Emergency Department Syndromic Surveillance to Monitor Tick-Borne Diseases: A 6-Year Small-Area Analysis in Northeastern Italy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Steinbrink, A.; Brugger, K.; Margos, G.; Kraiczy, P.; Klimpel, S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 2022, 121, 781–803. [Google Scholar] [CrossRef]
- Van Heuverswyn, J.; Hallmaier-Wacker, L.K.; Beauté, J.; Dias, J.G.; Haussig, J.M.; Busch, K.; Kerlik, J.; Markowicz, M.; Mäkelä, H.; Nygren, T.M.; et al. Spatiotemporal spread of tick-borne encephalitis in the EU/EEA, 2012 to 2020. Eurosurveillance 2023, 28, 2200543. [Google Scholar] [CrossRef]
- Kunze, M.; Banović, P.; Bogovič, P.; Briciu, V.; Čivljak, R.; Dobler, G.; Hristea, A.; Kerlik, J.; Kuivanen, S.; Kynčl, J.; et al. Recommendations to Improve Tick-Borne Encephalitis Surveillance and Vaccine Uptake in Europe. Microorganisms 2022, 10, 1283. [Google Scholar] [CrossRef]
- Burn, L.; Vyse, A.; Pilz, A.; Tran, T.M.P.; Fletcher, M.A.; Angulo, F.J.; Gessner, B.D.; Moïsi, J.C.; Stark, J.H. Incidence of Lyme Borreliosis in Europe: A Systematic Review (2005–2020). Vector-Borne Zoonotic Dis. 2023, 23, 172–194. [Google Scholar] [CrossRef]
- Burn, L.; Pilz, A.; Vyse, A.; Rabá, A.V.G.; Angulo, F.J.; Tran, T.M.P.; Fletcher, M.A.; Gessner, B.D.; Moïsi, J.C.; Stark, J.H. Seroprevalence of Lyme Borreliosis in Europe: Results from a Systematic Literature Review (2005–2020). Vector-Borne Zoonotic Dis. 2023, 23, 195–220. [Google Scholar] [CrossRef]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2016, 39, 74–81. [Google Scholar] [CrossRef]
- Hönig, V.; Švec, P.; Marek, L.; Mrkvička, T.; Dana, Z.; Wittmann, M.; Masař, O.; Szturcová, D.; Růžek, D.; Pfister, K.; et al. Model of Risk of Exposure to Lyme Borreliosis and Tick-Borne Encephalitis Virus-Infected Ticks in the Border Area of the Czech Republic (South Bohemia) and Germany (Lower Bavaria and Upper Palatinate). Int. J. Environ. Res. Public Health 2019, 16, 1173. [Google Scholar] [CrossRef]
- Chiffi, G.; Grandgirard, D.; Leib, S.L.; Chrdle, A.; Růžek, D. Tick-borne encephalitis: A comprehensive review of the epidemiology, virology, and clinical picture. Rev. Med. Virol. 2023, 33, e2470. [Google Scholar] [CrossRef]
- Erber, W.; Schmitt, H.J.; Vukovic, J.T.; Dobler, G.; Broker, M. The TBE Book; Global Health Press: Singapore, 2019. [Google Scholar]
- Cocchio, S.; Bertoncello, C.; Napoletano, G.; Claus, M.; Furlan, P.; Fonzo, M.; Gagliani, A.; Saia, M.; Russo, F.; Baldovin, T.; et al. Do We Know the True Burden of Tick-Borne Encephalitis? A Cross-Sectional Study. Neuroepidemiology 2020, 54, 227–234. [Google Scholar] [CrossRef]
- Rossi, B.; Barreca, F.; Benvenuto, D.; Braccialarghe, N.; Campogiani, L.; Lodi, A.; Aguglia, C.; Cavasio, R.A.; Giacalone, M.L.; Kontogiannis, D.; et al. Human Arboviral Infections in Italy: Past, Current, and Future Challenges. Viruses 2023, 15, 368. [Google Scholar] [CrossRef]
- Olsen, J.; Angulo, F.J.; Pilz, A.; Halsby, K.; Kelly, P.; Turunen, J.; Åhman, H.; Stark, J.; Jodar, L. Estimated Number of Symptomatic Lyme Borreliosis Cases in Adults in Finland in 2021 Using Seroprevalence Data to Adjust the Number of Surveillance-Reported Cases: A General Framework for Accounting for Underascertainment by Public Health Surveillance. Vector-Borne Zoonotic Dis. 2023, 23, 265–272. [Google Scholar] [CrossRef]
- Blanchard, L.; Jones-Diette, J.; Lorenc, T.; Sutcliffe, K.; Sowden, A.; Thomas, J. Comparison of national surveillance systems for Lyme disease in humans in Europe and North America: A policy review. BMC Public Health 2022, 22, 1307. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, A.; Aziz, U.; Song, B.; Zeb, J.; George, D.; Li, J.; Sparagano, O. The Role of Ticks in the Emergence of Borrelia burgdorferi as a Zoonotic Pathogen and Its Vector Control: A Global Systemic Review. Microorganisms 2021, 9, 2412. [Google Scholar] [CrossRef]
- Saydam, F.N.; Erdem, H.; Ankarali, H.; Ramadan, M.E.E.-A.; El-Sayed, N.M.; Civljak, R.; Pshenichnaya, N.; Moroti, R.V.; Mahmuodabad, F.M.; Maduka, A.V.; et al. Vector-borne and zoonotic infections and their relationships with regional and socioeconomic statuses: An ID-IRI survey in 24 countries of Europe, Africa and Asia. Travel Med. Infect. Dis. 2021, 44, 102174. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Cutler, S.; Potkonjak, A.; Vassier-Tussaut, M.; Van Bortel, W.; Zeller, H.; Fernández-Ruiz, N.; Mihalca, A.D. An updated meta-analysis of the distribution and prevalence of Borrelia burgdorferi s.l. in ticks in Europe. Int. J. Health Geogr. 2018, 17, 41. [Google Scholar] [CrossRef]
- Jenkins, V.A.; Silbernagl, G.; Baer, L.R.; Hoet, B. The epidemiology of infectious diseases in Europe in 2020 versus 2017–2019 and the rise of tick-borne encephalitis (1995–2020). Ticks Tick-Borne Dis. 2022, 13, 101972. [Google Scholar] [CrossRef]
- Della Salute, M. Piano Nazionale di Prevenzione, Sorveglianza e Risposta Alle Arbovirosi (PNA) 2020–2025. (Italian Ministry of Health. National Plan for Prevention, Surveillance and Response to Arboviruses 2020–2025). Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?id=2947&lingua=italiano (accessed on 21 July 2023).
- Daly, E.R.; Fredette, C.; Mathewson, A.A.; Dufault, K.; Swenson, D.J.; Chan, B.P. Tick bite and Lyme disease-related emergency department encounters in New Hampshire, 2010–2014. Zoonoses Public Health 2017, 64, 655–661. [Google Scholar] [CrossRef]
- Quarsten, H.; Henningsson, A.; Krogfelt, K.A.; Strube, C.; Wennerås, C.; Mavin, S. Tick-borne diseases under the radar in the North Sea Region. Ticks Tick-Borne Dis. 2023, 14, 102185. [Google Scholar] [CrossRef]
- Nagarajan, A.; Skufca, J.; Vyse, A.; Pilz, A.; Begier, E.; Riera-Montes, M.; Gessner, B.D.; Stark, J.H. The Landscape of Lyme Borreliosis Surveillance in Europe. Vector-Borne Zoonotic Dis. 2023, 23, 142–155. [Google Scholar] [CrossRef]
- Wint, G.W.; Balenghien, T.; Berriatua, E.; Braks, M.; Marsboom, C.; Medlock, J.; Schaffner, F.; Van Bortel, W.; Alexander, N.; Alten, B.; et al. VectorNet: Collaborative mapping of arthropod disease vectors in Europe and surrounding areas since 2010. Euro. Surveill. 2023, 28, 2200666. [Google Scholar] [CrossRef]
- Martin, L.J.; Hjertqvist, M.; Straten, E.V.; Bjelkmar, P. Investigating novel approaches to tick-borne encephalitis surveillance in Sweden, 2010-2017. Ticks Tick-Borne Dis. 2020, 11, 101486. [Google Scholar] [CrossRef]
- Vu Hai, V.; Almeras, L.; Socolovschi, C.; Raoult, D.; Parola, P.; Pagès, F. Monitoring human tick-borne disease risk and tick bite exposure in Europe. Available tools and promising future methods. Ticks Tick-Borne Dis. 2014, 5, 607–619. [Google Scholar] [CrossRef]
- Riccò, M. Epidemiology of Tick-borne encephalitis in North-Eastern Italy (2017–2020): International insights from national notification reports. Acta Biomed. Atenei. Parm. 2021, 92, e2021229. [Google Scholar] [CrossRef]
- Zanzani, S.A.; Rimoldi, S.G.; Manfredi, M.; Grande, R.; Gazzonis, A.L.; Merli, S.; Olivieri, E.; Giacomet, V.; Antinori, S.; Cislaghi, G.; et al. Lyme borreliosis incidence in Lombardy, Italy (2000–2015): Spatiotemporal analysis and environmental risk factors. Ticks Tick-Borne Dis. 2019, 10, 101257. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Synergies in Community and Institutional Public Health Emergency Preparedness for Tick-Borne Diseases in the Netherlands: A Case Study on Tick-Borne Encephalitis and Lyme Borreliosis; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2018. [CrossRef]
- Smith, G.E.; Elliot, A.J.; Lake, I.; Edeghere, O.; Morbey, R.; Catchpole, M.; Heymann, D.L.; Hawker, J.; Ibbotson, S.; McCloskey, B.; et al. Syndromic surveillance: Two decades experience of sustainable systems—Its people not just data! Epidemiol. Infect. 2019, 147, e101. [Google Scholar] [CrossRef]
- Yoon, P.W.; Ising, A.I.; Gunn, J.E. Using Syndromic Surveillance for All-Hazards Public Health Surveillance, Successes, Challenges, and the Future. Public Health Rep. 2017, 132, 3S–6S. [Google Scholar] [CrossRef]
- ISTAT. Principali Statistiche Geografiche Sui Comuni 2023. Available online: https://www.istat.it/it/archivio/156224 (accessed on 21 July 2023).
- Hook, S.A.; Nawrocki, C.C.; Meek, J.I.; Feldman, K.A.; White, J.L.; Connally, N.P.; Hinckley, A.F. Human-tick encounters as a measure of tickborne disease risk in lyme disease endemic areas. Zoonoses Public Health 2021, 68, 384–392. [Google Scholar] [CrossRef]
- Marx, G.E.; Spillane, M.; Beck, A.; Stein, Z.; Powell, A.K.; Hinckley, A.F. Emergency Department Visits for Tick Bites—United States, January 2017–December 2019. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 612–616. [Google Scholar] [CrossRef]
- Newitt, S.; Elliot, A.J.; Morbey, R.; Durnall, H.; Pietzsch, M.E.; Medlock, J.M.; Leach, S.; Smith, G.E. The use of syndromic surveillance to monitor the incidence of arthropod bites requiring healthcare in England, 2000–2013: A retrospective ecological study. Epidemiol. Infect. 2016, 144, 2251–2259. [Google Scholar] [CrossRef]
- Beltrame, A.; Rodari, P.; Mauroner, L.; Zanella, F.; Moro, L.; Bertoli, G.; Da Re, F.; Russo, F.; Napoletano, G.; Silva, R. Emergence of Lyme borreliosis in the province of Verona, Northern Italy: Five-years of sentinel surveillance. Ticks Tick-Borne Dis. 2021, 12, 101628. [Google Scholar] [CrossRef]
- del Veneto, R. Bur n. 53 of 24 May 2019. Deliberation of the Regional Council no. 612 of 14 May 2019. Approval of the Technical Document “Vaccination against Tick Borne Encephalitis (TBE) in the Veneto Region” and Amendment of the “Regional Vaccination Tariff”, Connected to the “Single Regional Tariff for Services Rendered by the Prevention Departments of the Veneto Local Health Units”, in the Part Relating to the Offer of Tick-Borne Encephalitis (TBE) Vaccination. Available online: https://bur.regione.veneto.it/BurvServices/pubblica/DettaglioDgr.aspx?id=394640 (accessed on 21 July 2023).
- Hansen, M.F.; Sørensen, P.K.; Sørensen, A.E.; Krogfelt, K.A. Can protection motivation theory predict protective behavior against ticks? BMC Public Health 2023, 23, 1214. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Ruscio, M.; Cinco, M.; Nan, K.; Forgione, P.; Di Meo, N.; Tranchini, P.; Nacca, M.; Trincone, S.; Rimoldi, S.G.; et al. The history of Lyme disease in Italy and its spread in the Italian territory. Front. Pharmacol. 2023, 14, 1128142. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.F.; Holding, M.L.; Sprong, H.; Jansen, P.A.; Esser, H.J. Biodiversity in the Lyme-light: Ecological restoration and tick-borne diseases in Europe. Trends Parasitol. 2023, 39, 373–385. [Google Scholar] [CrossRef] [PubMed]
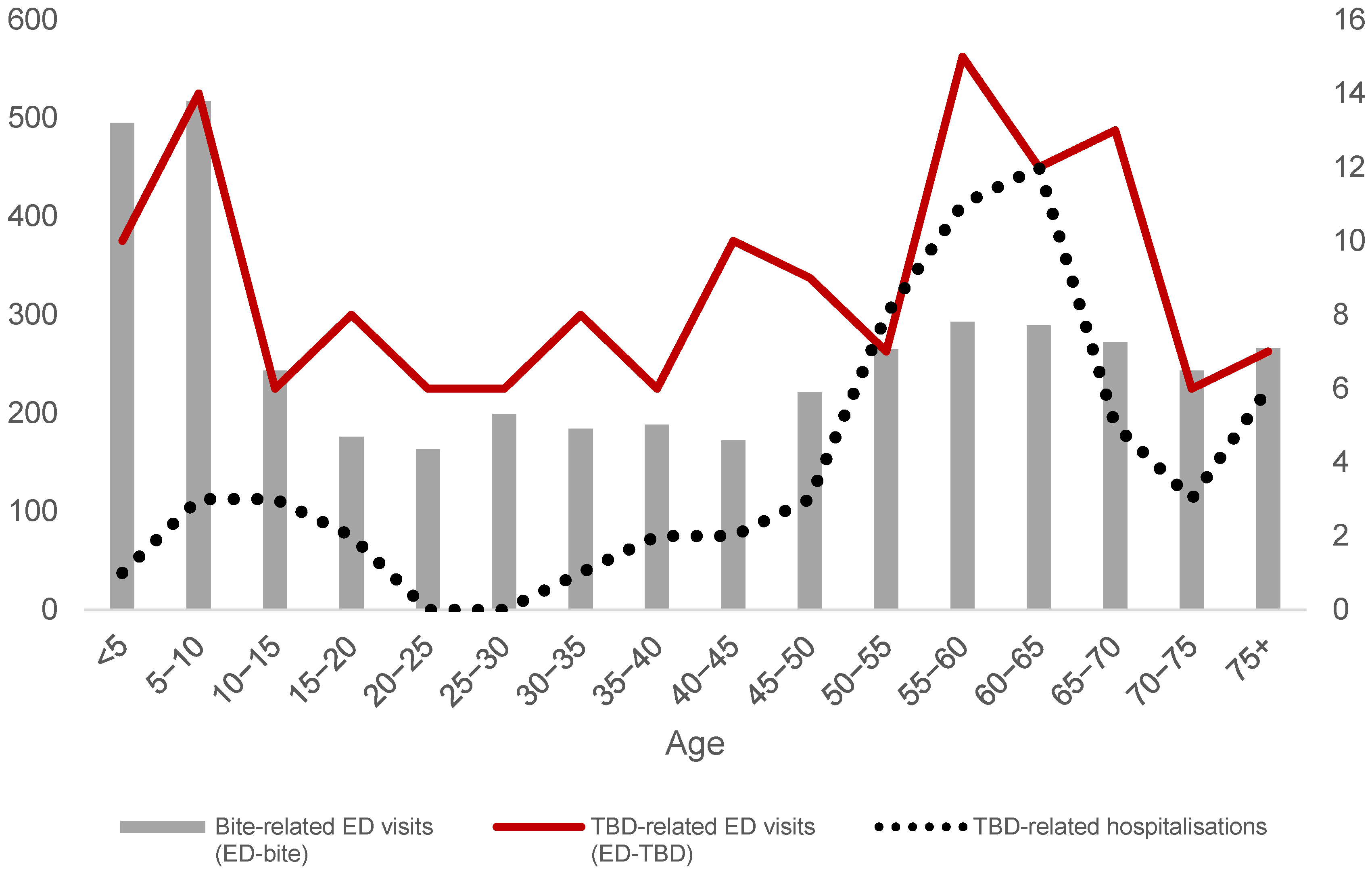


| Emergency Department Visits | Hospitalisations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bite-Related * | TBD-Related ** | Total ED Visits | LB-Related | TBE-Related | Total Hosp. | ||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Total | 4187 | 100% | 143 | 100% | 4330 | 100% | 31 | 100% | 31 | 100% | 62 | 100% | |
| Month | January | 20 | 0.5% | 1 | 0.7% | 21 | 0.5% | 1 | 3.2% | 0 | 0.0% | 1 | 1.6% |
| February | 60 | 1.4% | 4 | 2.8% | 64 | 1.5% | 2 | 6.5% | 0 | 0.0% | 2 | 3.2% | |
| March | 202 | 4.8% | 1 | 0.7% | 203 | 4.7% | 1 | 3.2% | 1 | 3.2% | 2 | 3.2% | |
| April | 605 | 14.4% | 12 | 8.4% | 617 | 14.2% | 2 | 6.5% | 1 | 3.2% | 3 | 4.8% | |
| May | 1061 | 25.3% | 28 | 19.6% | 1089 | 25.2% | 5 | 16.1% | 1 | 3.2% | 6 | 9.7% | |
| June | 1029 | 24.6% | 30 | 21.0% | 1059 | 24.5% | 3 | 9.7% | 2 | 6.5% | 5 | 8.1% | |
| July | 487 | 11.6% | 21 | 14.7% | 508 | 11.7% | 6 | 19.4% | 6 | 19.4% | 12 | 19.4% | |
| August | 259 | 6.2% | 15 | 10.5% | 274 | 6.3% | 5 | 16.1% | 4 | 12.9% | 9 | 14.5% | |
| September | 140 | 3.3% | 10 | 7.0% | 150 | 3.5% | 0 | 0.0% | 6 | 19.4% | 6 | 9.7% | |
| October | 231 | 5.5% | 12 | 8.4% | 243 | 5.6% | 1 | 3.2% | 3 | 9.7% | 4 | 6.5% | |
| November | 72 | 1.7% | 7 | 4.9% | 79 | 1.8% | 3 | 9.7% | 7 | 22.6% | 10 | 16.1% | |
| December | 21 | 0.5% | 2 | 1.4% | 23 | 0.5% | 2 | 6.5% | 0 | 0.0% | 2 | 3.2% | |
| Year | 2017 | 668 | 16.0% | 22 | 15.4% | 690 | 15.9% | 5 | 16.1% | 2 | 6.5% | 7 | 11.3% |
| 2018 | 953 | 22.8% | 20 | 14.0% | 973 | 22.5% | 5 | 16.1% | 0 | 0.0% | 5 | 8.1% | |
| 2019 | 740 | 17.7% | 26 | 18.2% | 766 | 17.7% | 8 | 25.8% | 7 | 22.6% | 15 | 24.2% | |
| 2020 | 615 | 14.7% | 20 | 14.0% | 635 | 14.7% | 7 | 22.6% | 3 | 9.7% | 10 | 16.1% | |
| 2021 | 515 | 12.3% | 26 | 18.2% | 541 | 12.5% | 4 | 12.9% | 8 | 25.8% | 12 | 19.4% | |
| 2022 | 696 | 16.6% | 29 | 20.3% | 725 | 16.7% | 2 | 6.5% | 11 | 35.5% | 13 | 21.0% | |
| Sex | Male | 2490 | 59.5% | 89 | 62.2% | 2579 | 59.6% | 17 | 54.8% | 21 | 67.7% | 38 | 61.3% |
| Female | 1696 | 40.5% | 54 | 37.8% | 1750 | 40.4% | 14 | 45.2% | 10 | 32.3% | 24 | 38.7% | |
| Age | <5 | 495 | 11.8% | 10 | 7.0% | 505 | 11.7% | 0 | 0.0% | 1 | 3.2% | 1 | 1.6% |
| 5–10 | 517 | 12.4% | 14 | 9.8% | 531 | 12.3% | 3 | 9.7% | 0 | 0.0% | 3 | 4.8% | |
| 10–15 | 243 | 5.8% | 6 | 4.2% | 249 | 5.8% | 2 | 6.5% | 1 | 3.2% | 3 | 4.8% | |
| 15–20 | 176 | 4.2% | 8 | 5.6% | 184 | 4.3% | 2 | 6.5% | 0 | 0.0% | 2 | 3.2% | |
| 20–25 | 163 | 3.9% | 6 | 4.2% | 169 | 3.9% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| 25–30 | 199 | 4.8% | 6 | 4.2% | 205 | 4.7% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| 30–35 | 184 | 4.4% | 8 | 5.6% | 192 | 4.4% | 1 | 3.2% | 0 | 0.0% | 1 | 1.6% | |
| 35–40 | 188 | 4.5% | 6 | 4.2% | 194 | 4.5% | 1 | 3.2% | 1 | 3.2% | 2 | 3.2% | |
| 40–45 | 172 | 4.1% | 10 | 7.0% | 182 | 4.2% | 1 | 3.2% | 1 | 3.2% | 2 | 3.2% | |
| 45–50 | 221 | 5.3% | 9 | 6.3% | 230 | 5.3% | 1 | 3.2% | 2 | 6.5% | 3 | 4.8% | |
| 50–55 | 265 | 6.3% | 7 | 4.9% | 272 | 6.3% | 6 | 19.4% | 2 | 6.5% | 8 | 12.9% | |
| 55–60 | 293 | 7.0% | 15 | 10.5% | 308 | 7.1% | 3 | 9.7% | 8 | 25.8% | 11 | 17.7% | |
| 60–65 | 289 | 6.9% | 12 | 8.4% | 301 | 7.0% | 7 | 22.6% | 5 | 16.1% | 12 | 19.4% | |
| 65–70 | 272 | 6.5% | 13 | 9.1% | 285 | 6.6% | 2 | 6.5% | 3 | 9.7% | 5 | 8.1% | |
| 70–75 | 243 | 5.8% | 6 | 4.2% | 249 | 5.8% | 0 | 0.0% | 3 | 9.7% | 3 | 4.8% | |
| 75+ | 266 | 6% | 7 | 4.9% | 273 | 6.3% | 2 | 6.5% | 4 | 12.9% | 6 | 9.7% | |
| Area | LB-Related Hospitalisation Rate (per 100,000/Year) | TBE-Related Hospitalisation Rate (per 100,000/Year) |
|---|---|---|
| North-west subarea | 2.08 | 1.84 |
| South-west subarea | 1.60 | 0.00 |
| Rest of LHA district | 0.34 | 0.46 |
| Entire LHA district | 0.99 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colucci, M.; Fonzo, M.; Miccolis, L.; Amoruso, I.; Mondino, S.; Trevisan, A.; Cazzaro, R.; Baldovin, T.; Bertoncello, C. Emergency Department Syndromic Surveillance to Monitor Tick-Borne Diseases: A 6-Year Small-Area Analysis in Northeastern Italy. Int. J. Environ. Res. Public Health 2023, 20, 6822. https://doi.org/10.3390/ijerph20196822
Colucci M, Fonzo M, Miccolis L, Amoruso I, Mondino S, Trevisan A, Cazzaro R, Baldovin T, Bertoncello C. Emergency Department Syndromic Surveillance to Monitor Tick-Borne Diseases: A 6-Year Small-Area Analysis in Northeastern Italy. International Journal of Environmental Research and Public Health. 2023; 20(19):6822. https://doi.org/10.3390/ijerph20196822
Chicago/Turabian StyleColucci, Massimiliano, Marco Fonzo, Liana Miccolis, Irene Amoruso, Sara Mondino, Andrea Trevisan, Romina Cazzaro, Tatjana Baldovin, and Chiara Bertoncello. 2023. "Emergency Department Syndromic Surveillance to Monitor Tick-Borne Diseases: A 6-Year Small-Area Analysis in Northeastern Italy" International Journal of Environmental Research and Public Health 20, no. 19: 6822. https://doi.org/10.3390/ijerph20196822
APA StyleColucci, M., Fonzo, M., Miccolis, L., Amoruso, I., Mondino, S., Trevisan, A., Cazzaro, R., Baldovin, T., & Bertoncello, C. (2023). Emergency Department Syndromic Surveillance to Monitor Tick-Borne Diseases: A 6-Year Small-Area Analysis in Northeastern Italy. International Journal of Environmental Research and Public Health, 20(19), 6822. https://doi.org/10.3390/ijerph20196822










