologies on Nursing Care: Results of an Overview of Systematic Reviews. J. Med. Internet Res. 2017, 19, e122. [Google Scholar] [CrossRef]
- Sprogis, S.K.; Currey, J.; Considine, J. Patient acceptability of wearable vital sign monitoring technologies in the acute care setting: A systematic review. J. Clin. Nurs. 2019, 28, 2732–2744. [Google Scholar] [CrossRef]
- Dall’Ora, C.; Griffiths, P.; Hope, J.; Barker, H.; Smith, G.B. What is the nursing time and workload involved in taking and recording patients’ vital signs? A systematic review. J. Clin. Nurs. 2020, 29, 2053–2068. [Google Scholar] [CrossRef]
- Leenen, J.P.L.; Dijkman, E.M.; van Hout, A.; Kalkman, C.J.; Schoonhoven, L.; Patijn, G.A. Nurses’ experiences with continuous vital sign monitoring on the general surgical ward: A qualitative study based on the Behaviour Change Wheel. BMC Nurs. 2022, 21, 60. [Google Scholar] [CrossRef]
- Sotera Wireless. ViSi Mobile System General Information. Available online: https://www.soterawireless.com/faqs (accessed on 27 July 2021).
- World Medical Association. WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving HUMAN subjects; WMA: Ferney-Voltaire, France, 2018. [Google Scholar]
- Sharma, A.; Minh Duc, N.T.; Luu Lam Thang, T.; Nam, N.H.; Ng, S.J.; Abbas, K.S.; Huy, N.T.; Marušić, A.; Paul, C.L.; Kwok, J.; et al. A Consensus-Based Checklist for Reporting of Survey Studies (CROSS). J. Gen. Intern. Med. 2021, 36, 3179–3187. [Google Scholar] [CrossRef]
- Leenen, J.P.L.; Leerentveld, C.; van Dijk, J.D.; van Westreenen, H.L.; Schoonhoven, L.; Patijn, G.A. Current Evidence for Continuous Vital Signs Monitoring by Wearable Wireless Devices in Hospitalized Adults: Systematic Review. J. Med. Internet Res. 2020, 22, e18636. [Google Scholar] [CrossRef]
- Weenk, M.; van Goor, H.; Frietman, B.; Engelen, L.J.; van Laarhoven, C.J.; Smit, J.; Bredie, S.J.; van de Belt, T.H. Continuous Monitoring of Vital Signs Using Wearable Devices on the General Ward: Pilot Study. JMIR mHealth uHealth 2017, 5, e91. [Google Scholar] [CrossRef] [PubMed]
- Schoot, T.S.; Weenk, M.; van de Belt, T.H.; Engelen, L.J.; van Goor, H.; Bredie, S.J. A New Cuffless Device for Measuring Blood Pressure: A Real-Life Validation Study. J. Med. Internet. Res. 2016, 18, e85. [Google Scholar] [CrossRef]
- Haahr-Raunkjaer, C.; Skovbye, M.; Rasmussen, S.M.; Elvekjaer, M.; Sørensen, H.B.D.; Meyhoff, C.S.; Aasvang, E.K. Agreement between standard and continuous wireless vital sign measurements after major abdominal surgery: A clinical comparison study. Physiol. Meas. 2022, 43, 115007. [Google Scholar] [CrossRef]
- Soller, B.; McCombie, D.; Kanter, B. A Blood Pressure Study Demonstrating Equivalence of the ViSi Mobile® System and GE DINAMAP™ CARESCAPE™ V100. 2015. Available online: https://cdn2.hubspot.net/hubfs/5599582/SoteraWireless-April2020/PDF/White-Paper-ViSi-Mobile-and-GE-Dinamap-Carescape-White-Paper-3.pdf?hsCtaTracking=69d7ffd3-0839-4199-87b5-8637d3bf6196%7Ce347d48c-2f99-4db4-ada4-e17a1d32cc1f (accessed on 18 January 2023).
- Nayak, M.S.D.P.; Narayan, K. Strengths and weaknesses of online surveys. IOSR-JHSS 2019, 24, 31–38. [Google Scholar]
- Williams, M.; Moser, T. The art of coding and thematic exploration in qualitative research. Int. Manag. Rev. 2019, 15, 45–55. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Proot, I.; Van der Lyke, S.; Smits, M. Qualitative research [Kwalitatief onderzoek]. In Evidence-Based Practice for Nurses: Shared Decision-Making [Evidence-Based Practice Voor Verpleegkundigen: Gezamenlijke Geïnformeerde Besluitvorming], 4th ed.; Munten, G., Verhoef, J., Kuiper, C., Eds.; Boom: Amsterdam, The Netherlands, 2016; pp. 191–208. [Google Scholar]
- Zwieten, M.; Willems, D. Valuation of qualitative research [Waardering van kwalitatief onderzoek]. Huisarts Wet. 2004, 47, 38–43. [Google Scholar] [CrossRef]
- Gagnon, M.P.; Desmartis, M.; Labrecque, M.; Car, J.; Pagliari, C.; Pluye, P.; Fremont, P.; Gagnon, J.; Tremblay, N.; Legare, F. Systematic review of factors influencing the adoption of information and communication technologies by healthcare professionals. J. Med. Syst. 2012, 36, 241–277. [Google Scholar] [CrossRef]
- Schoville, R.; Titler, M.G. Integrated Technology Implementation Model: Examination and Enhancements. Comput. Inform. Nurs. 2020, 38, 579–589. [Google Scholar] [CrossRef]
- Areia, C.; King, E.; Ede, J.; Young, L.; Tarassenko, L.; Watkinson, P.; Vollam, S. Experiences of current vital signs monitoring practices and views of wearable monitoring: A qualitative study in patients and nurses. J. Adv. Nurs. 2021, 78, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pope, N.; Bosco, A.M.; Mason, J.; Morgan, A. Issues affecting nurses’ capability to use digital technology at work: An integrative review. J. Clin. Nurs. 2020, 29, 2801–2819. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on the Robert Wood Johnson Foundation Initiative on the Future of Nursing, at the Institute of Medicine. The Future of Nursing: Leading Change, Advancing Health; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Hamerlijnck, D.; Tummers, M.; Kvaerner, K.; Sampietro-Colom, L.; Siebert, M.; Krahn, M.; Melien, O.; Abrishami, P.; Grutters, J. On the integration of early health technology assessment in the innovation process: Reflections from five stakeholders. Int. J. Technol. Assess. Health Care 2020, 36, 481–485. [Google Scholar] [CrossRef]
- Downey, C.L.; Tahir, W.; Randell, R.; Brown, J.M.; Jayne, D.G. Strengths and limitations of early warning scores: A systematic review and narrative synthesis. Int. J. Nurs. Stud. 2017, 76, 106–119. [Google Scholar] [CrossRef] [PubMed]
- van der Weegen, S.; Galle, I. Implementation Toolkit TECHNOLOGY in Healthcare [Implementatie Toolkit Technologie in de zorg]; Vilans: Utrecht, The Netherlands. Available online: https://www.waardigheidentrots.nl/wp-content/uploads/2017/12/Implementatie-toolkit-technologie-definitief.pdf (accessed on 18 January 2023).
- Kooij, L.; Peters, G.M.; Doggen, C.J.M.; van Harten, W.H. Remote continuous monitoring with wireless wearable sensors in clinical practice, nurses perspectives on factors affecting implementation: A qualitative study. BMC Nurs. 2022, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, J.A.; Woltjer, H.; Kool, R.B. Identification of Factors Influencing the Adoption of Health Information Technology by Nurses Who Are Digitally Lagging: In-Depth Interview Study. J. Med. Internet Res. 2020, 22, e15630. [Google Scholar] [CrossRef]
- Khanna, A.K.; Hoppe, P.; Saugel, B. Automated continuous noninvasive ward monitoring: Future directions and challenges. Crit. Care 2019, 23, 194. [Google Scholar] [CrossRef]
- Vears, D.F.; Gillam, L. Inductive content analysis: A guide for beginning qualitative researchers. FoHPE 2022, 23, 111–127. Available online: https://search.informit.org/doi/10.3316/informit.455663644555599 (accessed on 18 January 2023). [CrossRef]
- Al-rawashdeh, M.; Keikhosrokiani, P.; Belaton, B.; Alawida, M.; Zwiri, A. IoT Adoption and Application for Smart Healthcare: A Systematic Review. Sensors 2022, 22, 5377. [Google Scholar] [CrossRef]
- Dwivedi, Y.K.; Rana, N.P.; Tamilmani, K.; Raman, R. A meta-analysis based modified unified theory of acceptance and use of technology (meta-UTAUT): A review of emerging literature. Curr. Opin. Psychol. 2020, 36, 13–18. [Google Scholar] [CrossRef]
- Dwivedi, Y.K.; Rana, N.P.; Jeyaraj, A.; Clement, M.; Williams, M.D. Re-examining the Unified Theory of Acceptance and Use of Technology (UTAUT): Towards a Revised Theoretical Model. Inf. Syst. Front. 2019, 21, 719–734. [Google Scholar] [CrossRef]
- Banks, J.; McArthur, J.; Gordon, G. Flexible monitoring in the management of patient care processes: A pilot study. Lippincotts Case Manag. 2000, 5, 94–103, quiz 104–106. [Google Scholar]
- Haddad, L.M.; Annamaraju, P.; Toney-Butler, T.J. Nursing Shortage; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. QJM 2001, 94, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Sotera Wireless. Patient Monitoring System. User Manual; Sotera Wireless: San Diego, CA, USA, 2016; pp. 1–292. [Google Scholar]
| Respondents n = 58 (%) | Non-Respondents n = 53 (%) | |
|---|---|---|
| Gender | ||
| Male (%) | 9 (15.5) | 8 (15.1) |
| Female (%) | 49 (84.5) | 45 (84.9) |
| Education | ||
| Vocational educated nurse (%) | 12 (20.7) | 25 (47.2) |
| Registered nurse (%) | 46 (79.3) | 28 (52.8) |
| Ward | ||
| Abdominal/oncological surgery (%) | 22 (37.9) | 18 (34.0) |
| Internal medicine/rheumatology (%) | 27 (46.6) | 25 (47.2) |
| Gastroenterology (%) | 9 (15.5) | 10 (18.9) |
| Age group | ||
| 21–30 years (%) | 42 (72.4) | 26 (49.1) |
| 31–40 years (%) | 10 (17.2) | 9 (17.0) |
| 41–50 years (%) | 3 (5.2) | 8 (15.1) |
| 51–60 years (%) | 3 (5.2) | 7 (13.2) |
| 61–64 years (%) | 0 (0) | 3 (5.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becking-Verhaar, F.L.; Verweij, R.P.H.; de Vries, M.; Vermeulen, H.; van Goor, H.; Huisman-de Waal, G.J. Continuous Vital Signs Monitoring with a Wireless Device on a General Ward: A Survey to Explore Nurses’ Experiences in a Post-Implementation Period. Int. J. Environ. Res. Public Health 2023, 20, 5794. https://doi.org/10.3390/ijerph20105794
Becking-Verhaar FL, Verweij RPH, de Vries M, Vermeulen H, van Goor H, Huisman-de Waal GJ. Continuous Vital Signs Monitoring with a Wireless Device on a General Ward: A Survey to Explore Nurses’ Experiences in a Post-Implementation Period. International Journal of Environmental Research and Public Health. 2023; 20(10):5794. https://doi.org/10.3390/ijerph20105794
Chicago/Turabian StyleBecking-Verhaar, Femke L., Robin P. H. Verweij, Marjan de Vries, Hester Vermeulen, Harry van Goor, and Getty J. Huisman-de Waal. 2023. "Continuous Vital Signs Monitoring with a Wireless Device on a General Ward: A Survey to Explore Nurses’ Experiences in a Post-Implementation Period" International Journal of Environmental Research and Public Health 20, no. 10: 5794. https://doi.org/10.3390/ijerph20105794
APA StyleBecking-Verhaar, F. L., Verweij, R. P. H., de Vries, M., Vermeulen, H., van Goor, H., & Huisman-de Waal, G. J. (2023). Continuous Vital Signs Monitoring with a Wireless Device on a General Ward: A Survey to Explore Nurses’ Experiences in a Post-Implementation Period. International Journal of Environmental Research and Public Health, 20(10), 5794. https://doi.org/10.3390/ijerph20105794







