Plastics and Micro/Nano-Plastics (MNPs) in the Environment: Occurrence, Impact, and Toxicity
Abstract
:1. Introduction
2. Entry and Occurrence of Micro and Nano-Plastics (MNPs) in the Environment
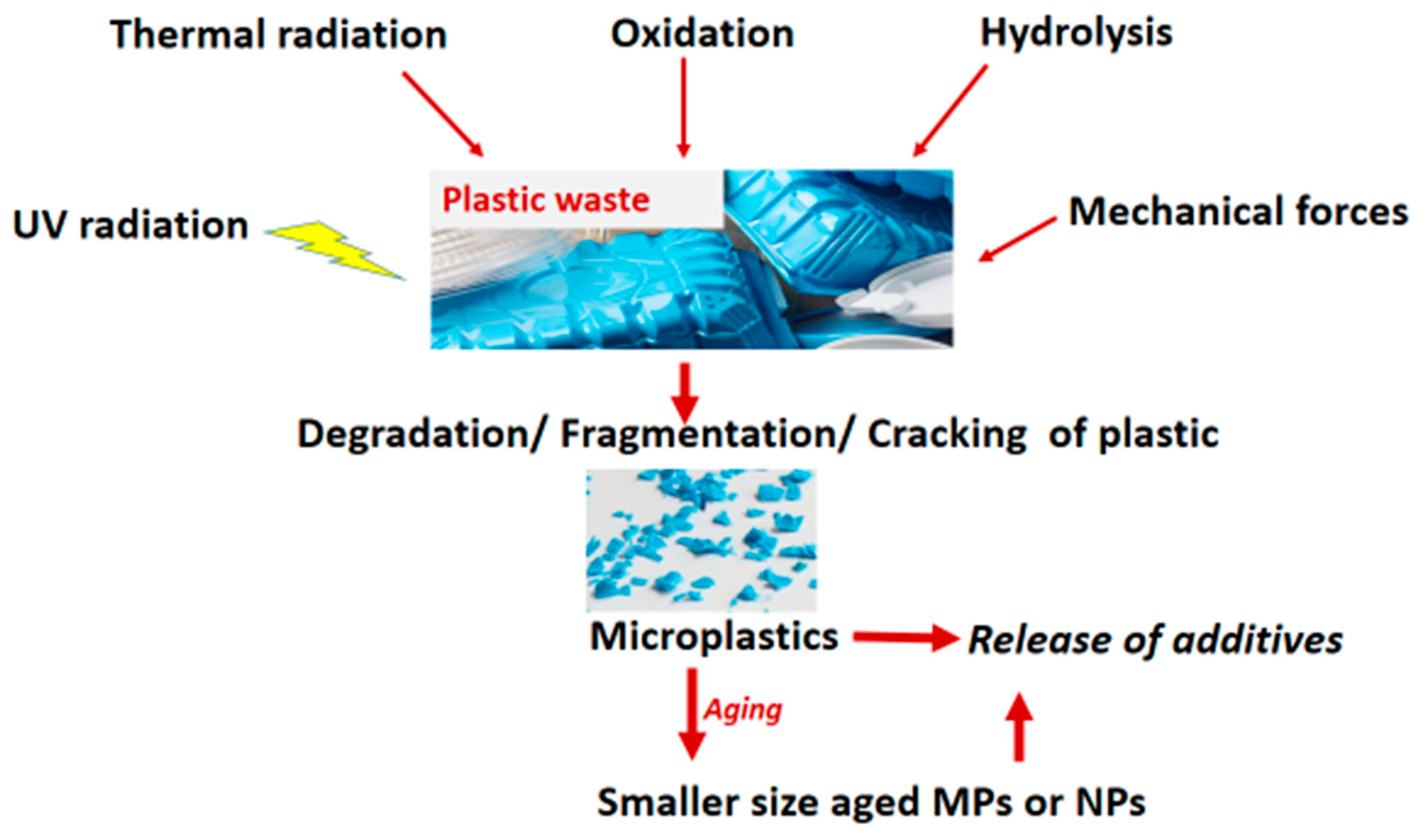
2.1. Primary MNPs and Secondary MNPs
2.1.1. Primary MNPs
2.1.2. Secondary MNPs
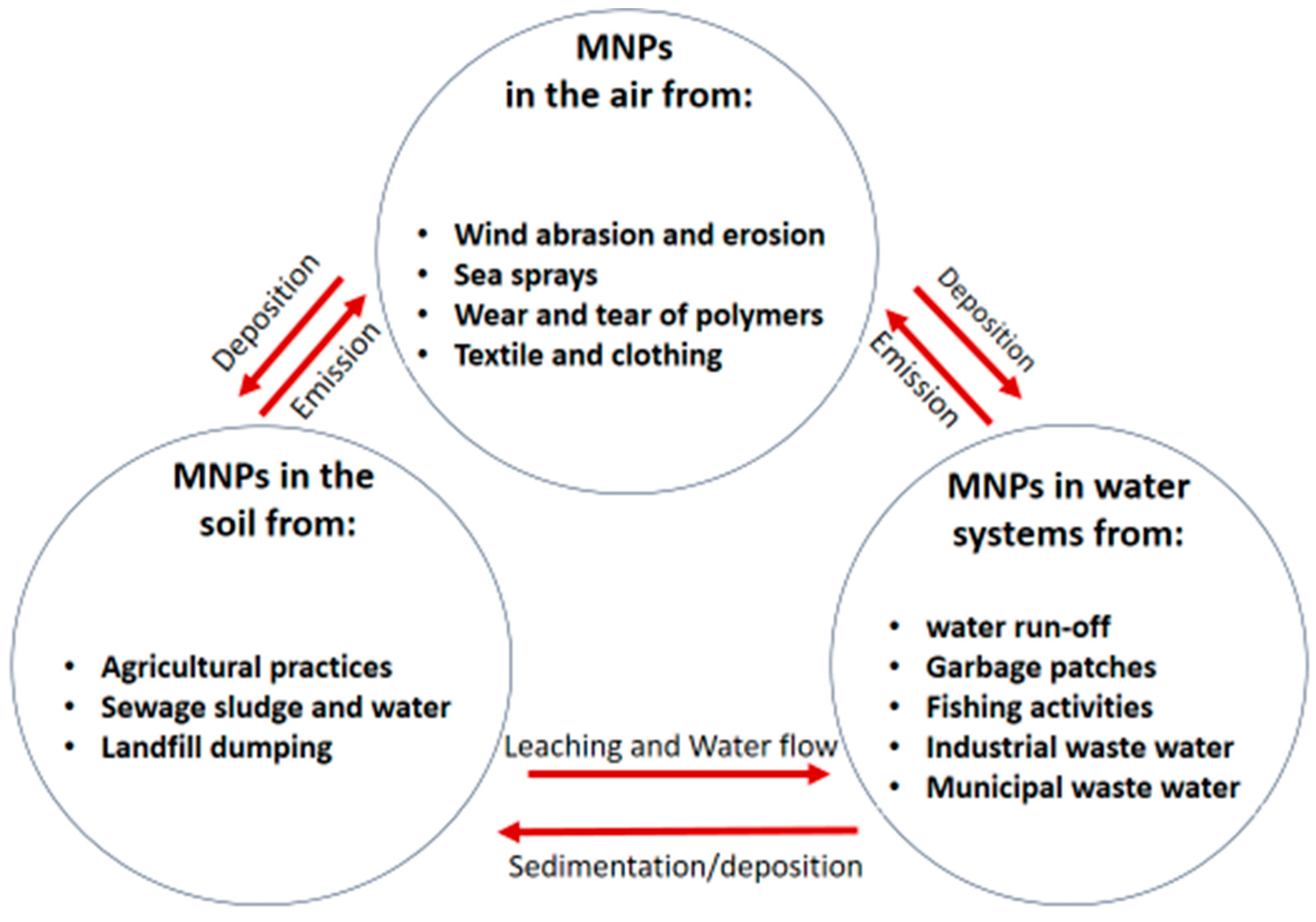
2.2. Entry and Occurrence of MNPs in the Soil, Water and Air
2.2.1. Entry and Occurrence of MNPs in the Soil
- i.
- MNPs from landfill dumping
- ii.
- MNPs from sewage sludge
- iii.
- MNPs from agricultural practices
- iv.
- MNPs from other sources
2.2.2. Entry and Occurrence of MNPs in the Air
- i.
- MNPs from the wear and tear of plastic or polymeric materials
- ii.
- MNPs from synthetic textiles and clothing
- iii.
- MNPs from the sea spray
- iv.
- MNPs from wind abrasion and erosion
2.2.3. Entry and Occurrence of MNPs in Water Systems
- i.
- MNPs from water runoff
- ii.
- MNPs from industrial and municipal waste
- iii.
- MNPs from fishing activities
- iv.
- MNPs from garbage patches
2.3. Aging of Microplastics
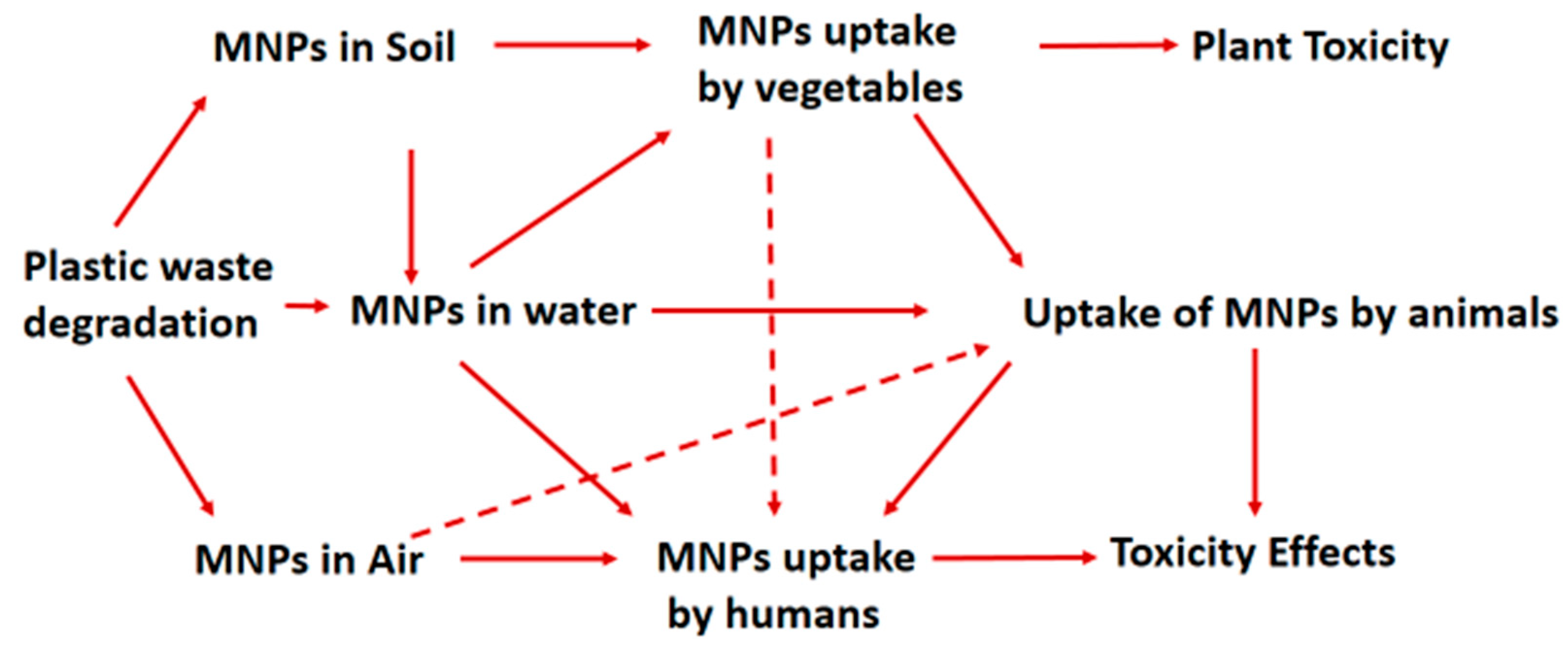
3. Uptake and Internalisation of MNPs by Living Organisms
3.1. MNP Uptake by Plants
3.2. MNP Uptake by Animals
3.3. MNPs Uptake by Humans
4. Mechanism of MNP Toxicity
4.1. Cytotoxicity of MNPs
4.2. Genotoxicity of MNPs
4.3. Immunotoxicity of MNPs
4.4. Oxidative Stress and Inflammation Induced by MNPs
4.5. Gastrointestinal Alterations and Membrane Injury due to MNPs
4.6. Neurotoxicity of MNPs
4.7. Carcinogenicity of MNPs
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Oosten, T.B. Properties of Plastics: A Guide for Conservators; Getty Publications: Los Angeles, CA, USA, 2022. [Google Scholar]
- Wang, C.-C. Material Properties of Plastics. In Molding Simulation: Theory and Practice; Klein, R., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2022; pp. 23–72. [Google Scholar] [CrossRef]
- Rodrigues, C.C.; Salla, R.F.; Rocha, T.L. Bioaccumulation and Ecotoxicological Impact of Micro(Nano)Plastics in Aquatic and Land Snails: Historical Review, Current Research and Emerging Trends. J. Hazard. Mater. 2023, 444, 130382. [Google Scholar] [CrossRef]
- Janssens, V. Plastics—The Facts 2022; Plastics Europe: Brussels, Belgium, 2022; pp. 1–81. [Google Scholar]
- OECD. Plastic Pollution Is Growing Relentlessly as Waste Management and Recycling Fall Short. OECD Rep. 2022. Available online: https://www.oecd.org/environment/plastics/ (accessed on 13 March 2023).
- Adeniran, A.A.; Ayesu-Koranteng, E.; Shakantu, W. A Review of the Literature on the Environmental and Health Impact of Plastic Waste Pollutants in Sub-Saharan Africa. Pollutants 2022, 2, 531–545. [Google Scholar] [CrossRef]
- Sadan, Z.; de Kock, L. Plastic Pollution in Africa: Identifying Policy Gaps and Opportunities; WWF South Africa: Cape Town, South Africa, 2021; pp. 1–44. [Google Scholar]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; Volume 17. [Google Scholar] [CrossRef]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the Soil Environment: A Critical Review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Dankwa Ampah, J.; Soudagar, M.E.M.; Veza, I.; Kingsley, U.; Afrane, S.; Jin, C.; Liu, H.; Elfasakhany, A.; Buyondo, K.A. Effects of Hybrid Nanoparticle Additives in N-Butanol/Waste Plastic Oil/Diesel Blends on Combustion, Particulate and Gaseous Emissions from Diesel Engine Evaluated with Entropy-Weighted PROMETHEE II and TOPSIS: Environmental and Health Risks of Plastic Wa. Energy Convers. Manag. 2022, 264, 115758. [Google Scholar] [CrossRef]
- Wu, D.; Li, Q.; Shang, X.; Liang, Y.; Ding, X.; Sun, H.; Li, S.; Wang, S.; Chen, Y.; Chen, J. Commodity Plastic Burning as a Source of Inhaled Toxic Aerosols. J. Hazard. Mater. 2021, 416, 125820. [Google Scholar] [CrossRef] [PubMed]
- Tait, P.W.; Brew, J.; Che, A.; Costanzo, A.; Danyluk, A.; Davis, M.; Khalaf, A.; McMahon, K.; Watson, A.; Rowcliff, K.; et al. The Health Impacts of Waste Incineration: A Systematic Review. Aust. N. Z. J. Public Health 2020, 44, 40–48. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Raveendran, S.; Singh, S.; Pillai, S. Environmental Impacts of Microplastics and Nanoplastics: A Current Overview. Front. Microbiol. 2021, 12, 768297. [Google Scholar] [CrossRef]
- Gündoğdu, S. Polymer Types of Microplastic in Coastal Areas. In Microplastic Pollution: Environmental Occurrence and Treatment Technologies; Hashmi, M.Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 77–87. [Google Scholar]
- Ziani, K.; Ioniță-Mîndrican, C.B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Dalela, M.; Shrivastav, T.G.; Kharbanda, S.; Singh, H. PH-Sensitive Biocompatible Nanoparticles of Paclitaxel-Conjugated Poly(Styrene-Co-Maleic Acid) for Anticancer Drug Delivery in Solid Tumors of Syngeneic Mice. ACS Appl. Mater. Interfaces 2015, 7, 26530–26548. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, L.; Dong, J.; Wu, L.; Fang, F.; Wang, Y.; Li, H.; Xie, C.; Li, W.; Wei, Z.; et al. A One-Step Surface Modification Technique Improved the Nutrient Release Characteristics of Controlled-Release Fertilizers and Reduced the Use of Coating Materials. J. Clean. Prod. 2022, 369, 133331. [Google Scholar] [CrossRef]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.M.; Kimiko, S.; Mak, C.W.; Fang, J.K.H.; Gonçalves, D. Personal Care and Cosmetic Products as a Potential Source of Environmental Contamination by Microplastics in a Densely Populated Asian City. Front. Mar. Sci. 2021, 8, 683482. [Google Scholar] [CrossRef]
- Hazlehurst, A.; Tiffin, L.; Sumner, M.; Taylor, M. Quantification of Microfibre Release from Textiles during Domestic Laundering. Environ. Sci. Pollut. Res. 2023, 30, 43932–43949. [Google Scholar] [CrossRef] [PubMed]
- Paruta, P.; Pucino, M.; Boucher, J. Plastic Paints the Environment; EA-Environmental Action: Lausanne, Switzerland, 2022; pp. 1–142. [Google Scholar]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Tamayo-Belda, M.; Pulido-Reyes, G.; González-Pleiter, M.; Martín-Betancor, K.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Identification and Toxicity towards Aquatic Primary Producers of the Smallest Fractions Released from Hydrolytic Degradation of Polycaprolactone Microplastics. Chemosphere 2022, 303, 134966. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Schöpfer, L.; Schnepf, U.; Marhan, S.; Brümmer, F.; Kandeler, E.; Pagel, H. Hydrolyzable Microplastics in Soil—Low Biodegradation but Formation of a Specific Microbial Habitat? Biol. Fertil. Soils 2022, 58, 471–486. [Google Scholar] [CrossRef]
- Meides, N.; Mauel, A.; Menzel, T.; Altstädt, V.; Ruckdäschel, H.; Senker, J.; Strohriegl, P. Quantifying the Fragmentation of Polypropylene upon Exposure to Accelerated Weathering. Microplastics Nanoplastics 2022, 2, 1–13. [Google Scholar] [CrossRef]
- Egger, M.; Sulu-Gambari, F.; Lebreton, L. First Evidence of Plastic Fallout from the North Pacific Garbage Patch. Sci. Rep. 2020, 10, 7495. [Google Scholar] [CrossRef]
- Meides, N.; Menzel, T.; Poetzschner, B.; Löder, M.G.J.; Mansfeld, U.; Strohriegl, P.; Altstaedt, V.; Senker, J. Reconstructing the Environmental Degradation of Polystyrene by Accelerated Weathering. Environ. Sci. Technol. 2021, 55, 7930–7938. [Google Scholar] [CrossRef]
- Lim, X.Z. Microplastics Are Everywhere–but Are They Harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef] [PubMed]
- García Rellán, A.; Vázquez Ares, D.; Vázquez Brea, C.; Francisco López, A.; Bello Bugallo, P.M. Sources, Sinks and Transformations of Plastics in Our Oceans: Review, Management Strategies and Modelling. Sci. Total Environ. 2023, 854, 158745. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Delaune, T.; Silva, J.V.; van Wijk, M.; Hammond, J.; Descheemaeker, K.; van de Ven, G.; Schut, A.G.T.; Taulya, G.; Chikowo, R.; et al. Small Farms and Development in Sub-Saharan Africa: Farming for Food, for Income or for Lack of Better Options? Food Secur. 2021, 13, 1431–1454. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in Soil: A Review on Methods, Occurrence, Sources, and Potential Risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef]
- Mahesh, S.; Gowda, N.K.; Mahesh, S. Identification of Microplastics from Urban Informal Solid Waste Landfill Soil; MP Associations with COD and Chloride. Water Sci. Technol. 2023, 87, 115–129. [Google Scholar] [CrossRef]
- Afrin, S.; Uddin, M.K.; Rahman, M.M. Microplastics Contamination in the Soil from Urban Landfill Site, Dhaka, Bangladesh. Heliyon 2020, 6, e05572. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic Transport in Soil by Earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Bullard, J.E.; Ockelford, A.; O’Brien, P.; McKenna Neuman, C. Preferential Transport of Microplastics by Wind. Atmos. Environ. 2021, 245, 118038. [Google Scholar] [CrossRef]
- Di Bella, G.; Corsino, S.F.; De Marines, F.; Lopresti, F.; La Carrubba, V.; Torregrossa, M.; Viviani, G. Occurrence of Microplastics in Waste Sludge of Wastewater Treatment Plants: Comparison between Membrane Bioreactor (MBR) and Conventional Activated Sludge (CAS) Technologies. Membranes 2022, 12, 371. [Google Scholar] [CrossRef]
- Hassan, F.; Daffa, K.; Nabilah, J.; Manh, H. Microplastic Contamination in Sewage Sludge: Abundance, Characteristics, and Impacts on the Environment and Human Health. Environ. Technol. Innov. 2023, 31, 103176. [Google Scholar] [CrossRef]
- Bretas Alvim, C.; Mendoza-Roca, J.A.; Bes-Piá, A. Wastewater Treatment Plant as Microplastics Release Source–Quantification and Identification Techniques. J. Environ. Manag. 2020, 255, 109739. [Google Scholar] [CrossRef]
- Franco, A.A.; Martín-García, A.P.; Egea-Corbacho, A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.M.; Coello, M.D. Assessment and Accumulation of Microplastics in Sewage Sludge at Wastewater Treatment Plants Located in Cádiz, Spain. Environ. Pollut. 2023, 317, 120689. [Google Scholar] [CrossRef]
- Harley-Nyang, D.; Memon, F.A.; Jones, N.; Galloway, T. Investigation and Analysis of Microplastics in Sewage Sludge and Biosolids: A Case Study from One Wastewater Treatment Works in the UK. Sci. Total Environ. 2022, 823, 153735. [Google Scholar] [CrossRef]
- Weber, C.J.; Santowski, A.; Chifflard, P. Investigating the Dispersal of Macro- and Microplastics on Agricultural Fields 30 Years after Sewage Sludge Application. Sci. Rep. 2022, 12, 6401. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Brandes, E.; Fischer, F.; Fischer, D.; Brandt, J.; Labrenz, M. Agricultural Application of Microplastic-Rich Sewage Sludge Leads to Further Uncontrolled Contamination. Sci. Total Environ. 2022, 806, 150611. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. The Input–Output Balance of Microplastics Derived from Coated Fertilizer in Paddy Fields and the Timing of Their Discharge during the Irrigation Season. Chemosphere 2021, 279, 130574. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, J.; Zhai, W.; Liu, D.; Zhou, Z.; Wang, P. The Influence of Polyethylene Microplastics on Pesticide Residue and Degradation in the Aquatic Environment. J. Hazard. Mater. 2020, 394, 122517. [Google Scholar] [CrossRef]
- van Schothorst, B.; Beriot, N.; Huerta Lwanga, E.; Geissen, V. Sources of Light Density Microplastic Related to Two Agricultural Practices: The Use of Compost and Plastic Mulch. Environments 2021, 8, 36. [Google Scholar] [CrossRef]
- Gui, J.; Sun, Y.; Wang, J.; Chen, X.; Zhang, S.; Wu, D. Microplastics in Composting of Rural Domestic Waste: Abundance, Characteristics, and Release from the Surface of Macroplastics. Environ. Pollut. 2021, 274, 116553. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Beriot, N.; Corradini, F.; Silva, V.; Yang, X.; Baartman, J.; Rezaei, M.; van Schaik, L.; Riksen, M.; Geissen, V. Review of Microplastic Sources, Transport Pathways and Correlations with Other Soil Stressors: A Journey from Agricultural Sites into the Environment. Chem. Biol. Technol. Agric. 2022, 9, 20. [Google Scholar] [CrossRef]
- Li, S.; Ding, F.; Flury, M.; Wang, Z.; Xu, L.; Li, S.; Jones, D.L.; Wang, J. Macro- and Microplastic Accumulation in Soil after 32 Years of Plastic Film Mulching. Environ. Pollut. 2022, 300, 118945. [Google Scholar] [CrossRef]
- Mokhtarzadeh, Z.; Keshavarzi, B.; Moore, F.; Busquets, R.; Rezaei, M.; Padoan, E.; Ajmone-Marsan, F. Microplastics in Industrial and Urban Areas in South-West Iran. Int. J. Environ. Sci. Technol. 2022, 19, 10199–10210. [Google Scholar] [CrossRef]
- Fernandes, E.M.S.; de Souza, A.G.; Barbosa, R.F. da S.; Rosa, D. dos S. Municipal Park Grounds and Microplastics Contamination. J. Polym. Environ. 2022, 30, 5202–5210. [Google Scholar] [CrossRef]
- Yang, Z.; Lü, F.; Zhang, H.; Wang, W.; Shao, L.; Ye, J.; He, P. Is Incineration the Terminator of Plastics and Microplastics? J. Hazard. Mater. 2021, 401, 123429. [Google Scholar] [CrossRef]
- Caracci, E.; Vega-Herrera, A.; Dachs, J.; Berrojalbiz, N.; Buonanno, G.; Abad, E.; Llorca, M.; Moreno, T.; Farré, M. Micro(Nano)Plastics in the Atmosphere of the Atlantic Ocean. J. Hazard. Mater. 2023, 450, 131036. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Banerjee, T.; Badola, N.; Chauhan, J.S. Evidences of Microplastics in Aerosols and Street Dust: A Case Study of Varanasi City, India. Environ. Sci. Pollut. Res. 2022, 29, 82006–82013. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, K.; Janus, R.; Wądrzyk, M.; Wilczyńska-Michalik, W.; Natkański, P.; Michalik, M. Airborne Microplastic in the Atmospheric Deposition and How to Identify and Quantify the Threat: Semi-Quantitative Approach Based on Kraków Case Study. Int. J. Environ. Res. Public Health 2022, 19, 12252. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Uddin, S.; Fowler, S.W.; Behbehani, M. Microplastics in the Atmosphere: A Review. J. Environ. Expo. Assess. 2022, 1, 6. [Google Scholar] [CrossRef]
- Sun, X.; Song, R.; Liu, J.; Yan, S.; Li, Y.; Jin, X.; Liang, Y.; Wu, Y.; Mei, L.; Pan, R.; et al. Characterization of Airborne Microplastics at Different Workplaces of the Poly(Ethylene:Propylene:Diene) (EPDM) Rubber Industry. Environ. Sci. Pollut. Res. 2023, 30, 78839–78848. [Google Scholar] [CrossRef]
- Padha, S.; Kumar, R.; Dhar, A.; Sharma, P. Microplastic Pollution in Mountain Terrains and Foothills: A Review on Source, Extraction, and Distribution of Microplastics in Remote Areas. Environ. Res. 2022, 207, 112232. [Google Scholar] [CrossRef]
- Allen, D.; Allen, S.; Abbasi, S.; Baker, A.; Bergmann, M.; Brahney, J.; Butler, T.; Duce, R.A.; Eckhardt, S.; Evangeliou, N.; et al. Microplastics and Nanoplastics in the Marine-Atmosphere Environment. Nat. Rev. Earth Environ. 2022, 3, 393–405. [Google Scholar] [CrossRef]
- Jan Kole, P.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Goßmann, I.; Süßmuth, R.; Scholz-Böttcher, B.M. Plastic in the Air?!-Spider Webs as Spatial and Temporal Mirror for Microplastics Including Tire Wear Particles in Urban Air. Sci. Total Environ. 2022, 832, 155008. [Google Scholar] [CrossRef] [PubMed]
- Sieber, R.; Kawecki, D.; Nowack, B. Dynamic Probabilistic Material Flow Analysis of Rubber Release from Tires into the Environment. Environ. Pollut. 2020, 258, 113573. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Okoffo, E.D.; O’Brien, J.W.; Ribeiro, F.; Wang, X.; Wright, S.L.; Samanipour, S.; Rauert, C.; Toapanta, T.Y.A.; Albarracin, R.; et al. Airborne Emissions of Microplastic Fibres from Domestic Laundry Dryers. Sci. Total Environ. 2020, 747, 141175. [Google Scholar] [CrossRef]
- Pinlova, B.; Hufenus, R.; Nowack, B. Systematic Study of the Presence of Microplastic Fibers during Polyester Yarn Production. J. Clean. Prod. 2022, 363, 132247. [Google Scholar] [CrossRef]
- Dalla Fontana, G.; Mossotti, R.; Montarsolo, A. Influence of Sewing on Microplastic Release from Textiles during Washing. Water Air Soil Pollut. 2021, 232, 50. [Google Scholar] [CrossRef]
- Chen, E.Y.; Lin, K.T.; Jung, C.C.; Chang, C.L.; Chen, C.Y. Characteristics and Influencing Factors of Airborne Microplastics in Nail Salons. Sci. Total Environ. 2022, 806, 151472. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Moss, K.; Le Roux, G.; Phoenix, V.R.; Sonke, J.E. Examination of the Ocean as a Source for Atmospheric Microplastics. PLoS ONE 2020, 15, e0232746. [Google Scholar] [CrossRef]
- Harb, C.; Pokhrel, N.; Foroutan, H. Quantification of the Emission of Atmospheric Microplastics and Nanoplastics via Sea Spray. Environ. Sci. Technol. Lett. 2023, 10, 513–519. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, T.; Gan, Y.; Lu, X.; Chen, H.; Chen, J.; Yang, X.; Wang, X. Constraining Microplastic Particle Emission Flux from the Ocean. Environ. Sci. Technol. Lett. 2022, 9, 513–519. [Google Scholar] [CrossRef]
- Long, X.; Fu, T.M.; Yang, X.; Tang, Y.; Zheng, Y.; Zhu, L.; Shen, H.; Ye, J.; Wang, C.; Wang, T.; et al. Efficient Atmospheric Transport of Microplastics over Asia and Adjacent Oceans. Environ. Sci. Technol. 2022, 56, 6243–6252. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.A.; Neuman, C.L.M.; Aherne, J. Effects of Shape and Size on Microplastic Atmospheric Settling Velocity. Environ. Sci. Technol. 2023, 57, 11937–11947. [Google Scholar] [CrossRef]
- Ren, S.Y.; Ni, H.G. A Method for Measuring the Emissions of in Situ Agricultural Plastic Film Microplastics by Ultraviolet and Mechanical Abrasion. Sci. Total Environ. 2022, 819, 152041. [Google Scholar] [CrossRef]
- Bullard, J.E.; Zhou, Z.; Davis, S.; Fowler, S. Breakdown and Modification of Microplastic Beads by Aeolian Abrasion. Environ. Sci. Technol. 2023, 57, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Abbasi, S.; Pourmahmood, H.; Oleszczuk, P.; Ritsema, C.; Turner, A. Microplastics in Agricultural Soils from a Semi-Arid Region and Their Transport by Wind Erosion. Environ. Res. 2022, 212, 113213. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Riksen, M.J.P.M.; Sirjani, E.; Sameni, A.; Geissen, V. Wind Erosion as a Driver for Transport of Light Density Microplastics. Sci. Total Environ. 2019, 669, 273–281. [Google Scholar] [CrossRef]
- Yang, M.; Tian, X.; Guo, Z.; Chang, C.; Li, J.; Guo, Z.; Li, H.; Liu, R.; Wang, R.; Li, Q.; et al. Effect of Dry Soil Aggregate Size on Microplastic Distribution and Its Implications for Microplastic Emissions Induced by Wind Erosion. Environ. Sci. Technol. Lett. 2022, 9, 618–624. [Google Scholar] [CrossRef]
- Tian, X.; Yang, M.; Guo, Z.; Chang, C.; Li, J.; Guo, Z.; Wang, R.; Li, Q.; Zou, X. Plastic Mulch Film Induced Soil Microplastic Enrichment and Its Impact on Wind-Blown Sand and Dust. Sci. Total Environ. 2022, 813, 152490. [Google Scholar] [CrossRef]
- Fisher, S.; Bellinger, D.C.; Cropper, M.L.; Kumar, P.; Binagwaho, A.; Koudenoukpo, J.B.; Park, Y.; Taghian, G.; Landrigan, P.J. Air Pollution and Development in Africa: Impacts on Health, the Economy, and Human Capital. Lancet Planet. Health 2021, 5, e681–e688. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Visalli, G.; Ciarello, M.P.; Di Pietro, A. Newly Emerging Airborne Pollutants: Current Knowledge of Health Impact of Micro and Nanoplastics. Int. J. Environ. Res. Public Health 2021, 18, 2997. [Google Scholar] [CrossRef] [PubMed]
- Kiran, B.R.; Kopperi, H.; Venkata Mohan, S. Micro/Nano-Plastics Occurrence, Identification, Risk Analysis and Mitigation: Challenges and Perspectives; Springer: Dordrecht, The Netherlands, 2022; Volume 21. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Misra, M. Microplastics in Ecosystems: Their Implications and Mitigation Pathways. Environ. Sci. Adv. 2022, 1, 9–29. [Google Scholar] [CrossRef]
- Apetogbor, K.; Pereao, O.; Sparks, C.; Opeolu, B. Spatio-Temporal Distribution of Microplastics in Water and Sediment Samples of the Plankenburg River, Western Cape, South Africa. Environ. Pollut. 2023, 323, 121303. [Google Scholar] [CrossRef]
- Khan, L.; Ghias, S.; Zafar, M.I.; Alhodaib, A.; Fatima, H.; Ur-Rehman, T.; Waseem, A.; Howari, H. Exploration of Microplastic Pollution with Particular Focus on Source Identification and Spatial Patterns in Riverine Water, Sediment and Fish of the Swat River, Pakistan. RSC Adv. 2022, 12, 9556–9566. [Google Scholar] [CrossRef]
- Ayeleru, O.O.; Dlova, S.; Akinribide, O.J.; Ntuli, F.; Kupolati, W.K.; Marina, P.F.; Blencowe, A.; Olubambi, P.A. Challenges of Plastic Waste Generation and Management in Sub-Saharan Africa: A Review. Waste Manag. 2020, 110, 24–42. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, R.; Saxena, A.; Sekar, S. Microplastics from Face Masks: A Potential Hazard Post COVID-19 Pandemic. Chemosphere 2022, 302, 134805. [Google Scholar] [CrossRef] [PubMed]
- Arimiyaw, A.W.; Abass, K.; Morgan, A.K. Minimizing the Long-Term Impact of COVID-19 on Environmental Pollution in Sub-Saharan Africa. Sustain. Sci. Pract. Policy 2021, 17, 82–85. [Google Scholar] [CrossRef]
- Morgana, S.; Casentini, B.; Amalfitano, S. Uncovering the Release of Micro/Nanoplastics from Disposable Face Masks at Times of COVID-19. J. Hazard. Mater. 2021, 419, 126507. [Google Scholar] [CrossRef]
- Wang, Z.; An, C.; Chen, X.; Lee, K.; Zhang, B.; Feng, Q. Disposable Masks Release Microplastics to the Aqueous Environment with Exacerbation by Natural Weathering. J. Hazard. Mater. 2021, 417, 126036. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; O’Connor, D.; Wang, L.; Wu, W.M.; Luo, J.; Hou, D. Microplastics in Urban Runoff: Global Occurrence and Fate. Water Res. 2022, 225, 119129. [Google Scholar] [CrossRef] [PubMed]
- Beni, N.N.; Karimifard, S.; Gilley, J.; Messer, T.; Schmidt, A.; Bartelt-Hunt, S. Higher Concentrations of Microplastics in Runoff from Biosolid-Amended Croplands than Manure-Amended Croplands. Commun. Earth Environ. 2023, 4, 42. [Google Scholar] [CrossRef]
- Ross, M.S.; Loutan, A.; Groeneveld, T.; Molenaar, D.; Kroetch, K.; Bujaczek, T.; Kolter, S.; Moon, S.; Huynh, A.; Khayam, R.; et al. Estimated Discharge of Microplastics via Urban Stormwater during Individual Rain Events. Front. Environ. Sci. 2023, 11, 1090267. [Google Scholar] [CrossRef]
- Kretzmann, S. Communities Turned into Sewage Swamps. Cent. Collab. Investig. J. 2022. Available online: https://www.circleofblue.org/2022/world/communities-turned-into-sewage-swamps/ (accessed on 28 March 2023).
- Laville, S. ‘Streams as Toilets’: Thames Water’s Real-Time Map Shows Scale of Sewage Dumps. Guardian 2023. Available online: https://www.theguardian.com/environment/2023/jan/05/interactive-map-shows-thames-water-raw-sewage-discharges-in-england-rivers (accessed on 28 March 2023).
- Chan, C.K.M.; Park, C.; Chan, K.M.; Mak, D.C.W.; Fang, J.K.H.; Mitrano, D.M. Microplastic Fibre Releases from Industrial Wastewater Effluent: A Textile Wet-Processing Mill in China. Environ. Chem. 2021, 18, 93–100. [Google Scholar] [CrossRef]
- Barkmann-Metaj, L.; Weber, F.; Bitter, H.; Wolff, S.; Lackner, S.; Kerpen, J.; Engelhart, M. Quantification of Microplastics in Wastewater Systems of German Industrial Parks and Their Wastewater Treatment Plants. Sci. Total Environ. 2023, 881, 163349. [Google Scholar] [CrossRef] [PubMed]
- Bitter, H.; Lackner, S. First Quantification of Semi-Crystalline Microplastics in Industrial Wastewaters. Chemosphere 2020, 258, 127388. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P.; Suttie, M.; Lewis, V.T.; Smith, A.P.; Singer, A.C. Detection of Untreated Sewage Discharges to Watercourses Using Machine Learning. NPJ Clean Water 2021, 4, 18. [Google Scholar] [CrossRef]
- Kretzmann, S. Sewage Seeps into Vaal Dam as Mpumalanga Water Treatment Plants Fail. GroundUp 2022. Available online: https://www.dailymaverick.co.za/article/2022-09-09-sewage-continually-draining-into-vaal-dam-as-overburdened-underserviced-water-treatment-plants-fail/ (accessed on 6 April 2023).
- Dharmaraj, I.; Appavoo, M.S. Occurrence of Coliforms in Microplastic Associated Biofilm in Estuarine Ecosystem. Polish J. Environ. Stud. 2023, 32, 547–557. [Google Scholar] [CrossRef]
- Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G.; Shruti, V.C. A Central Role for Fecal Matter in the Transport of Microplastics: An Updated Analysis of New Findings and Persisting Questions. J. Hazard. Mater. Adv. 2021, 4, 100021. [Google Scholar] [CrossRef]
- Syversen, T.; Lilleng, G.; Vollstad, J.; Hanssen, B.J.; Sønvisen, S.A. Oceanic Plastic Pollution Caused by Danish Seine Fishing in Norway. Mar. Pollut. Bull. 2022, 179, 113711. [Google Scholar] [CrossRef]
- Syversen, T.; Lilleng, G. Microplastics Derived from Commercial Fishing Activities. In Advances and Challenges in Microplastics; Salama, E.-S., Ed.; IntechOpen: London, UK, 2022; pp. 1–19. [Google Scholar] [CrossRef]
- Vitale, D.; Spinelli, A.; Picó, Y. Microplastics Detected in Sediments and Rocks Substrate of Marine Areas with Ghost Nets. J. Mar. Sci. Eng. 2023, 11, 750. [Google Scholar] [CrossRef]
- Kuczenski, B.; Vargas Poulsen, C.; Gilman, E.L.; Musyl, M.; Geyer, R.; Wilson, J. Plastic Gear Loss Estimates from Remote Observation of Industrial Fishing Activity. Fish Fish. 2022, 23, 22–33. [Google Scholar] [CrossRef]
- Wright, L.S.; Napper, I.E.; Thompson, R.C. Potential Microplastic Release from Beached Fishing Gear in Great Britain’s Region of Highest Fishing Litter Density. Mar. Pollut. Bull. 2021, 173, 113115. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Hunt, J.; Kovaleva, M. Garbage Patches and Their Environmental Implications in a Plastisphere. J. Mar. Sci. Eng. 2021, 9, 1289. [Google Scholar] [CrossRef]
- Zhao, S.; Mincer, T.J.; Lebreton, L.; Egger, M. Pelagic Microplastics in the North Pacific Subtropical Gyre: A Prevalent Anthropogenic Component of the Particulate Organic Carbon Pool. Proc. Natl. Acad. Sci. USA Nexus 2023, 2, pgad070. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, F.; Zhang, D.; Cao, W.; Zhao, C. Zonal Distribution Characteristics of Microplastics in the Southern Indian Ocean and the Influence of Ocean Current. J. Mar. Sci. Eng. 2022, 10, 290. [Google Scholar] [CrossRef]
- Hajbane, S.; Calmanovici, B.; Reisser, J.; Jolly, A.; Summers, V.; Ferrari, F.; Ghadouani, A.; Pattiaratchi, C. Coastal Garbage Patches: Fronts Accumulate Plastic Films at Ashmore Reef Marine Park (Pulau Pasir), Australia. Front. Mar. Sci. 2021, 8, 613399. [Google Scholar] [CrossRef]
- Fauvelle, V.; Garel, M.; Tamburini, C.; Nerini, D.; Castro-Jiménez, J.; Schmidt, N.; Paluselli, A.; Fahs, A.; Papillon, L.; Booth, A.M.; et al. Organic Additive Release from Plastic to Seawater Is Lower under Deep-Sea Conditions. Nat. Commun. 2021, 12, 4426. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, B.; Guo, J.; Sun, C.; Shi, C.; Huang, S.; Liu, W.; Wu, C.; Zhang, Y. Aging Process of Microplastics in the Aquatic Environments: Aging Pathway, Characteristic Change, Compound Effect, and Environmentally Persistent Free Radicals Formation. Water 2022, 14, 3515. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Q.; Xing, X.; Chen, W.; She, Z.; Luo, Z. Raman Spectra and Surface Changes of Microplastics Weathered under Natural Environments. Sci. Total Environ. 2020, 739, 139990. [Google Scholar] [CrossRef]
- Al Harraq, A.; Bharti, B. Microplastics through the Lens of Colloid Science. ACS Environ. Au 2022, 2, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X. Environmental Behaviors of Microplastics in Aquatic Systems: A Systematic Review on Degradation, Adsorption, Toxicity and Biofilm under Aging Conditions. J. Hazard. Mater. 2022, 423, 126915. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lei, C.; Yuan, Y.; Xu, J.; Han, M. Nanoplastic Impacts on the Foliar Uptake, Metabolism and Phytotoxicity of Phthalate Esters in Corn (Zea mays L.) Plants. Chemosphere 2022, 304, 135309. [Google Scholar] [CrossRef]
- Sendra, M.; Saco, A.; Yeste, M.P.; Romero, A.; Novoa, B.; Figueras, A. Nanoplastics: From Tissue Accumulation to Cell Translocation into Mytilus galloprovincialis Hemocytes. Resilience of Immune Cells Exposed to Nanoplastics and Nanoplastics plus Vibrio Splendidus Combination. J. Hazard. Mater. 2020, 388, 121788. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Huang, Q.; Dong, S.; Wang, X.; Yan, C. Combined Effects of Micro-/Nano-Plastics and Oxytetracycline on the Intestinal Histopathology and Microbiome in Zebrafish (Danio rerio). Sci. Total Environ. 2022, 843, 156917. [Google Scholar] [CrossRef]
- Liu, S.J.; Huang, Z.Q.; Yang, C.; Yao, Q.; Dang, Z. Effect of Polystyrene Microplastics on the Degradation of Sulfamethazine: The Role of Persistent Free Radicals. Sci. Total Environ. 2022, 833, 155024. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Zhao, S.; Xia, T.; Guo, X.; Wang, T.; Zhu, L. Formation of Environmentally Persistent Free Radicals on Microplastics under Light Irradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef]
- Liu, S.; Huang, W.; Yang, J.; Xiong, Y.; Huang, Z.; Wang, J.; Cai, T.; Dang, Z.; Yang, C. Formation of Environmentally Persistent Free Radicals on Microplastics under UV Irradiations. J. Hazard. Mater. 2023, 453, 131277. [Google Scholar] [CrossRef]
- Völkl, M.; Jérôme, V.; Weig, A.; Jasinski, J.; Meides, N.; Strohriegl, P.; Scheibel, T.; Freitag, R. Pristine and Artificially-Aged Polystyrene Microplastic Particles Differ in Regard to Cellular Response. J. Hazard. Mater. 2022, 435, 128955. [Google Scholar] [CrossRef]
- Guerrera, M.C.; Aragona, M.; Porcino, C.; Fazio, F.; Laurà, R.; Levanti, M.; Montalbano, G.; Germanà, G.; Abbate, F.; Germanà, A. Micro and Nano Plastics Distribution in Fish as Model Organisms: Histopathology, Blood Response and Bioaccumulation in Different Organs. Appl. Sci. 2021, 11, 5768. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, Y.; Peijnenburg, W.J.G.M.; Li, R.; Yang, J.; Zhou, Q. Confocal Measurement of Microplastics Uptake by Plants. MethodsX 2020, 7, 100750. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective Uptake of Submicrometre Plastics by Crop Plants via a Crack-Entry Mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Zhang, W.; Li, Q.; Lin, B.; Wei, C. An Unrecognized Entry Pathway of Submicrometre Plastics into Crop Root: The Split of Hole in Protective Layer. J. Hazard. Mater. 2023, 457, 131732. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Uptake of Microplastics by Carrots in Presence of As (III): Combined Toxic Effects. J. Hazard. Mater. 2021, 411, 125055. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; Sun, H.; Wu, J.; Xua, J.; Sun, J. Foliar Uptake and In-Leaf Translocation of Micro(Nano)Plastics and Their Interaction with Epicuticular Wax. Environ. Sci. Nano 2023, 10, 1126–1137. [Google Scholar] [CrossRef]
- Falsini, S.; Colzi, I.; Chelazzi, D.; Dainelli, M.; Schiff, S.; Papini, A.; Coppi, A.; Gonnelli, C.; Ristori, S. Plastic Is in the Air: Impact of Micro-Nanoplastics from Airborne Pollution on Tillandsia usneoides (L.) L. (Bromeliaceae) as a Possible Green Sensor. J. Hazard. Mater. 2022, 437, 129314. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-Applied Polystyrene Nanoplastics (PSNPs) Reduce the Growth and Nutritional Quality of Lettuce (Lactuca sativa L.). Environ. Pollut. 2021, 280, 116978. [Google Scholar] [CrossRef]
- Wardlaw, C.M.; Corcoran, P.L.; Neff, B.D. Factors Influencing the Variation of Microplastic Uptake in Demersal Fishes from the Upper Thames River Ontario. Environ. Pollut. 2022, 313, 120095. [Google Scholar] [CrossRef]
- Justino, A.K.S.; Ferreira, G.V.B.; Schmidt, N.; Eduardo, L.N.; Fauvelle, V.; Lenoble, V.; Sempéré, R.; Panagiotopoulos, C.; Mincarone, M.M.; Frédou, T.; et al. The Role of Mesopelagic Fishes as Microplastics Vectors across the Deep-Sea Layers from the Southwestern Tropical Atlantic. Environ. Pollut. 2022, 300, 118988. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Khan, F.R.; Crowther, C.; Mitrano, D.M.; Thompson, R.C. Uptake, Distribution and Elimination of Palladium-Doped Polystyrene Nanoplastics in Rainbow Trout (Oncorhynchus mykiss) Following Dietary Exposure. Sci. Total Environ. 2023, 854, 158765. [Google Scholar] [CrossRef] [PubMed]
- van-der Veen, I.; van Mourik, L.M.; Van-Velzen, M.J.M.; Groenewoud, Q.R.; Leslie, H.A. Plastic Particles in Livestock Feed, Milk, Meat and Blood. Environ. Health 2022. Available online: https://www.plasticsoupfoundation.org/wp-content/uploads/2022/07/Final-Report-pilot-study-plastic-particles-in-livestock-feed-milk-meat-and-blood-SIGNED-1.pdf (accessed on 28 April 2023).
- Da, P.A.; Filho, C.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; et al. Detection and Characterization of Small-Sized Microplastics (≥5 Μm) in Milk Products. Sci. Rep. 2021, 11, 24046. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zhang, Z.; Halimu, G.; Li, Y.; Li, Y.; Gu, W.; Zhang, B.; Wang, X. In Vitro Study on the Toxicity of Nanoplastics with Different Charges to Murine Splenic Lymphocytes. J. Hazard. Mater. 2022, 424, 127508. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zuo, J.; Pan, M.; Nie, H.; Shen, J.; Yang, Q.; Hung, T.C.; Li, G. The Presence of Polystyrene Nanoplastics Enhances the MCLR Uptake in Zebrafish Leading to the Exacerbation of Oxidative Liver Damage. Sci. Total Environ. 2022, 818, 151749. [Google Scholar] [CrossRef]
- Kim, L.; Cui, R.; Il Kwak, J.; An, Y.J. Trophic Transfer of Nanoplastics through a Microalgae–Crustacean–Small Yellow Croaker Food Chain: Inhibition of Digestive Enzyme Activity in Fish. J. Hazard. Mater. 2022, 440, 129715. [Google Scholar] [CrossRef]
- Cary, C.M.; DeLoid, G.M.; Yang, Z.; Bitounis, D.; Polunas, M.; Goedken, M.J.; Buckley, B.; Cheatham, B.; Stapleton, P.A.; Demokritou, P. Ingested Polystyrene Nanospheres Translocate to Placenta and Fetal Tissues in Pregnant Rats: Potential Health Implications. Nanomaterials 2023, 13, 720. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Engineered Nanoparticles in Aquatic Systems: Toxicity and Mechanism of Toxicity in Fish. Emerg. Contam. 2023, 9, 100212. [Google Scholar] [CrossRef]
- Yee, M.S.L.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Brandts, I.; Cánovas, M.; Tvarijonaviciute, A.; Llorca, M.; Vega, A.; Farré, M.; Pastor, J.; Roher, N.; Teles, M. Nanoplastics Are Bioaccumulated in Fish Liver and Muscle and Cause DNA Damage after a Chronic Exposure. Environ. Res. 2022, 212, 113433. [Google Scholar] [CrossRef] [PubMed]
- Khoshnamv, M.; Hanachi, P.; Ashtiani, S.; Walker, T.R. Toxic Effects of Polystyrene Nanoplastics on Microalgae chlorella Vulgaris: Changes in Biomass, Photosynthetic Pigments and Morphology. Chemosphere 2021, 280, 130725. [Google Scholar] [CrossRef]
- Gomes, T.; Almeida, A.C.; Georgantzopoulou, A. Characterization of Cell Responses in Rhodomonas baltica Exposed to PMMA Nanoplastics. Sci. Total Environ. 2020, 726, 138547. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Silva, G.; Galleguillos Madrid, F.M.; Hernández, D.; Pincheira, G.; Peralta, A.K.; Urrestarazu Gavilán, M.; Vergara-Carmona, V.; Fuentes-Peñailillo, F. Microplastics and Their Effect in Horticultural Crops: Food Safety and Plant Stress. Agronomy 2021, 11, 1528. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Yao, Q.; Feng, X.; Shen, T.; Guo, P.; Wang, P.; Bai, Y.; Li, B.; Wang, P.; et al. Toxicological Effects of Micro/Nano-Plastics on Mouse/Rat Models: A Systematic Review and Meta-Analysis. Front. Public Health 2023, 11, 1103289. [Google Scholar] [CrossRef]
- Yasir, A.M.; Ma, J.; Ouyang, X.; Zhao, J.; Zhao, Y.; Weng, L.; Islam, M.S.; Chen, Y.; Li, Y. Effects of Selected Functional Groups on Nanoplastics Transport in Saturated Media under Diethylhexyl Phthalate Co-Contamination Conditions. Chemosphere 2022, 286, 131965. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Xu, Y.; Ren, L.; Yang, L.; Liu, X.; Dai, Y.; Zhao, J.; Yue, T. Distinguishing the Nanoplastic-Cell Membrane Interface by Polymer Type and Aging Properties: Translocation, Transformation and Perturbation. Environ. Sci. Nano 2023, 10, 440–453. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.; Liu, Y.; Mei, A.X.; Wang, X.; Shi, Q. Cellular Absorption of Polystyrene Nanoplastics with Different Surface Functionalization and the Toxicity to RAW264.7 Macrophage Cells. Ecotoxicol. Environ. Saf. 2023, 252, 114574. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhang, H.; Yuan, S. Understanding the Transformations of Nanoplastic onto Phospholipid Bilayers: Mechanism, Microscopic Interaction and Cytotoxicity Assessment. Sci. Total Environ. 2023, 859, 160388. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Huang, R. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef]
- Cheng, S.; Ye, Z.; Wang, X.; Lian, C.; Shang, Y.; Liu, H. Colloids and Surfaces B: Biointerfaces The Effects of Adsorbed Benzo (a) Pyrene on Dynamic Behavior of Polystyrene Nanoplastics through Phospholipid Membrane: A Molecular Simulation Study. Colloids Surf. B Biointerfaces 2023, 224, 113211. [Google Scholar] [CrossRef]
- Yan, L.; Yu, Z.; Lin, P.; Qiu, S.; He, L.; Wu, Z.; Ma, L.; Gu, Y.; He, L.; Dai, Z.; et al. Polystyrene Nanoplastics Promote the Apoptosis in Caco-2 Cells Induced by Okadaic Acid More than Microplastics. Ecotoxicol. Environ. Saf. 2023, 249, 114375. [Google Scholar] [CrossRef]
- Soto-Bielicka, P.; Tejeda, I.; Peropadre, A.; Hazen, M.J.; Fernández Freire, P. Detrimental Effects of Individual versus Combined Exposure to Tetrabromobisphenol A and Polystyrene Nanoplastics in Fish Cell Lines. Environ. Toxicol. Pharmacol. 2023, 98, 104072. [Google Scholar] [CrossRef]
- Barguilla, I.; Domenech, J.; Rubio, L.; Marcos, R.; Hernández, A. Nanoplastics and Arsenic Co-Exposures Exacerbate Oncogenic Biomarkers under an In Vitro Long-Term Exposure Scenario. Int. J. Mol. Sci. 2022, 23, 2958. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Y. Association between Microorganisms and Microplastics: How Does It Change the Host–Pathogen Interaction and Subsequent Immune Response? Int. J. Mol. Sci. 2023, 24, 4065. [Google Scholar] [CrossRef]
- Cheng, H.; Duan, Z.; Wu, Y.; Wang, Y.; Zhang, H.; Shi, Y.; Zhang, H.; Wei, Y.; Sun, H. Immunotoxicity Responses to Polystyrene Nanoplastics and Their Related Mechanisms in the Liver of Zebrafish (Danio rerio) Larvae. Environ. Int. 2022, 161, 107128. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, W.; Tang, Y.; Shi, W.; Shao, Y.; Ren, P.; Zhang, J.; Xiao, G.; Sun, H.; Liu, G. Microplastics Aggravate the Bioaccumulation of Three Veterinary Antibiotics in the Thick Shell Mussel Mytilus Coruscus and Induce Synergistic Immunotoxic Effects. Sci. Total Environ. 2021, 770, 145273. [Google Scholar] [CrossRef] [PubMed]
- Yedier, S.; Yalçınkaya, S.K.; Bostancı, D. Exposure to Polypropylene Microplastics via Diet and Water Induces Oxidative Stress in Cyprinus Carpio. Aquat. Toxicol. 2023, 259, 106540. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Fan, X.; Xu, T.; He, Y.; Chi, Q.; Li, Z.; Li, S. Polystyrene Nanoplastics Exacerbated Lipopolysaccharide-Induced Necroptosis and Inflammation via the ROS/MAPK Pathway in Mice Spleen. Environ. Toxicol. 2022, 37, 2552–2565. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xuan, Y.; Shen, H.; Tang, Y.; Zhang, T.; Xu, J. Surface Functionalization-Dependent Inflammatory Potential of Polystyrene Nanoplastics through the Activation of MAPK/ NF-ΚB Signaling Pathways in Macrophage Raw 264.7. Ecotoxicol. Environ. Saf. 2023, 251, 114520. [Google Scholar] [CrossRef]
- Woo, J.H.; Seo, H.J.; Lee, J.Y.; Lee, I.; Jeon, K.; Kim, B.; Lee, K. Polypropylene Nanoplastic Exposure Leads to Lung Inflammation through P38-Mediated NF-ΚB Pathway Due to Mitochondrial Damage. Part. Fibre Toxicol. 2023, 20, 2. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal Exposure to Polystyrene Nanoplastics during Gestation and Lactation Induces Hepatic and Testicular Toxicity in Male Mouse Offspring. Food Chem. Toxicol. 2022, 160, 112803. [Google Scholar] [CrossRef]
- Ho, Y.W.; Lim, J.Y.; Yeoh, Y.K.; Chiou, J.C.; Zhu, Y.; Lai, K.P.; Li, L.; Chan, P.K.S.; Fang, J.K.H. Preliminary Findings of the High Quantity of Microplastics in Faeces of Hong Kong Residents. Toxics 2022, 10, 6–12. [Google Scholar] [CrossRef]
- Wang, W.; Do, A.T.N.; Kwon, J.H. Ecotoxicological Effects of Micro- and Nanoplastics on Terrestrial Food Web from Plants to Human Beings. Sci. Total Environ. 2022, 834, 155333. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Gao, Y.; Li, Z.-C.; Zhou, X.-X.; Yan, B. Size-Dependent Uptake and Depuration of Nanoplastics in Tilapia (Oreochromis niloticus) and Distinct Intestinal Impacts. Environ. Sci. Technol. 2023, 57, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Z.; Zhou, X.; Su, L.; Wang, M.; Wang, T.; Zhang, H. Nanoplastic-Induced Vascular Endothelial Injury and Coagulation Dysfunction in Mice. Sci. Total Environ. 2023, 865, 161271. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene Nanoplastics Penetrate across the Blood-Brain Barrier and Induce Activation of Microglia in the Brain of Mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Dou, J.; Hou, Q.; Cheng, J.; Jiang, X. Bioeffects of Inhaled Nanoplastics on Neurons and Alteration of Animal Behaviors through Deposition in the Brain. Nano Lett. 2022, 22, 1091–1099. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, D.; Yang, S.; Shi, Z.; Pan, H.; Jin, Q.; Ding, Z. Neurotoxicity of Polystyrene Nanoplastics with Different Particle Sizes at Environment-Related Concentrations on Early Zebrafish Embryos. Sci. Total Environ. 2023, 872, 162096. [Google Scholar] [CrossRef]
- Barguilla, I.; Domenech, J.; Ballesteros, S.; Rubio, L.; Marcos, R.; Hernández, A. Long-Term Exposure to Nanoplastics Alters Molecular and Functional Traits Related to the Carcinogenic Process. J. Hazard. Mater. 2022, 438, 129470. [Google Scholar] [CrossRef] [PubMed]
- Sulukan, E.; Şenol, O.; Baran, A.; Kankaynar, M.; Yıldırım, S.; Kızıltan, T.; Bolat, İ.; Ceyhun, S.B. Nano-Sized Polystyrene Plastic Particles Affect Many Cancer-Related Biological Processes Even in the next Generations; Zebrafish Modeling. Sci. Total Environ. 2022, 838, 156391. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Parkin, D.M. Cancer in Sub-Saharan Africa in 2020: A Review of Current Estimates of the National Burden, Data Gaps, and Future Needs. Lancet Oncol. 2022, 23, 719–728. [Google Scholar] [CrossRef]
- Henderson, E. Cancer Mortality in Sub-Saharan Africa Could Reach 1 Million by 2030 without Rapid Interventions. Medicall News, 9 May 2022; 1–6. [Google Scholar]
- Ngwa, W.; Addai, B.W.; Adewole, I.; Ainsworth, V.; Alaro, J.; Alatise, O.I.; Ali, Z.; Anderson, B.O.; Anorlu, R.; Avery, S.; et al. Cancer in Sub-Saharan Africa: A Lancet Oncology Commission. Lancet Oncol. 2022, 23, e251–e312. [Google Scholar]
- Baj, J.; Dring, J.C.; Czeczelewski, M.; Kozyra, P.; Forma, A.; Flieger, J.; Kowalska, B.; Buszewicz, G.; Teresiński, G. Derivatives of Plastics as Potential Carcinogenic Factors: The Current State of Knowledge. Cancers 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez-Andrade, M.; Chirino, Y.I.; González-Ramírez, I.; Sánchez-Pérez, Y.; García-Cuellar, C.M. Deciphering the Code between Air Pollution and Disease: The Effect of Particulate Matter on Cancer Hallmarks. Int. J. Mol. Sci. 2020, 21, 136. [Google Scholar] [CrossRef]
- Koual, M.; Tomkiewicz, C.; Cano-Sancho, G.; Antignac, J.P.; Bats, A.S.; Coumoul, X. Environmental Chemicals, Breast Cancer Progression and Drug Resistance. Environ. Health A Glob. Access Sci. Source 2020, 19, 117. [Google Scholar] [CrossRef]
- Chen, Q.; Allgeier, A.; Yin, D.; Hollert, H. Leaching of Endocrine Disrupting Chemicals from Marine Microplastics and Mesoplastics under Common Life Stress Conditions. Environ. Int. 2019, 130, 104938. [Google Scholar] [CrossRef]
- Calaf, G.M.; Ponce-Cusi, R.; Aguayo, F.; Muñoz, J.P.; Bleak, T.C. Endocrine Disruptors from the Environment Affecting Breast Cancer (Review). Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Bhan, A.; Hussain, I.; Ansari, K.I.; Bobzean, S.A.; Pandita, T.K.; Perrotti, L.I.; Mandal, S.S. Endocrine Disrupting Chemical, Bisphenol-A, Induces Breast Cancer Associated Gene HOXB9 Expression In Vitro and In Vivo. Gene 2016, 590, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Martinou, E.; Moller-levet, C.; Karamanis, D.; Bagwan, I.; Angelidi, A.M. HOXB9 Overexpression Promotes Colorectal Cancer Progression and Is Associated with Worse Survival in Liver Resection Patients for Colorectal Liver Metastases. Int. J. Mol. Sci. 2022, 23, 2281. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 1–17. [Google Scholar] [CrossRef]
- Greenpeace Africa. Plastic Bans in Africa | A Reality Check; Greenpeace Africa: Randburg, South Africa, 2020. [Google Scholar]
- Iroegbu, A.O.C.; Ray, S.S.; Mbarane, V.; Bordado, J.C.; Sardinha, J.P. Plastic Pollution: A Perspective on Matters Arising: Challenges and Opportunities. ACS Omega 2021, 6, 19343–19355. [Google Scholar] [CrossRef]
- Syberg, K.; Nielsen, M.B.; Oturai, N.B.; Clausen, L.P.W.; Ramos, T.M.; Hansen, S.F. Circular Economy and Reduction of Micro(Nano)Plastics Contamination. J. Hazard. Mater. Adv. 2022, 5, 100044. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, E.; Okuthe, G.E. Plastics and Micro/Nano-Plastics (MNPs) in the Environment: Occurrence, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2023, 20, 6667. https://doi.org/10.3390/ijerph20176667
Dube E, Okuthe GE. Plastics and Micro/Nano-Plastics (MNPs) in the Environment: Occurrence, Impact, and Toxicity. International Journal of Environmental Research and Public Health. 2023; 20(17):6667. https://doi.org/10.3390/ijerph20176667
Chicago/Turabian StyleDube, Edith, and Grace Emily Okuthe. 2023. "Plastics and Micro/Nano-Plastics (MNPs) in the Environment: Occurrence, Impact, and Toxicity" International Journal of Environmental Research and Public Health 20, no. 17: 6667. https://doi.org/10.3390/ijerph20176667
APA StyleDube, E., & Okuthe, G. E. (2023). Plastics and Micro/Nano-Plastics (MNPs) in the Environment: Occurrence, Impact, and Toxicity. International Journal of Environmental Research and Public Health, 20(17), 6667. https://doi.org/10.3390/ijerph20176667





