Analysis of the 2007–2018 National Health Interview Survey (NHIS): Examining Neurological Complications among Children with Sickle Cell Disease in the United States
Abstract
1. Introduction
2. Methods
2.1. Study Design
Secondary Analysis of NHIS Dataset
2.2. Data Source
NHIS Data
2.3. Sample Child Core Questionnaire
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Sample
3.2. Neuro-Developmental Conditions, Health Status, and Healthcare Utilization
3.3. Association of SCD Status and Demographics/SES with Neurological Comorbidities, and SDoH Outcomes including Healthcare, and Educational Service Utilization
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karkoska, K.A.; Haber, K.; Elam, M.; Strong, S.; McGann, P.T. Academic Challenges and School Service Utilization in Children with Sickle Cell Disease. J. Pediatr. 2021, 230, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Children, H. Sickle Cell Disease: Information for Parents. 2019. Available online: https://www.healthychildren.org/English/health-issues/conditions/chronic/Pages/Sickle-Cell-Disease-in-Children.aspx#:~:text=Every%20year%2C%20roughly%202%2C000%20babies,pain%20and%20ongoing%20medical%20challenges (accessed on 16 September 2022).
- Brousseau, D.C.; Panepinto, J.A.; Nimmer, M.; Hoffmann, R.G. The number of people with sickle-cell disease in the United States: National and state estimates. Am. J. Hematol. 2010, 85, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Bou-Maroun, L.M.; Meta, F.; Hanba, C.J.; Campbell, A.D.; Yanik, G.A. An analysis of inpatient pediatric sickle cell disease: Incidence, costs, and outcomes. Pediatr. Blood Cancer 2018, 65, e26758. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Data & Statistics on Sickle Cell Disease. 2020. Available online: https://www.cdc.gov/ncbddd/sicklecell/data.html (accessed on 16 September 2022).
- Peterson, E.E.; Salemi, J.L.; Dongarwar, D.; Salihu, H.M. Acute care utilization in pediatric sickle cell disease and sickle cell trait in the USA: Prevalence, temporal trends, and cost. Eur. J. Pediatr. 2020, 179, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.L.; Yanni, E.A.; Creary, M.S.; Olney, R.S. Health status and healthcare use in a national sample of children with sickle cell disease. Am. J. Prev. Med. 2010, 38 (Suppl. S4), S528–S535. [Google Scholar] [CrossRef]
- Pecker, L.H.; Darbari, D.S. Psychosocial and affective comorbidities in sickle cell disease. Neurosci. Lett. 2019, 705, 1–6. [Google Scholar] [CrossRef]
- Osunkwo, I.; Manwani, D.; Kanter, J. Current and novel therapies for the prevention of vaso-occlusive crisis in sickle cell disease. Ther. Adv. Hematol. 2020, 11, 2040620720955000. [Google Scholar] [CrossRef]
- Takaoka, K.; Cyril, A.C.; Jinesh, S.; Radhakrishnan, R. Mechanisms of pain in sickle cell disease. Br. J. Pain 2021, 15, 213–220. [Google Scholar] [CrossRef]
- Wang, Y.; Hardy, S.J.; Ichesco, E.; Zhang, P.; Harris, R.E.; Darbari, D.S. Alteration of grey matter volume is associated with pain and quality of life in children with sickle cell disease. Transl. Res. 2022, 240, 17–25. [Google Scholar] [CrossRef]
- Zaidi, A.U.; Glaros, A.K.; Lee, S.; Wang, T.; Bhojwani, R.; Morris, E.; Donohue, B.; Paulose, J.; Iorga, Ş.R.; Nellesen, D. A systematic literature review of frequency of vaso-occlusive crises in sickle cell disease. Orphanet. J. Rare Dis. 2021, 16, 460. [Google Scholar] [CrossRef]
- Bakri, M.H.; Ismail, E.A.; Elsedfy, G.O.; Amr, M.A.; Ibrahim, A. Behavioral impact of sickle cell disease in young children with repeated hospitalization. Saudi J. Anaesth. 2014, 8, 504. [Google Scholar] [CrossRef]
- Dale, J.C.; Cochran, C.J.; Roy, L.; Jernigan, E.; Buchanan, G.R. Health-related quality of life in children and adolescents with sickle cell disease. J. Pediatr. Health Care 2011, 25, 208–215. [Google Scholar] [CrossRef]
- Bonner, M.J.; Schumacher, E.; Gustafson, K.E.; Thompson, R.J., Jr. The impact of sickle cell disease on cognitive functioning and learning. Sch. Psychol. Rev. 1999, 28, 182–193. [Google Scholar] [CrossRef]
- Rabiner, D.L.; Carrig, M.M.; Dodge, K.A. Attention Problems and Academic Achievement: Do Persistent and Earlier-Emerging Problems Have More Adverse Long-Term Effects? J. Atten. Disord. 2016, 20, 946–957. [Google Scholar] [CrossRef]
- Raphael, J.L.; Dietrich, C.L.; Whitmire, D.; Mahoney, D.H.; Mueller, B.U.; Giardino, A.P. Healthcare utilization and expenditures for low income children with sickle cell disease. Pediatr. Blood Cancer 2009, 52, 263–267. [Google Scholar] [CrossRef]
- Lance, E.I.; Comi, A.M.; Johnston, M.V.; Casella, J.F.; Shapiro, B.K. Risk factors for attention and behavioral issues in pediatric sickle cell disease. Clin. Pediatr. 2015, 54, 1087–1093. [Google Scholar] [CrossRef]
- Khan, H.; Krull, M.; Hankins, J.S.; Wang, W.C.; Porter, J.S. Sickle cell disease and social determinants of health: A scoping review. Pediatr. Blood Cancer 2023, 70, e30089. [Google Scholar] [CrossRef]
- Nero, A.; Bozzo, J. Economics of Sickle Cell Disease and Evidence to Support Comprehensive Care. Hematol. Oncol. Clin. N. Am. 2022, 36, 1125–1135. [Google Scholar] [CrossRef]
- Raphael, J.L. Addressing social determinants of health in sickle cell disease: The role of Medicaid policy. Pediatr. Blood Cancer 2020, 67, e28202. [Google Scholar] [CrossRef]
- Treadwell, M.J.; Anie, K.A. Quality of Life in Sickle Cell Disease: What Matters. Hematol. Oncol. Clin. N. Am. 2022, 36, 1137–1149. [Google Scholar] [CrossRef]
- Braveman, P.; Gottlieb, L. The social determinants of health: It’s time to consider the causes of the causes. Public Health Rep. 2014, 129 (Suppl. S2), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, J.; Tampubolon, S.; Lee, J.T.; Islam, F.; Ojo, T.; Opeyemi, J.; Qiao, W.; Mai, A.; Wang, C.; Vieira, D.; et al. Characterisation of medical conditions of children with sickle cell disease in the USA: Findings from the 2007–2018 National Health Interview Survey (NHIS). BMJ Open 2023, 13, e069075. [Google Scholar] [CrossRef] [PubMed]
- 2021 Federal Poverty Levels/Guidelines & How They Determine Medicaid Eligibility. 2021. Available online: https://www.medicaidplanningassistance.org/federal-poverty-guidelines/ (accessed on 29 October 2021).
- Centers for Disease Control and Prevention. About the National Health Interview Survey. 2023. Available online: https://www.cdc.gov/nchs/nhis/about_nhis.htm (accessed on 15 March 2023).
- Stata Corp. Stata Statistical Software: Release 15; StataCorp LLC: College Station, TX, USA, 2017. [Google Scholar]
- Dickman, S.L.; Himmelstein, D.U.; Woolhandler, S. Inequality and the health-care system in the USA. Lancet 2017, 389, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Kayle, M.; Valle, J.; Paulukonis, S.; Holl, J.L.; Tanabe, P.; French, D.D.; Garg, R.; Liem, R.I.; Badawy, S.M.; Treadwell, M.J. Impact of Medicaid expansion on access and healthcare among individuals with sickle cell disease. Pediatr. Blood Cancer 2020, 67, e28152. [Google Scholar] [CrossRef]
- Jesus, A.; Konstantyner, T.; Lobo, I.K.V.; Braga, J.A.P. Socioeconomic and Nutritional Characteristics of Children and Adolescents with Sickle Cell Anemia: A Systematic Review. Rev. Paul. Pediatr. 2018, 36, 491–499. [Google Scholar] [CrossRef]
- Loo, S.; Brochier, A.; Wexler, M.G.; Long, K.; Kavanagh, P.L.; Garg, A.; Drainoni, M.-L. Addressing unmet basic needs for children with sickle cell disease in the United States: Clinic and staff perspectives. BMC Health Serv. Res. 2021, 21, 55. [Google Scholar] [CrossRef]
- Bulgin, D.; Tanabe, P.; Jenerette, C. Stigma of Sickle Cell Disease: A Systematic Review. Issues Ment. Health Nurs. 2018, 39, 675–686. [Google Scholar] [CrossRef]
- Lee, L.; Smith-Whitley, K.; Banks, S.; Puckrein, G. Reducing Health Care Disparities in Sickle Cell Disease: A Review. Public Health Rep. 2019, 134, 599–607. [Google Scholar] [CrossRef]
- DeLaune, J.; Close, J.; Murphy, M. Addressing bias towards patients with sickle cell disease. Lancet Haematol. 2020, 7, e508. [Google Scholar] [CrossRef]
- Abdallah, K.; Buscetta, A.; Cooper, K.; Byeon, J.; Crouch, A.; Pink, S.; Minniti, C.; Bonham, V.L. Emergency Department Utilization for Patients Living with Sickle Cell Disease: Psychosocial Predictors of Health Care Behaviors. Ann. Emerg. Med. 2020, 76, S56–S63. [Google Scholar] [CrossRef]
- Reich, J.; Cantrell, M.A.; Smeltzer, S.C. An Integrative Review: The Evolution of Provider Knowledge, Attitudes, Perceptions and Perceived Barriers to Caring for Patients with Sickle Cell Disease 1970-Now. J. Pediatr. Hematol. Oncol. Nurs. 2023, 40, 43–64. [Google Scholar] [CrossRef]
- Haywood, C., Jr.; Diener-West, M.; Strouse, J.; Carroll, C.P.; Bediako, S.; Lanzkron, S.; Haythornthwaite, J.; Onojobi, G.; Beach, M.C. Perceived discrimination in health care is associated with a greater burden of pain in sickle cell disease. J. Pain Symptom. Manag. 2014, 48, 934–943. [Google Scholar] [CrossRef]
- Martin, S.R.; Cohen, L.L.; Mougianis, I.; Griffin, A.; Sil, S.; Dampier, C. Stigma and Pain in Adolescents Hospitalized for Sickle Cell Vasoocclusive Pain Episodes. Clin. J. Pain 2018, 34, 438–444. [Google Scholar] [CrossRef]
- Stanton, M.V.; Jonassaint, C.R.; Bartholomew, F.B.; Edwards, C.; Richman, L.; DeCastro, L.; Williams, R. The association of optimism and perceived discrimination with health care utilization in adults with sickle cell disease. J. Natl. Med. Assoc. 2010, 102, 1056–1063. [Google Scholar] [CrossRef]
- Brennan-Cook, J.; Bonnabeau, E.; Aponte, R.; Augustin, C.; Tanabe, P. Barriers to Care for Persons with Sickle Cell Disease: The Case Manager’s Opportunity to Improve Patient Outcomes. Prof. Case Manag. 2018, 23, 213–219. [Google Scholar] [CrossRef]
- Maxwell, K.; Streetly, A.; Bevan, D. Experiences of hospital care and treatment seeking for pain from sickle cell disease: Qualitative study. BMJ 1999, 318, 1585–1590. [Google Scholar] [CrossRef]
- Masese, R.V.; Bulgin, D.; Douglas, C.; Shah, N.; Tanabe, P. Barriers and facilitators to care for individuals with sickle cell disease in central North Carolina: The emergency department providers’ perspective. PLoS ONE 2019, 14, e0216414. [Google Scholar] [CrossRef]
- Brown, R.T.; Armstrong, F.D.; Eckman, J.R. Neurocognitive aspects of pediatric sickle cell disease. J. Learn. Disabil. 1993, 26, 33–45. [Google Scholar] [CrossRef]
- Jabine, T.B. Reporting Chronic Conditions in the National Health Interview Survey: A Review of Findings from Evaluation Studies and Methodological Test; Vital Health Stat 2; U.S. Government Printing Office: Washington, DC, USA, 1987. [Google Scholar]
- Schmier, J.K.; Halpern, M.T. Patient recall and recall bias of health state and health status. Expert Rev. Pharm. Outcomes Res. 2004, 4, 159–163. [Google Scholar] [CrossRef]
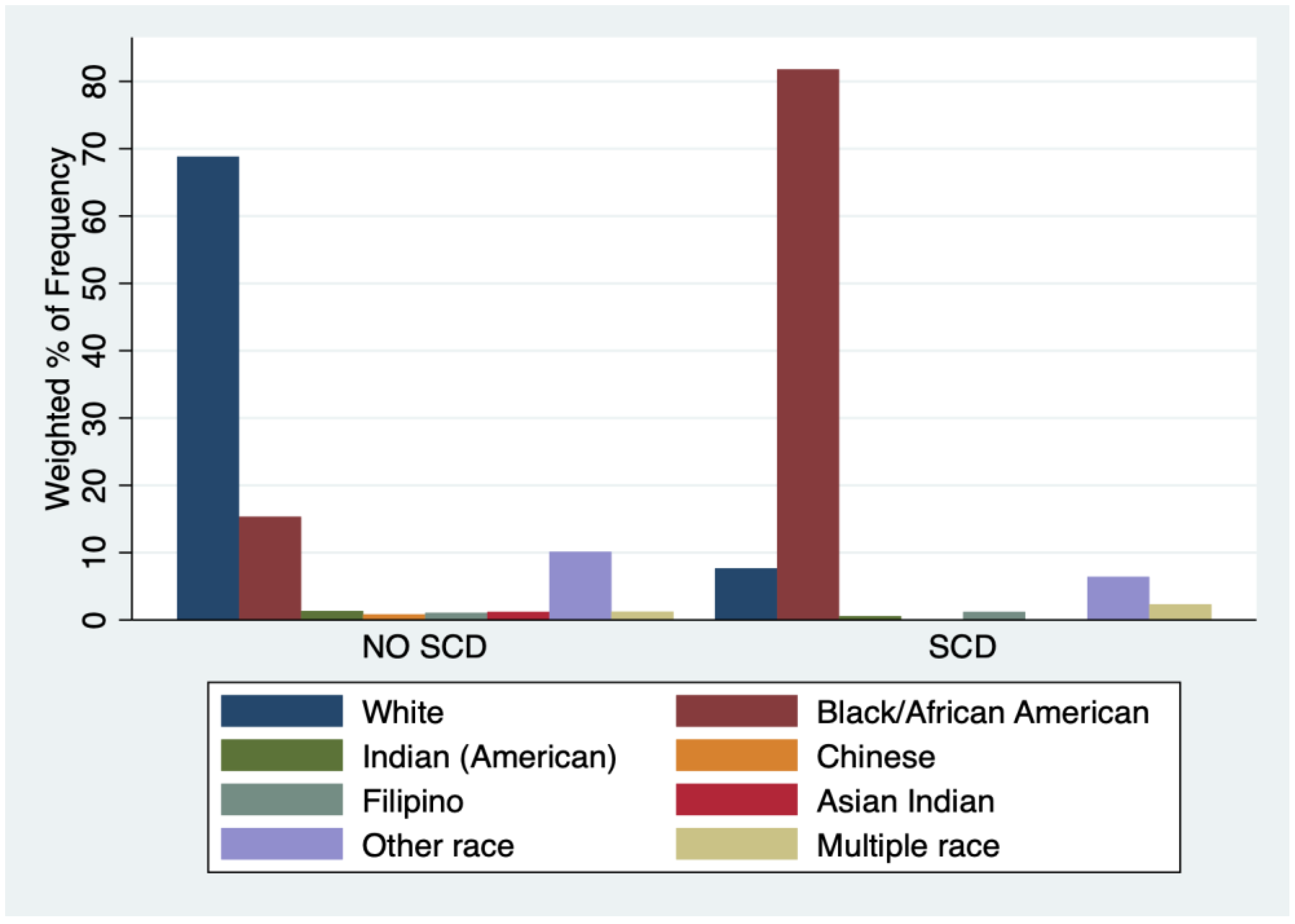
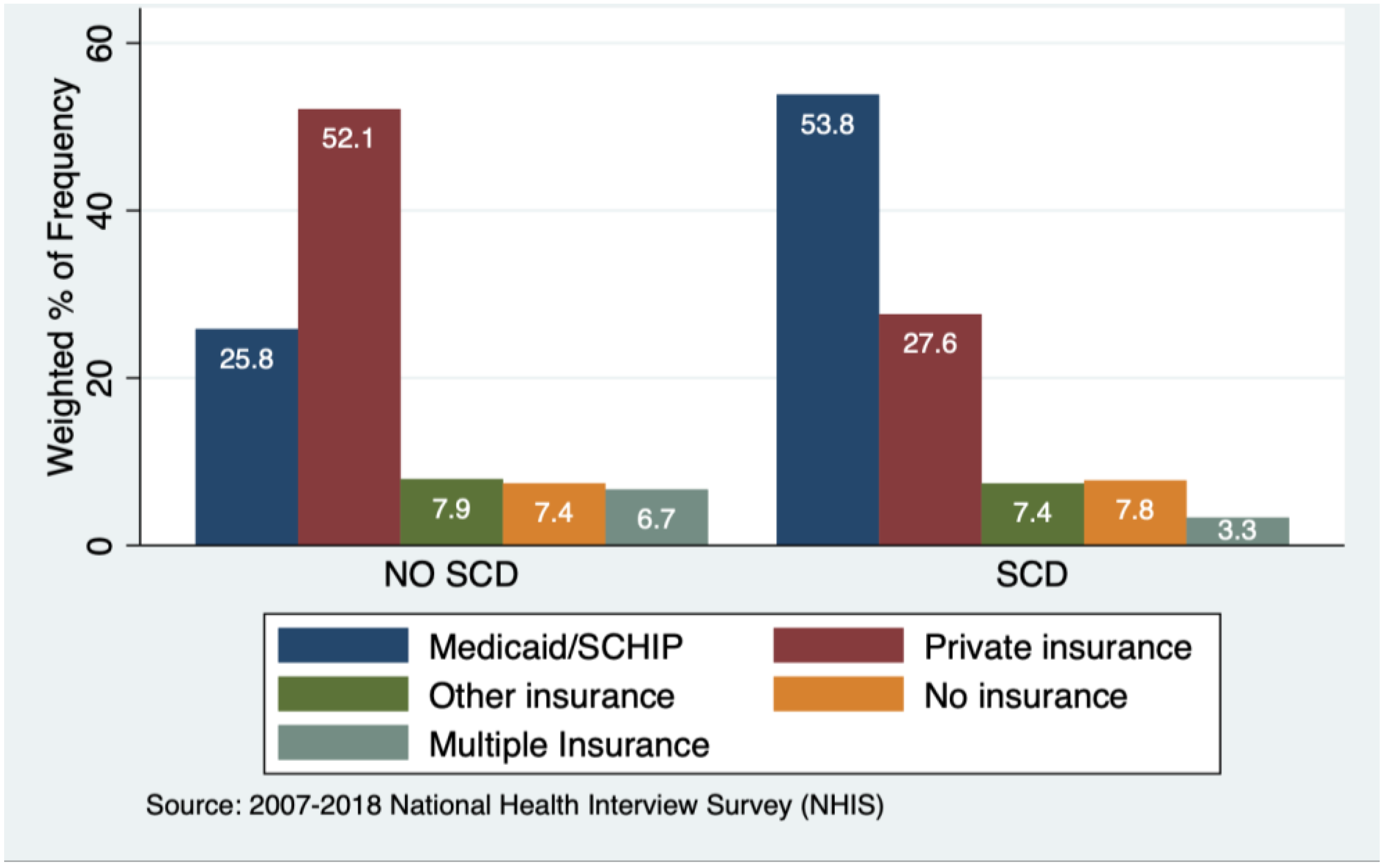
| Table 1(a) | ||||||
|---|---|---|---|---|---|---|
| Children with SCD (n = 215) | Children without SCD (n = 133,266) | AOR for SCD vs. No SCD (95% CI) | p Value | |||
| Developmental Conditions | Unweighted | Weighted % | Unweighted n | Weighted % | ||
| ADD/ADHD | 27 | 14.32 | 9754 | 8.34 | 0.8 (0.3, 2.0) | 0.657 |
| Learning Disability | 28 | 17.08 | 8497 | 7.70 | 1.5 (0.6, 3.5) | 0.350 |
| Intellectual Disability | 5 | 4.11 | 1230 | 0.99 | 4.1 (1.3, 13.3) | 0.018 |
| Trouble Hearing | 10 | 5.28 | 2679 | 2.09 | 2.5 (1.0, 6.0) | 0.039 |
| Trouble Seeing | 10 | 5.67 | 3718 | 2.80 | 2.0 (0.8, 4.6) | 0.114 |
| Other developmental delay | 2 | 5.21 | 268 | 1.97 | 0.9 (0.1, 7.3) | 0.947 |
| Table 1(b) | ||||||
| Children with SCD Unweighted (n = 215) | Children without SCD Unweighted (n = 133,266) | AOR for SCD vs. No SCD (95% CI) | p Value | |||
| Health Impact | Unweighted n | Weighted % | Unweighted n | Weighted % | ||
| Limited in ability to crawl, walk, run, or play | 22 | 12.70 | 2340 | 1.75 | 7.2 (3.9, 13.3) | <0.001 |
| Needed special equipment | 4 | 1.47 | 1566 | 1.16 | 1.3 (0.4, 4.4) | 0.647 |
| Took prescription medication for at least three months | 78 | 36.44 | 17,308 | 12.95 | 3.8 (2.4, 6.2) | <0.001 |
| Reported health status fair or poor | ||||||
| Better | 69 | 33.42 | 29,475 | 20.73 | 1.5 (1.0, 2.3) | 0.035 |
| Worse | 10 | 4.63 | 1792 | 1.33 | 4.1 (1.8, 9.0) | 0.001 |
| About the same | 135 | 61.95 | 101,648 | 77.95 | 0.5 (0.4, 0.8) | 0.003 |
| Miss school because of illness or injury | ||||||
| 0 | 29 | 24.85 | 28,969 | 30.12 | 0.4 (0.2, 0.8) | 0.007 |
| 1–10 | 78 | 59.37 | 60,803 | 65.45 | 1.1 (0.7, 1.8) | 0.723 |
| 11–20 | 14 | 8.82 | 3176 | 3.26 | 3.8 (1.8, 7.7) | <0.001 |
| 21–30 | 5 | 2.84 | 746 | 0.71 | 6.5 (2.4, 17.6) | <0.001 |
| 31–40 | 0 | - | 174 | 0.15 | - | - |
| 40+ | 5 | 4.11 | 328 | 0.31 | 14.0 (4.5, 43.1) | <0.001 |
| Healthcare and special education services use (past 12 months) | ||||||
| Saw a medical specialist | 56 | 23.56 | 18,836 | 14.29 | 2.3 (1.5, 3.7) | <0.001 |
| Saw mental health professional | 22 | 9.82 | 9219 | 7.81 | 1.04 (0.5, 2.2) | 0.911 |
| Saw a therapist | 22 | 13.20 | 8558 | 7.55 | 2.0 (1.0, 4.1) | 0.040 |
| Saw an optometrist, ophthalmologist, or eye doctor | 57 | 31.20 | 31,873 | 26.89 | 1.4 (0.9, 2.3) | 0.126 |
| How many times have you seen a doctor within 12 months? | ||||||
| none | 9 | 3.06 | 12,651 | 9.19 | 0.2 (0.1, 0.6) | 0.003 |
| 1–5 | 142 | 68.47 | 99,137 | 75.91 | 0.5 (0.4, 0.8) | 0.004 |
| 6–9 | 28 | 14.72 | 11,842 | 8.82 | 2.6 (1.4, 4.8) | 0.002 |
| 10–15 | 21 | 9.55 | 5239 | 3.86 | 3.5 (1.9, 6.4) | <0.001 |
| 16 or more | 12 | 4.20 | 2921 | 2.22 | 2.2 (0.9, 5.2) | 0.073 |
| Multiple visits to emergency room | ||||||
| none | 125 | 55.32 | 106,956 | 81.24 | 0.4 (0.2, 0.6) | <0.001 |
| 1 | 34 | 15.75 | 16,969 | 12.50 | 1.0 (0.5, 1.7) | 0.935 |
| 2–3 | 32 | 17.28 | 6869 | 5.01 | 2.9 (1.6, 5.2) | 0.001 |
| 4–5 | 13 | 6.54 | 1089 | 0.79 | 4.8 (2.3, 10.2) | <0.001 |
| 6–16+ | 11 | 5.11 | 634 | 0.45 | 9.3 (3.6, 24.0) | <0.001 |
| Had surgery or another surgical procedure | 15 | 3.92 | 6286 | 4.76 | 1.0 (0.5, 2.0) | 0.957 |
| Healthcare Barrier | ||||||
| Couldn’t use telephone | 5 | 3.62 | 2330 | 1.74 | 1.4 (0.4, 5.5) | 0.613 |
| Couldn’t get an appointment | 11 | 5.66 | 5882 | 4.45 | 1.1 (0.5, 2.5) | 0.858 |
| Waited too long at the doctor’s office | 11 | 2.63 | 5457 | 3.95 | 0.6 (0.2, 1.3) | 0.163 |
| No transportation | 12 | 4.42 | 2416 | 1.83 | 0.8 (0.3, 1.9) | 0.646 |
| Couldn’t afford prescription medicines | 1 | 5.45 | 230 | 1.37 | 5.1 (0.9, 28.3) | 0.065 |
| Table 2(a) | ||||||
|---|---|---|---|---|---|---|
| Black Children with SCD Unweighted (n = 170) | Black Children without SCD Unweighted (n = 21,395) | AOR for SCD vs. No SCD (95% CI) | p Value | |||
| Developmental Conditions | Unweighted n | Weighted % | Unweighted n | Weighted % | ||
| ADD/ADHD | 23 | 14.05 | 1806 | 9.43 | 5 (0.2, 1.2) | 0.117 |
| Learning Disability | 23 | 17.64 | 1563 | 8.96 | 1.3 (0.5, 3.1) | 0.620 |
| Intellectual Disability | 2 | 2.49 | 239 | 1.25 | 2.4 (0.6, 9.9) | 0.232 |
| Trouble Hearing | 8 | 3.87 | 524 | 2.56 | 1.6 (0.6, 4.1) | 0.303 |
| Trouble Seeing | 10 | 6.93 | 698 | 3.64 | 2.3 (1.0, 5.4) | 0.047 |
| Other developmental delay | 2 | 7.36 | 38 | 1.58 | 1.9 (0.2, 23.0) | 0.618 |
| Table 2(b) | ||||||
| Black Children with SCD Unweighted (n = 170) | Black Children without SCD Unweighted (n = 21,395) | AOR for SCD vs. No SCD (95% CI) | p Value | |||
| Health Impact | Unweighted n | Weighted % | Unweighted n | Weighted % | ||
| Limited in ability to crawl, walk, run, or play | 21 | 13.63 | 399 | 1.83 | 7.8 (4.2, 14.4) | <0.001 |
| Needed special equipment | 3 | 1.45 | 263 | 1.23 | 1.3 (0.3, 5.5) | 0.707 |
| Took prescription medication for at least three months | 69 | 38.90 | 3139 | 14.00 | 3.9 (2.3, 6.4) | <0.001 |
| Reported health status fair or poor | Unweighted n | Unweighted n | ||||
| Better | 58 | 36.73 | 5124 | 24.10 | 1.8 (1.2, 2.9) | 0.008 |
| Worse | 7 | 3.74 | 258 | 1.35 | 3.1 (1.0, 9.2) | 0.041 |
| About the same | 104 | 59.52 | 15,920 | 74.54 | 0.5 (0.3, 0.8) | 0.001 |
| Miss school because of illness or injury (days) | Unweighted n | Unweighted n | ||||
| 0 | 24 | 26.78 | 5944 | 39.56 | 0.5 (0.2, 0.9) | 0.028 |
| 1–10 | 64 | 55.81 | 8749 | 57.10 | 0.9 (0.5, 1.6) | 0.758 |
| 11–20 | 13 | 9.38 | 407 | 2.42 | 4.1 (1.9, 9.0) | <0.001 |
| 21–30 | 5 | 3.28 | 102 | 0.55 | 8.4 (2.9, 24.3) | <0.001 |
| 31–40 | 0 | - | 17 | 0.01 | - | - |
| 40+ | 5 | 4.75 | 39 | 0.28 | 19.0 (5.5, 65.5) | <0.001 |
| Healthcare and special education services use (past 12 months) | Unweighted n | Unweighted n | ||||
| Saw a medical specialist | 47 | 23.52 | 2507 | 11.56 | 2.5 (1.5, 4.2) | 0.001 |
| Saw mental health professional | 19 | 10.74 | 1417 | 7.38 | 1.1 (0.5, 2.3) | 0.900 |
| Saw a therapist | 16 | 12.56 | 1258 | 6.83 | 2.0 (0.9, 4.1) | 0.074 |
| Saw an optometrist, ophthalmologist, or eye doctor | 50 | 32.18 | 4878 | 25.37 | 1.5 (0.9, 2.5) | 0.144 |
| How many times have you seen a doctor within 12 months)? | Unweighted n | Unweighted n | ||||
| none | 8 | 3.57 | 2022 | 9.25 | 0.2 (0.1, 0.7) | 0.009 |
| 1–5 | 111 | 69.22 | 16,583 | 79.38 | 0.6 (0.4, 0.9) | 0.019 |
| 6–9 | 22 | 13.30 | 1510 | 6.98 | 2.3 (1.2, 4.5) | 0.010 |
| 10–15 | 17 | 9.44 | 628 | 2.92 | 3.7 (1.9, 7.3) | <0.001 |
| 16 or more | 10 | 4.47 | 325 | 1.48 | 2.6 (1.0, 6.8) | 0.059 |
| Multiple visits to emergency room | Unweighted n | Unweighted n | ||||
| none | 91 | 52.68 | 16,058 | 76.34 | 0.3 (0.2, 0.6) | <0.001 |
| 1 | 30 | 17.42 | 3173 | 14.66 | 1.1 (0.6, 1.9) | 0.773 |
| 2–3 | 28 | 18.14 | 1572 | 7.15 | 2.8 (1.5, 5.4) | 0.002 |
| 4–5 | 11 | 7.31 | 266 | 1.15 | 5.1 (2.3, 11.3) | <0.001 |
| 6–16+ | 10 | 4.45 | 148 | 0.69 | 7.3 (3.0, 17.5) | <0.001 |
| Had surgery or another surgical procedure | 13 | 3.82 | 824 | 3.89 | 1.0 (0.4, 2.0) | 0.905 |
| Healthcare Barrier | Unweighted n | Unweighted n | ||||
| Couldn’t use telephone | 5 | 4.42 | 361 | 1.79 | 1.7 (0.4, 6.7) | 0.431 |
| Couldn’t get an appointment | 8 | 6.14 | 964 | 4.58 | 1.2 (0.5, 3.0) | 0.717 |
| Waited too long at the doctor’s office | 4 | 1.33 | 808 | 3.87 | 0.3 (0.1, 1.1) | 0.064 |
| No transportation | 10 | 4.70 | 622 | 3.24 | 0.8 (0.3, 2.1) | 0.632 |
| Couldn’t afford prescription medicines | 1 | 7.70 | 41 | 1.30 | 8.4 (2.4, 29.4) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peprah, E.; Gyamfi, J.; Lee, J.T.; Islam, F.; Opeyemi, J.; Tampubolon, S.; Ojo, T.; Qiao, W.; Mai, A.; Wang, C.; et al. Analysis of the 2007–2018 National Health Interview Survey (NHIS): Examining Neurological Complications among Children with Sickle Cell Disease in the United States. Int. J. Environ. Res. Public Health 2023, 20, 6137. https://doi.org/10.3390/ijerph20126137
Peprah E, Gyamfi J, Lee JT, Islam F, Opeyemi J, Tampubolon S, Ojo T, Qiao W, Mai A, Wang C, et al. Analysis of the 2007–2018 National Health Interview Survey (NHIS): Examining Neurological Complications among Children with Sickle Cell Disease in the United States. International Journal of Environmental Research and Public Health. 2023; 20(12):6137. https://doi.org/10.3390/ijerph20126137
Chicago/Turabian StylePeprah, Emmanuel, Joyce Gyamfi, Justin Tyler Lee, Farha Islam, Jumoke Opeyemi, Siphra Tampubolon, Temitope Ojo, Wanqiu Qiao, Andi Mai, Cong Wang, and et al. 2023. "Analysis of the 2007–2018 National Health Interview Survey (NHIS): Examining Neurological Complications among Children with Sickle Cell Disease in the United States" International Journal of Environmental Research and Public Health 20, no. 12: 6137. https://doi.org/10.3390/ijerph20126137
APA StylePeprah, E., Gyamfi, J., Lee, J. T., Islam, F., Opeyemi, J., Tampubolon, S., Ojo, T., Qiao, W., Mai, A., Wang, C., Vieira, D., Meda, S., Adenikinju, D., Osei-Tutu, N., Ryan, N., & Ogedegbe, G. (2023). Analysis of the 2007–2018 National Health Interview Survey (NHIS): Examining Neurological Complications among Children with Sickle Cell Disease in the United States. International Journal of Environmental Research and Public Health, 20(12), 6137. https://doi.org/10.3390/ijerph20126137











