Using the Multi-Theory Model (MTM) of Health Behavior Change to Explain the Seeking of Stool-Based Tests for Colorectal Cancer Screening
Abstract
1. Introduction
2. Materials and Methods
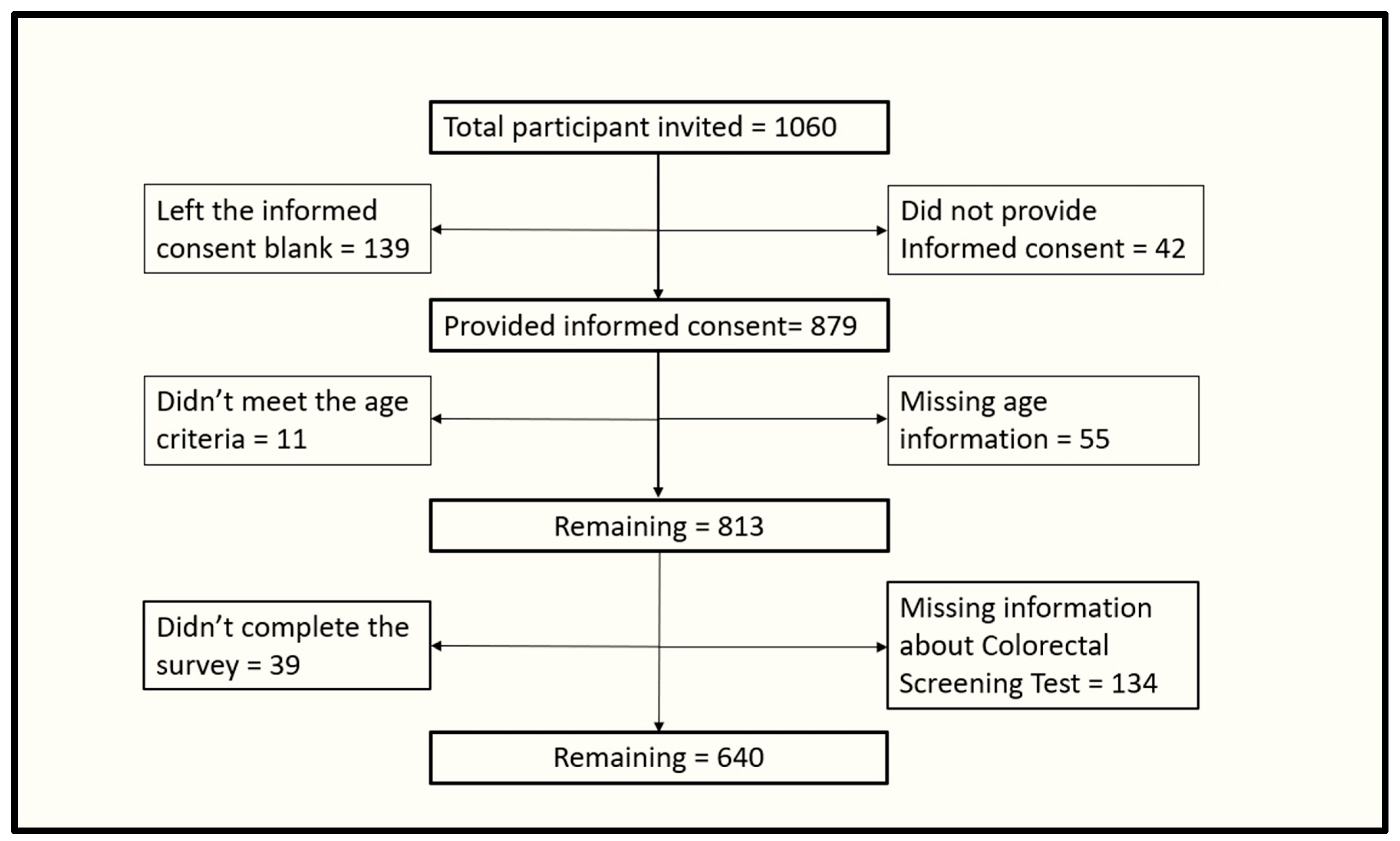
2.1. Study Design and Participants’ Recruitment
2.2. Sampling and Data Collection
2.3. Ethical Considerations
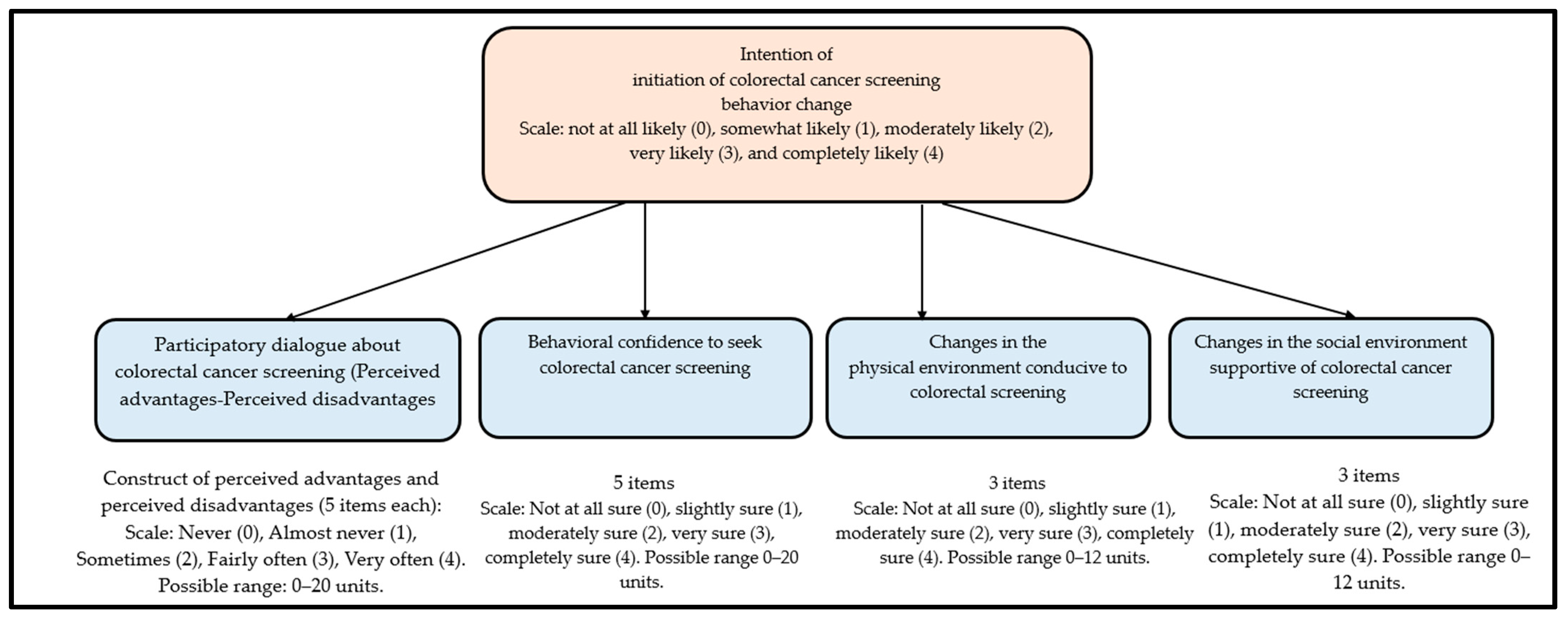
2.4. Questionaire
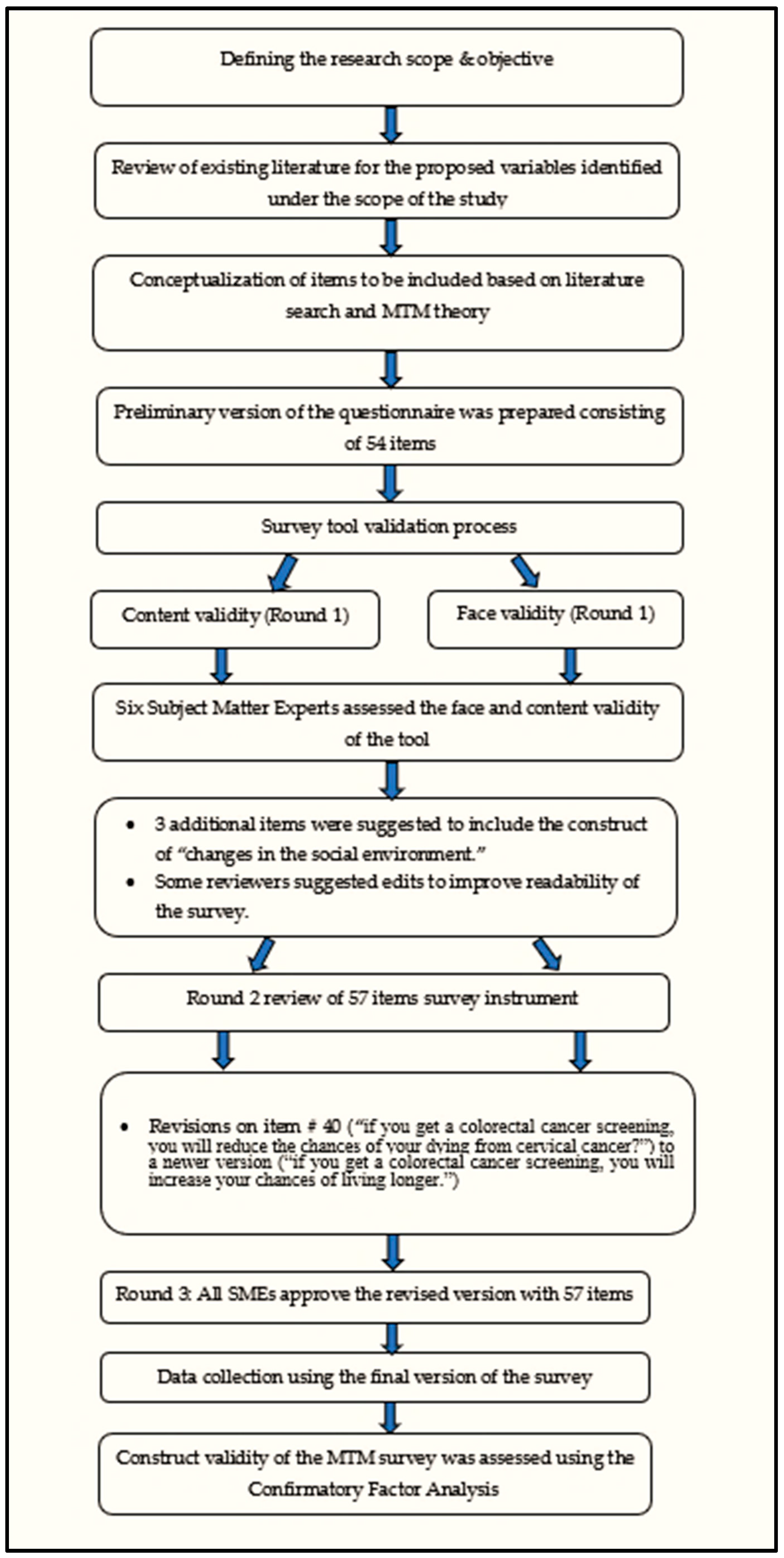
2.5. Survey Validation
2.6. Construct Validity
2.7. Testing of Assumptions
2.8. Data Analysis
3. Results
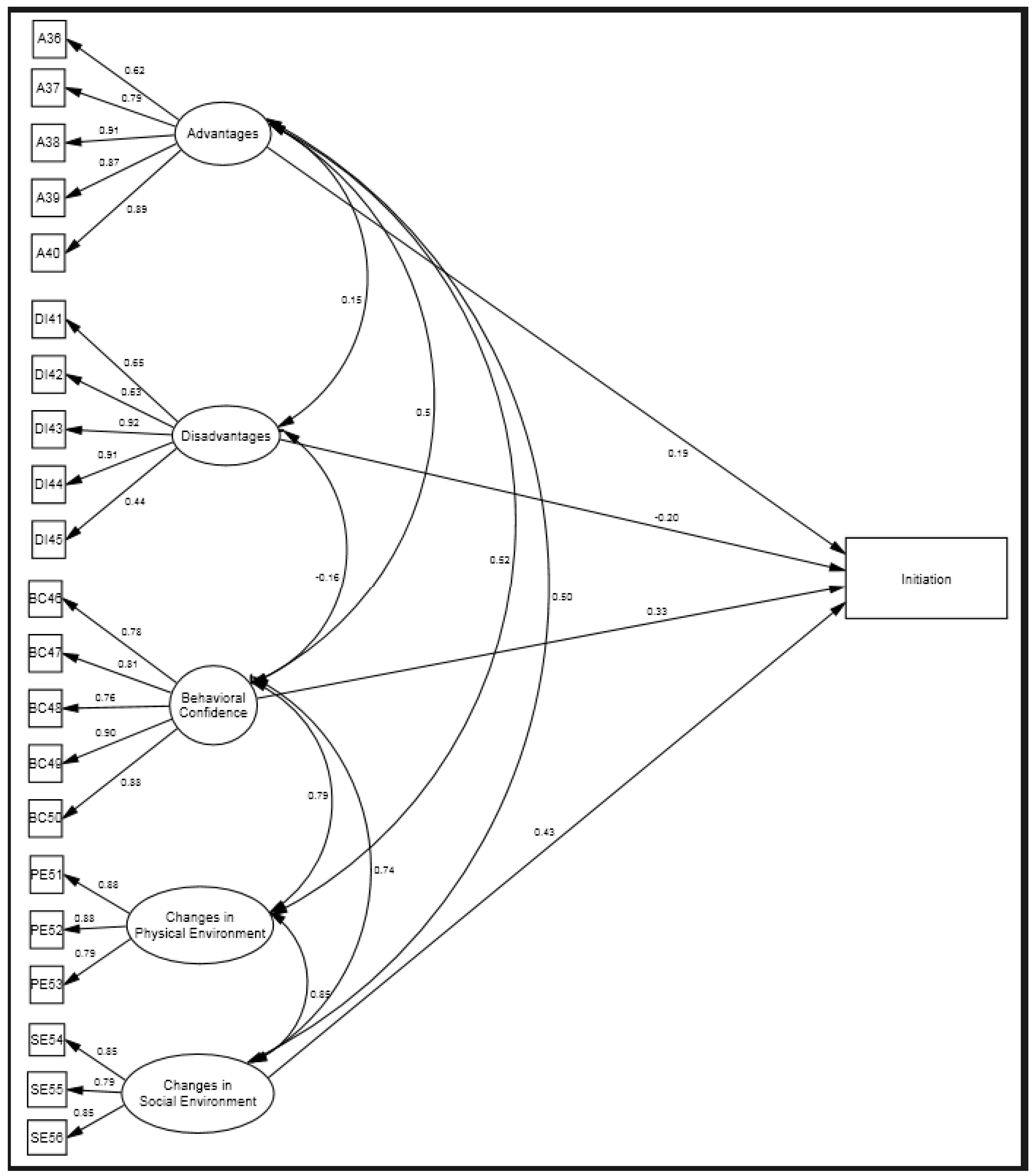
3.1. Construct Validity
3.2. Characteristics of the Sample
3.3. Comparison of the MTM Constructs
3.4. Correlation and Reliability Diagnostics
3.5. Hierarchical Regression
4. Discussion
4.1. Implications for Practice
4.2. Strengths and Limitations of Our Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Kanth, P.; Inadomi, J.M. Screening and Prevention of Colorectal Cancer. BMJ 2021, 374, n1855. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; Kubik, M.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA-J. Am. Med. Assoc. 2021, 325, 1965–1977. [Google Scholar]
- Tu, S.P.; Taylor, V.; Yasui, Y.; Chun, A.; Yip, M.P.; Acorda, E.; Li, L.; Bastani, R. Promoting Culturally Appropriate Colorectal Cancer Screening through a Health Educator: A Randomized Controlled Trial. Cancer 2006, 107, 959–966. [Google Scholar]
- Singal, A.G.; Gupta, S.; Tiro, J.A.; Skinner, C.S.; McCallister, K.; Sanders, J.M.; Bishop, W.P.; Agrawal, D.; Mayorga, C.A.; Ahn, C.; et al. Outreach Invitations for FIT and Colonoscopy Improve Colorectal Cancer Screening Rates: A Randomized Controlled Trial in a Safety-Net Health System. Cancer 2016, 122, 456–463. [Google Scholar] [CrossRef]
- Dougherty, M.K.; Brenner, A.T.; Crockett, S.D.; Gupta, S.; Wheeler, S.B.; Coker-Schwimmer, M.; Cubillos, L.; Malo, T.; Reuland, D.S. Evaluation of Interventions Intended to Increase Colorectal Cancer Screening Rates in the United States: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2018, 178, 1645–1658. [Google Scholar] [CrossRef]
- Goldman, S.N.; Liss, D.T.; Brown, T.; Lee, J.Y.; Buchanan, D.R.; Balsley, K.; Cesan, A.; Weil, J.; Garrity, B.H.; Baker, D.W. Comparative Effectiveness of Multifaceted Outreach to Initiate Colorectal Cancer Screening in Community Health Centers: A Randomized Controlled Trial. J. Gen. Intern. Med. 2015, 30, 1178–1184. [Google Scholar] [CrossRef]
- Holden, D.J.; Jonas, D.E.; Porterfield, D.S.; Reuland, D.; Harris, R. Systematic Review: Enhancing the Use and Quality of Colorectal Cancer Screening. Ann. Intern. Med. 2010, 152, 668–676. [Google Scholar]
- Leach, K.M.; Granzow, M.E.; Popalis, M.L.; Stoltzfus, K.C.; Moss, J.L. Promoting Colorectal Cancer Screening: A Scoping Review of Screening Interventions and Resources. Prev. Med. 2021, 147, 106517. [Google Scholar]
- Lemmo, D.; Martino, M.L.; Vallone, F.; Donizzetti, A.R.; Freda, M.F.; Palumbo, F.; Lorenzo, E.; D’Argenzio, A.; Caso, D. Clinical and Psychosocial Constructs for Breast, Cervical, and Colorectal Cancer Screening Participation: A Systematic Review. Int. J. Clin. Health Psychol. 2023, 23, 100354. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.B.; Phengrasamy, L.; Hudes, E.S.; McPhee, S.J.; Walsh, J.M.E. Offering Annual Fecal Occult Blood Tests at Annual Flu Shot Clinics Increases Colorectal Cancer Screening Rates. Ann. Fam. Med. 2009, 7, 17–23. [Google Scholar] [CrossRef]
- Potter, M.B.; Walsh, J.M.E.; Yu, T.M.; Gildengorin, G.; Green, L.W.; McPhee, S.J. The Effectiveness of the FLU-FOBT Program in Primary Care: A Randomized Trial. Am. J. Prev. Med. 2011, 41, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.E.; Salazar, R.; Nguyen, T.T.; Kaplan, C.; Nguyen, L.; Hwang, J.; McPhee, S.J.; Pasick, R.J. Healthy Colon, Healthy Life. A Novel Colorectal Cancer Screening Intervention. Am. J. Prev. Med. 2010, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, M.T.; Bennett, A.; Zaiter, M.; Marshall, J.R. Individual-Level Factors in Colorectal Cancer Screening: A Review of the Literature on the Relation of Individual-Level Health Behavior Constructs and Screening Behavior. Psycho-Oncology 2011, 20, 1023–1033. [Google Scholar]
- Basch, C.E.; Zybert, P.; Wolf, R.L.; Basch, C.H.; Ullman, R.; Shmukler, C.; King, F.; Neugut, A.I.; Shea, S. A Randomized Trial to Compare Alternative Educational Interventions to Increase Colorectal Cancer Screening in a Hard-to-Reach Urban Minority Population with Health Insurance. J. Community Health 2015, 40, 975–983. [Google Scholar] [CrossRef]
- Basch, C.E.; Wolf, R.L.; Brouse, C.H.; Shmukler, C.; Neugut, A.; DeCarlo, L.T.; Shea, S. Telephone Outreach to Increase Colorectal Cancer Screening in an Urban Minority Population. Am. J. Public Health 2006, 96, 2246–2253. [Google Scholar] [CrossRef]
- Sharma, M. Multi-Theory Model (MTM) for Health Behavior Change. Webmedcentral 2015, 6, WMC004982. [Google Scholar]
- Sharma, M.; Dai, C.-L.; Batra, K.; Chen, C.-C.; Pharr, J.R.; Coughenour, C.; Awan, A.; Catalano, H. Using the Multi-Theory Model (MTM) of Health Behavior Change to Explain the Correlates of Mammography Screening among Asian American Women. Pharmacy 2021, 9, 126. [Google Scholar] [CrossRef]
- Asare, M.; Agyei-Baffour, P.; Lanning, B.A.; Owusu, A.B.; Commeh, M.E.; Boozer, K.; Koranteng, A.; Spies, L.A.; Montealegre, J.R.; Paskett, E.D. Multi-Theory Model and Predictors of Likelihood of Accepting the Series of HPV Vaccination: A Cross-Sectional Study among Ghanaian Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 571. [Google Scholar] [CrossRef]
- Sharma, M.; Batra, K.; Johansen, C.; Raich, S. Explaining Correlates of Cervical Cancer Screening among Minority Women in the United States. Pharmacy 2022, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Petosa, R.L. Evaluation and Measurement in Health Promotion; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2023. [Google Scholar]
- Kline, R.B. Principles and Practice of Structural Equation Modeling; Guilford Publications: New York, NY, USA, 2015. [Google Scholar]
- Dimitrov, D.M. Statistical Methods for Validation of Assessment Scale Data in Counseling and Related Fields; American Counseling Association: Alexandria, VA, USA, 2012. [Google Scholar]
- Fan, X.; Thompson, B.; Wang, L. Effects of sample size, estimation method, and model specification on structural equation modeling fit indexes. Struct. Equ. Model. 1999, 6, 56–83. [Google Scholar] [CrossRef]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- CDC. QuickStats: Percentage of Adults Aged 50–75 Years Who Met Colorectal Cancer (CRC) Screening Recommendations—National Health Interview Survey, United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 314. [Google Scholar] [CrossRef]
- Wolf, E.J.; Harrington, K.M.; Clark, S.L.; Miller, M.W. Sample Size Requirements for Structural Equation Models: An Evaluation of Power, Bias, and Solution Propriety. Educ. Psychol. Meas. 2013, 76, 913–934. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.J.; Tangka, F.K.L.; Sabatino, S.A.; Thompson, T.D.; Graubard, B.I.; Breen, N. Patterns and Trends in Cancer Screening in the United States. Prev. Chronic Dis. 2018, 15, 170465. [Google Scholar] [CrossRef]
| Variable Name | Categories | Overall Sample | Had Any Form of Stool-Based Colorectal Cancer Screening | p Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| N = 640 | Group 1 Yes (n = 251) | Group 2 No (n = 389) | ||||
| Age in years (M ± SD) | - | 58.26 ± 8.96 | 59.08 ± 8.94 | 57.80 ± 8.94 | 0.1 | 57.56, 58.96 |
| Gender | Male | 271 (42.4) | 113 (45.0) | 158 (40.7) | 0.4 | 38.4, 46.2 |
| Female | 368 (57.6) | 138 (55.0) | 230 (59.3) | 53.5, 61.3 | ||
| Race | Black | 74 (11.6) | 28 (17.1) | 46 (15.3) | 0.6 | 9.0, 14.0 |
| White | 331 (51.7) | 116 (70.7) | 215 (71.7) | 47.7, 55.6 | ||
| AAPI | 23 (3.6) | 10 (6.1) | 13 (4.3) | 2.3, 5.3 | ||
| Other | 36 (5.6) | 10 (6.1) | 26 (8.7) | 3.8, 7.4 | ||
| Ethnicity | Hispanic | 86 (13.4) | 32 (15.0) | 54 (14.0) | 0.7 | 10.8, 16.0 |
| Non-Hispanic | 513 (80.2) | 182 (85.0) | 331 (86.0) | 77.0, 83.2 | ||
| Marital status | Married | 303 (47.3) | 114 (52.1) | 189 (49.1) | 0.4 | 43.5, 51.2 |
| Divorced/Separated | 123 (19.2) | 39 (17.8) | 84 (21.8) | 16.1, 22.2 | ||
| Widowed | 42 (6.5) | 19 (8.7) | 23 (6.0) | 4.6, 8.5 | ||
| Single, never married | 103 (16.1) | 38 (17.4) | 65 (16.9) | 13.2, 18.9 | ||
| Other | 33 (5.2) | 9 (4.1) | 24 (6.2) | 3.4, 6.8 | ||
| Education | Less than a high school | 17 (2.6) | 5 (2.3) | 12 (3.1) | 0.8 | 1.4, 3.9 |
| High school diploma or GED | 154 (24.0) | 53 (24.2) | 101 (26.2) | 20.7, 27.3 | ||
| Some college but not degree | 161 (25.2) | 64 (29.2) | 97 (25.1) | 21.7, 28.5 | ||
| College degree | 205 (32.0) | 71 (32.4) | 134 (34.7) | 28.4, 35.6 | ||
| Graduate level degree | 61 (9.5) | 24 (11.0) | 37 (9.6) | 7.2, 11.8 | ||
| Other | 7 (1.2) | 2 (0.9) | 5 (1.3) | 0.2, 1.9 | ||
| Health insurance | Yes | 567 (88.5) | 213 (97.3) | 354 (91.9) | 0.009 | 86.1, 91.0 |
| No | 37 (5.8) | 6 (2.7) | 31 (8.1) | 3.9, 7.6 | ||
| Region | Rural | 176 (27.5) | 63 (28.8) | 113 (29.3) | 0.4 | 24.0, 30.9 |
| Urban | 152 (23.7) | 62 (28.3) | 90 (23.3) | 20.5, 27.1 | ||
| Suburban | 277 (43.2) | 94 (42.9) | 183 (47.4) | 39.4, 47.1 | ||
| Employment status | Employed or self employed | 279 (43.5) | 97 (44.3) | 182 (47.2) | 0.8 | 39.7, 47.4 |
| Not working (e.g., out of work, homemaker, retired) | 260 (40.6) | 98 (44.7) | 162 (42.0) | 36.8, 44.4 | ||
| Unable to work | 66 (10.3) | 24 (11.0) | 42 (10.9) | 7.9, 12.7 | ||
| Religion | Christian | 429 (67.0) | 153 (69.9) | 276 (71.5) | 0.7 | 63.3, 70.6 |
| Non-Christian | 176 (27.5) | 66 (30.1) | 110 (28.5) | 24.0, 30.9 | ||
| Median income | <USD 25,000 | 143 (22.3) | 49 (22.9) | 94 (25.5) | 0.2 | 19.1, 25.5 |
| USD 25,000–USD 50,000 | 162 (25.3) | 61 (28.5) | 101 (27.4) | 21.9, 28.6 | ||
| USD 50,001–USD 75,000 | 111 (17.3) | 42 (19.6) | 69 (18.7) | 14.4, 20.3 | ||
| USD 75,001–USD 100,000 | 57 (8.9) | 24 (11.2) | 33 (8.9) | 6.7, 11.1 | ||
| USD 100,001–USD 125,000 | 40 (6.2) | 14 (6.5) | 26 (7.0) | 4.4, 8.1 | ||
| USD 125,001–USD 150,000 | 33 (5.2) | 17 (7.9) | 16 (4.3) | 3.4, 6.8 | ||
| >USD 150,000 | 37 (5.7) | 7 (3.3) | 30 (8.1) | 3.9, 7.6 | ||
| Variable Name | Categories | Overall Sample n (%) | Had Any Form of Stool-Based Colorectal Cancer Screening | p Value | |
|---|---|---|---|---|---|
| N = 640 | Group 1 Yes (n = 251) | Group 2 No (n = 389) | |||
| Personal history of colorectal cancer | Yes | 16 (2.7) | 12 (5.6) | 4 (1.1) | 0.001 |
| No | 577 (97.3) | 204 (94.4) | 373 (98.9) | ||
| Family history of colorectal cancer | Yes | 87 (15.3) | 37 (18.0) | 50 (13.8) | 0.2 |
| No | 481 (84.7) | 169 (82.0) | 312 (86.2) | ||
| Personal history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease) | Yes | 50 (8.7) | 27 (13.0) | 23 (6.2) | 0.005 |
| No | 528 (91.3) | 181 (87.0) | 347 (93.8) | ||
| Personal history of confirmed or suspected hereditary colorectal cancer syndrome | Yes | 20 (3.7) | 14 (7.0) | 6 (1.7) | 0.001 |
| No | 527 (96.3) | 185 (93.0) | 342 (98.3) | ||
| Personal history of getting radiation to the abdomen (belly) or pelvic area to treat any prior cancer | Yes | 30 (5.0) | 20 (9.2) | 10 (2.6) | <0.001 |
| No | 565 (95.0) | 197 (90.8) | 368 (97.4) | ||
| Recommended a colorectal screening by healthcare provider (HCP) | Yes | 196 (34.8) | 93 (45.4) | 103 (28.7) | <0.001 |
| No | 345 (61.2) | 110 (53.7) | 235 (65.5) | ||
| Do not have HCP | 23 (4.1) | 2 (1.0) | 21 (5.8) | ||
| Encouraged by a family member to undertake CRC screening | Yes | 195 (32.2) | 78 (35.5) | 117 (30.3) | 0.2 |
| No | 411 (67.8) | 142 (64.5) | 269 (69.7) | ||
| Have had a recent visit to a primary healthcare provider | Yes | 403 (68.7) | 171 (78.4) | 232 (62.9) | <0.001 |
| No | 184 (31.3) | 47 (21.6) | 137 (37.1) | ||
| MTM Construct | Had Any Form of Colorectal Cancer Screening | p Value | Mean Difference | 95% CI of Mean Difference | |
|---|---|---|---|---|---|
| Yes (n = 251) | No (n = 389) | ||||
| Overall Initiation Score | 2.75 ± 1.16 | 2.22 ± 1.36 | <0.001 | 0.538 | 0.331, 0.745 |
| Subscales | |||||
| Perceived Advantages | 14.19 ± 5.09 | 13.04 ± 5.47 | 0.01 | 1.14 | 0.260, 2.039 |
| Perceived Disadvantages | 9.50 ± 4.59 | 9.57 ± 4.80 | 0.85 | −0.076 | −0.864, 0.711 |
| Participatory Dialogue | 4.69 ± 6.43 | 3.46 ± 6.50 | 0.03 | 1.22 | 0.149, 2.303 |
| Behavioral Confidence | 12.10 ± 5.34 | 11.11 ± 5.53 | 0.03 | 0.987 | 0.757, 1.899 |
| Changes in the Physical Environment | 8.45 ± 3.31 | 7.64 ± 3.70 | 0.006 | 0.817 | 0.240, 1.393 |
| Change in the Social Environment | 7.51 ± 3.61 | 6.60 ± 3.87 | 0.004 | 0.916 | 0.286, 1.546 |
| Variables | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Participatory Dialogue | 1 | 0.510 ** [0.448, 0.567] | 0.447 ** [0.380, 0.508] | 0.417 ** [0.349, 0.481] |
| 2. Behavioral Confidence | 0.510 ** [0.448, 0.567] | 1 | 0.745 ** [0.707, 0.778] | 0.676 ** [0.631, 0.718] |
| 3. Changes in the Physical Environment | 0.447 ** [0.380, 0.508] | 0.745 ** [0.707, 0.778] | 1 | 0.765 ** [0.729, 0.796] |
| 4. Changes in the Social Environment | 0.417 ** [0.349, 0.481] | 0.676 ** [0.631, 0.718] | 0.765 ** [0.729, 0.796] | 1 |
| Cronbach’s Alpha | - | 0.913 | 0.874 | 0.873 |
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | β | B | β | B | β | B | β | B | β | |
| Constant | 0.120 | 1.065 * | 0.457 | 0.482 | 0.601 | |||||
| Age | 0.011 | 0.071 | 0.002 | 0.013 | 0.000 | −0.003 | −0.001 | −0.004 | −0.004 | −0.029 |
| Gender: Male (Ref: Female) | −0.071 | −0.025 | −0.197 | −0.070 | −0.101 | −0.036 | −0.106 | −0.038 | −0.049 | −0.017 |
| Race: White (Ref: Black) | 0.026 | 0.009 | −0.063 | −0.023 | −0.128 | −0.046 | −0.126 | −0.045 | −0.104 | −0.038 |
| AAPI | −1.023 * | −0.128 | −0.702 | −0.088 | −0.524 | −0.065 | −0.502 | −0.063 | −0.398 | −0.050 |
| Other | −0.225 | −0.042 | −0.127 | −0.024 | −0.240 | −0.044 | −0.232 | −0.043 | −0.227 | −0.042 |
| Ethnicity: Non-Hispanic (Ref: Hispanic) | 0.048 | 0.012 | −0.063 | −0.016 | −0.053 | −0.013 | −0.066 | −0.016 | −0.032 | −0.008 |
| Marital Status: Divorced/Separated (Ref: Married) | −0.229 | −0.068 | −0.219 | −0.065 | −0.166 | −0.049 | −0.180 | −0.053 | −0.068 | −0.020 |
| Widowed | −0.099 | −0.018 | 0.014 | 0.002 | −0.060 | −0.011 | −0.073 | −0.013 | 0.020 | 0.004 |
| Single, never married | −0.374 | −0.100 | −0.283 | −0.076 | −0.254 | −0.068 | −0.281 | −0.075 | −0.268 | −0.071 |
| Other | −0.193 | −0.034 | −0.034 | −0.006 | −0.027 | −0.005 | −0.090 | −0.016 | −0.164 | −0.029 |
| Education Less than a high school (Ref: High school diploma or GED) | −0.270 | −0.037 | −0.218 | −0.030 | −0.228 | −0.031 | −0.254 | −0.035 | −0.155 | −0.021 |
| Some college but not degree | 0.211 | 0.063 | 0.044 | 0.013 | −0.177 | −0.053 | −0.175 | −0.053 | −0.091 | −0.027 |
| College degree | 0.156 | 0.054 | −0.120 | −0.042 | −0.299 * | −0.104 | −0.322 * | −0.112 | −0.283 | −0.098 |
| Graduate level degree | −0.155 | −0.034 | −0.193 | −0.042 | −0.363 | −0.079 | −0.369 | −0.081 | −0.352 | −0.077 |
| Other | −0.247 | −0.020 | −0.445 | −0.036 | −0.615 | −0.049 | −0.656 | −0.052 | −0.710 | −0.057 |
| Health insurance: Yes (Ref: No) | 1.102 ** | 0.224 | 0.660 * | 0.134 | 0.415 * | 0.084 | 0.363 | 0.074 | 0.260 | 0.053 |
| Region: Urban (Ref: Rural) | 0.296 | 0.090 | 0.224 | 0.068 | 0.293 | 0.089 | 0.306 * | 0.093 | 0.358 * | 0.109 |
| Suburban | 0.428 * | 0.154 | 0.262 | 0.094 | 0.182 | 0.066 | 0.172 | 0.062 | 0.190 | 0.069 |
| Employment status: Not Working (Ref: Employed or self-employed) | 0.127 | 0.045 | −0.022 | −0.008 | −0.045 | −0.016 | −0.067 | −0.024 | −0.030 | −0.011 |
| Unable to work | −0.101 | −0.021 | −0.201 | −0.041 | −0.234 | −0.048 | −0.247 | −0.051 | −0.177 | −0.036 |
| Religion: Christian (Ref: Non-Christian) | 0.137 | 0.044 | 0.196 | 0.063 | 0.240 * | 0.077 | 0.218 | 0.070 | 0.162 | 0.052 |
| Income: USD 25,000–USD 50,000 (Ref: <USD 25,000) | −0.127 | −0.041 | −0.039 | −0.012 | −0.085 | −0.027 | −0.099 | −0.032 | −0.130 | −0.042 |
| USD 50,001–USD 75,000 | 0.003 | 0.001 | −0.003 | −0.001 | −0.140 | −0.039 | −0.167 | −0.046 | −0.241 | −0.067 |
| USD 75,001–USD 100,000 | 0.766 * | 0.145 | 0.459 | 0.087 | 0.153 | 0.029 | 0.111 | 0.021 | 0.056 | 0.011 |
| USD 100,001–USD 125,000 | 0.229 | 0.042 | 0.340 | 0.062 | 0.211 | 0.038 | 0.203 | 0.037 | 0.096 | 0.017 |
| USD 125,001–USD 150,000 | 0.471 | 0.067 | 0.545 | 0.077 | 0.336 | 0.048 | 0.280 | 0.040 | 0.285 | 0.040 |
| >USD 150,000 | 0.275 | 0.054 | 0.222 | 0.043 | −0.022 | −0.004 | −0.050 | −0.010 | −0.098 | −0.019 |
| Personal history of colorectal cancer: Yes (Ref: No) | 0.684 | 0.047 | 1.003 | 0.069 | 1.208 * | 0.084 | 1.181 * | 0.082 | 0.993 | 0.069 |
| Family history of colorectal cancer: Yes (Ref: No) | 0.195 | 0.046 | 0.003 | 0.001 | −0.132 | −0.031 | −0.147 | −0.035 | −0.120 | −0.028 |
| Personal history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease): Yes (Ref: No) | 0.194 | 0.033 | −0.069 | −0.012 | −0.038 | −0.006 | −0.032 | −0.006 | −0.057 | −0.010 |
| Participatory dialogue | - | - | 0.125 ** | 0.590 | 0.067 ** | 0.315 | 0.066 ** | 0.310 | 0.061 ** | 0.290 |
| Behavioral confidence | - | - | - | - | 0.125 ** | 0.506 | 0.109 ** | 0.438 | 0.096 ** | 0.388 |
| Changes in the physical environment | - | - | - | - | - | - | 0.039 | 0.102 | −0.014 | −0.036 |
| Changes in the Social Environment | - | - | - | - | - | - | - | - | 0.099 ** | 0.274 |
| R2 | 0.189 | - | 0.485 | - | 0.621 | - | 0.625 | - | 0.651 | - |
| F | 2.257 ** | - | 8.826 ** | - | 14.798 ** | - | 14.538 ** | - | 15.730 ** | - |
| ΔR2 | 0.189 | - | 0.297 | - | 0.136 | - | 0.004 | - | 0.026 | - |
| ΔF | 2.257 ** | - | 167.232 ** | - | 103.352 ** | - | 2.976 | - | 21.286 ** | - |
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | β | B | β | B | β | B | β | B | β | |
| Constant | 1.790 | 2.048 * | 0.874 | 1.076 | 1.171 | |||||
| Age | 0.005 | 0.038 | −0.003 | −0.025 | 0.007 | 0.053 | 0.002 | 0.016 | 0.000 | 0.003 |
| Gender: Male (Ref: Female) | −0.012 | −0.005 | 0.143 | 0.061 | 0.101 | 0.043 | 0.150 | 0.064 | 0.190 | 0.081 |
| Race: White (Ref: Black) | 0.063 | 0.027 | 0.043 | 0.018 | −0.054 | −0.023 | −0.062 | −0.026 | −0.034 | −0.014 |
| AAPI | 0.360 | 0.067 | 0.410 | 0.076 | 0.823 * | 0.153 | 0.647 | 0.120 | 0.726 * | 0.135 |
| Other | 0.332 | 0.065 | 0.297 | 0.058 | 0.140 | 0.027 | 0.134 | 0.026 | 0.125 | 0.024 |
| Ethnicity: Non-Hispanic (Ref: Hispanic) | −0.191 | −0.060 | −0.239 | −0.075 | −0.304 | −0.095 | −0.306 | −0.096 | −0.302 | −0.095 |
| Marital Status: Divorced/Separated (Ref: Married) | −0.199 | −0.064 | −0.012 | −0.004 | −0.077 | −0.025 | −0.071 | −0.023 | −0.069 | −0.022 |
| Widowed | 0.255 | 0.062 | 0.244 | 0.059 | 0.085 | 0.021 | 0.101 | 0.024 | 0.232 | 0.056 |
| Single, never married | −0.391 | −0.129 | −0.313 | −0.103 | −0.220 | −0.072 | −0.184 | −0.061 | −0.160 | −0.053 |
| Other | −0.504 | −0.077 | −0.269 | −0.041 | −0.441 | −0.067 | −0.306 | −0.047 | −0.286 | −0.044 |
| Education Less than a high school (Ref: High school diploma or GED) | −0.274 | −0.034 | 0.055 | 0.007 | 0.344 | 0.043 | 0.193 | 0.024 | 0.104 | 0.013 |
| Some college but not degree | −0.170 | −0.065 | −0.158 | −0.060 | −0.042 | −0.016 | −0.140 | −0.054 | −0.094 | −0.036 |
| College degree | 0.048 | 0.019 | −0.111 | −0.043 | −0.140 | −0.055 | −0.137 | −0.054 | 0.030 | 0.012 |
| Graduate level degree | 0.221 | 0.063 | 0.163 | 0.046 | 0.205 | 0.058 | 0.081 | 0.023 | 0.131 | 0.037 |
| Other | −2.249 * | −0.201 | −1.666 * | −0.149 | −1.437 * | −0.128 | −1.420 * | −0.127 | −1.166 | −0.104 |
| Health insurance: Yes (Ref: No) | 0.985 | 0.150 | 0.948 * | 0.145 | 0.568 | 0.087 | 0.382 | 0.058 | 0.273 | 0.042 |
| Region: Urban (Ref: Rural) | −0.154 | −0.058 | −0.216 | −0.082 | −0.194 | −0.074 | −0.227 | −0.086 | −0.261 | −0.099 |
| Suburban | −0.035 | −0.015 | −0.057 | −0.024 | −0.057 | −0.024 | −0.057 | −0.024 | −0.040 | −0.017 |
| Employment status: Not Working (Ref: Employed or self-employed) | −0.010 | −0.004 | −0.208 | −0.088 | −0.318 | −0.135 | −0.300 | −0.127 | −0.282 | −0.119 |
| Unable to work | 0.268 | 0.073 | 0.034 | 0.009 | 0.041 | 0.011 | −0.001 | 0.000 | −0.052 | −0.014 |
| Religion: Christian (Ref: Non-Christian) | −0.218 | −0.086 | −0.170 | −0.067 | −0.047 | −0.019 | −0.081 | −0.032 | −0.012 | −0.005 |
| Income: USD 25,000–USD 50,000 (Ref: <USD 25,000) | −0.116 | −0.045 | −0.220 | −0.085 | −0.166 | −0.064 | −0.191 | −0.074 | −0.318 | −0.123 |
| USD 50,001–USD 75,000 | 0.368 | 0.118 | 0.141 | 0.045 | 0.075 | 0.024 | 0.019 | 0.006 | −0.171 | −0.055 |
| USD 75,001–USD 100,000 | 0.157 | 0.042 | 0.205 | 0.055 | 0.143 | 0.038 | 0.044 | 0.012 | −0.088 | −0.024 |
| USD 100,001–USD 125,000 | 0.374 | 0.079 | 0.198 | 0.042 | 0.157 | 0.033 | 0.047 | 0.010 | −0.162 | −0.034 |
| USD 125,001–USD 150,000 | 0.860 | 0.202 | 0.581 | 0.137 | 0.470 | 0.111 | 0.406 | 0.096 | 0.254 | 0.060 |
| >USD 150,000 | 0.504 | 0.083 | 0.359 | 0.059 | 0.372 | 0.061 | 0.300 | 0.049 | 0.089 | 0.015 |
| Personal history of colorectal cancer: Yes (Ref: No) | −0.093 | −0.017 | 0.028 | 0.005 | −0.202 | −0.037 | −0.083 | −0.015 | 0.039 | 0.007 |
| Family history of colorectal cancer: Yes (Ref: No) | 0.039 | 0.012 | 0.065 | 0.021 | −0.106 | −0.034 | −0.055 | −0.017 | −0.030 | −0.009 |
| Personal history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease): Yes (Ref: No) | −0.255 | −0.074 | −0.156 | −0.045 | −0.099 | −0.029 | −0.105 | −0.030 | −0.100 | −0.029 |
| Participatory dialogue | - | - | 0.084 ** | 0.457 | 0.052 ** | 0.283 | 0.045 * | 0.244 | 0.042 * | 0.229 |
| Behavioral confidence | - | - | - | - | 0.099 ** | 0.446 | 0.063 * | 0.283 | 0.043 * | 0.193 |
| Changes in the physical environment | - | - | - | - | - | - | 0.096 * | 0.263 | 0.033 | 0.092 |
| Changes in the Social Environment | - | - | - | - | - | - | - | - | 0.108 * | 0.331 |
| R2 | 0.208 | - | 0.373 | - | 0.516 | - | 0.545 | - | 0.579 | - |
| F | 1.313 | - | 2.856 ** | - | 4.934 ** | - | 5.334 ** | - | 5.916 ** | - |
| ΔR2 | 0.208 | - | 0.165 | - | 0.143 | - | 0.029 | - | 0.035 | - |
| ΔF | 1.313 | - | 39.128 ** | - | 43.879 ** | - | 9.287 * | - | 11.977 * | - |
| MTM Construct | Crib Sheet Lines |
|---|---|
| Advantages | Cologuard is a non-invasive, home-based stool test for the early detection of polyps and colon cancer for people 45 years of age and older. |
| Behavioral confidence | It is very easy to use and comes with all detailed instructions. |
| Changes in the social environment | Should you decide to use it, our nurse will be able to explain all preliminary details to you. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.; Johansen, C.; Batra, K.; Dai, C.-L.; Batra, R.; Hayes, T.; Singh, A. Using the Multi-Theory Model (MTM) of Health Behavior Change to Explain the Seeking of Stool-Based Tests for Colorectal Cancer Screening. Int. J. Environ. Res. Public Health 2023, 20, 6553. https://doi.org/10.3390/ijerph20166553
Sharma M, Johansen C, Batra K, Dai C-L, Batra R, Hayes T, Singh A. Using the Multi-Theory Model (MTM) of Health Behavior Change to Explain the Seeking of Stool-Based Tests for Colorectal Cancer Screening. International Journal of Environmental Research and Public Health. 2023; 20(16):6553. https://doi.org/10.3390/ijerph20166553
Chicago/Turabian StyleSharma, Manoj, Christopher Johansen, Kavita Batra, Chia-Liang Dai, Ravi Batra, Traci Hayes, and Aditi Singh. 2023. "Using the Multi-Theory Model (MTM) of Health Behavior Change to Explain the Seeking of Stool-Based Tests for Colorectal Cancer Screening" International Journal of Environmental Research and Public Health 20, no. 16: 6553. https://doi.org/10.3390/ijerph20166553
APA StyleSharma, M., Johansen, C., Batra, K., Dai, C.-L., Batra, R., Hayes, T., & Singh, A. (2023). Using the Multi-Theory Model (MTM) of Health Behavior Change to Explain the Seeking of Stool-Based Tests for Colorectal Cancer Screening. International Journal of Environmental Research and Public Health, 20(16), 6553. https://doi.org/10.3390/ijerph20166553









